ΔFosB regulates rosiglitazone-induced milk fat synthesis and cell survival
Abstract
Rosiglitazone induces adipogenesis in adipocyte and regulates cell survival and differentiation in number of cell types. However, whether PPARγ regulates the synthesis of milk fat and cell survival in goat mammary gland remains unknown. Rosiglitazone strongly enhanced cellular triacylglycerol content and accumulation of lipid droplet in goat mammary epithelial cells (GMEC). Furthermore, ΔFosB decreased the expression of PPARγ at both mRNA and protein levels, and rosiglitazone-induced milk fat synthesis was abolished by ΔFosB overexpression. ΔFosB reduced milk fat synthesis and enhanced saturated fatty acid concentration. Rosiglitazone increased the number of GMEC in G0/G1 phase and inhibited cell proliferation, and these effects were improved by overexpression of ΔFosB. ΔFosB was found to promote the expression of Bcl-2 and suppress the expression of Bax, and protected GMEC from apoptosis induced by rosiglitazone. Intracellular calcium trafficking assay revealed that rosiglitazone markedly increased intracellular calcium concentration. ΔFosB protected GMEC from apoptosis induced by intracellular Ca2+ overload. ΔFosB increased MMP-9 gelatinolytic activity. SB-3CT, an MMP-9 inhibitor, suppressed the expression of Bcl-2, and increased intracellular calcium levels, and this effect was abolished by ΔFosB overexpression. SB-3CT induced GMEC apoptosis and this effect was inhibited by ΔFosB overexpression. These findings suggest that ΔFosB regulates rosiglitazone-induced milk fat synthesis and cell survival. Therefore, ΔFosB may be an important checkpoint to control milk fat synthesis and cell apoptosis.
Abbreviations
-
- ΔFosB
-
- FBJ murine osteosarcoma viral oncogene-like protein B
-
- AGPAT6
-
- 1-acylglycerol-3-phosphate O-acyltransferase 6
-
- Bad
-
- Bcl-2-associated death promoter
-
- Bax
-
- Bcl-2-associated X protein
-
- Bcl-2
-
- B-cell lymphoma 2
-
- Bcl-xL
-
- B-cell lymphoma-extra large
-
- CCK-8
-
- cell counting kit-8
-
- CD36
-
- thrombospondin receptor
-
- C/EBPα
-
- CCAAT/enhancer binding protein α
-
- DGAT1
-
- diacylglycerol acyl transferase 1
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- FA
-
- fatty acid
-
- FABP4
-
- fatty acid binding protein 4
-
- FASN
-
- fatty acid synthase
-
- FBS
-
- fetal bovine serum
-
- FITC
-
- fluorescein isothiocyanate
-
- Fra-1
-
- Fos-related antigen 1
-
- Fra-2
-
- Fos-related antigen 2
-
- GAPDH
-
- glyceraldehyde-3-phosphate dehydrogenase
-
- GC-MS
-
- gas chromatography-mass spectrometry
-
- LPL
-
- Lipoprotein lipase
-
- PBS
-
- phosphate buffered saline
-
- PCNA
-
- proliferating cell nuclear antigen
-
- PI
-
- propidium iodide
-
- PLIN2
-
- perilipin2
-
- PLIN3
-
- perilipin3
-
- PPARγ
-
- peroxisome proliferator-activated receptor γ
-
- MRPL39
-
- mitochondrial ribosomal protein L39
-
- NRIH3
-
- Liver X receptor alpha
-
- qPCR
-
- quantitative real time PCR
-
- SCD
-
- stearoyl-CoA desaturase
-
- SREBF1
-
- Sterol regulatory element-binding transcription factor 1
-
- TAG
-
- triacylglycerol
-
- UXT
-
- ubiquitously expressed transcript
1 INTRODUCTION
Peroxisome proliferator-activated receptor γ (PPARγ), a member of nuclear receptor, is predominantly expressed in adipose tissue and induced dramatically during adipogenesis (Chawla & Lazar, 1994; Tontonoz, Hu, Graves, Budavari, & Spiegelman, 1994). PPARγ binds near most adipogenic genes as a heterodimer with retinoid X receptor (Lefterova et al., 2008; Step et al., 2014). It is necessary (Rosen et al., 1999) and sufficient (Tontonoz, Hu, & Spiegelman, 1994) for adipogenesis and is also critical for the functions of mature adipocytes, including adipokine secretion, lipid metabolism, and insulin-stimulated glucose uptake (Rangwala & Lazar, 2004). Rosiglitazone, a gold standard PPARγ agonist, is effective drug to lower plasma glucose levels in Type II diabetic patients (Werner & Travaglini, 2001). Rosiglitazone induces adipogenesis, thus reducing glycerol, triacylglycerol, free fatty acids, and glucose in the circulation, and increasing insulin sensitivity of the liver, muscle, and other organs (Rong et al., 2007). However, a meta-analysis published in the New England Journal of Medicine in 2007 linked the drug's use to an increased risk of heart attack (Nissen & Wolski, 2007), and then this drug was used restrictively. Later, in 2013 the FDA lifted its earlier restrictions on rosiglitazone after reviewing the results of the 2009 RECORD clinical trial (a 6-year, open label randomized control trial), which failed to show heart attack risks associated with the drug (Food and Drugs Administration, 2013).
Although key role of PPARγ is demonstrated in the differentiation of adipocytes, PPARγ also regulates the growth, differentiation, and apoptosis in number of cell types including mammary epithelial cells (Jain et al., 1998), a majority of breast cancer cell lines (Elstner et al., 1998), and breast tumors (Mueller et al., 1998). Several studies have reported that PPARγ in vitro induces differentiation and suppresses growth in MDAMB-231 and MCF-7 cells (Elstner et al., 1998; Yu et al., 2008). Others using PPARγ agonists in vivo successfully induced regression of breast tumors in rodents (Apostoli et al., 2014; Pighetti et al., 2000; Suh et al., 1999; Yee, Young, Rosol, VanBuskirk, & Clinton, 2005). In addition, PPARγ agonist has been shown to exhibit an anti-inflammatory effect in vitro and in vivo (Cuzzocrea et al., 2004; Jiang, Ting, & Seed, 1998). These studies reflect that normal PPARγ expression is crucial to inhibiting breast tumor development; however, the role of PPARγ in cell survival of dairy goat mammary epithelial cells (GMEC) remains to be characterized.
Milk fat is an important component of milk production, and is necessary for neonatal growth and development (Bernard et al., 2005). Mammary gland epithelial cells which synthesize and secrete milk fat can be a cellular mode of research for mammary gland lipid synthesis mechanism (Zhu et al., 2015). The activator protein 1 (AP-1) family of transcription factors comprises various combinations of Fos (Fos, FosB, Fra-1, and Fra-2) and Jun (c-Jun, JunB, and JunD) which upon dimer formation regulate osteoblast differentiation and influence adipocyte commitment (Luther et al., 2011, 2014; Sabatakos et al., 2000). In ΔfosB (fosB product) transgenic mice, the phenotype exhibits a decreased mass of adipose tissue, reduced adipocyte numbers, and abdominal fat concentrations (Kveiborg et al., 2004; Rowe et al., 2009; Sabatakos et al., 2000). The cultured bone marrow stromal cell from ΔFosB mice shows few adipocytes differentiated with only few and small lipid droplets. Additionally, the expression of PPARγ2, C/EBPα and C/EBPβ, confirmed transcription factors associated with the adipocytic phenotype, is also decreased (Sabatakos et al., 2000). Overexpression of ΔFosB in the multipotential stromal cell line ST2 inhibits adipogenic differentiation, binding to and altering the DNA-binding capacity of C/EBPβ (Kveiborg et al., 2002, 2004). Moreover, ΔFosB has been reported to regulate cell proliferation and death (Kurushima et al., 2005; Miura et al., 2005; Tahara et al., 2003). For example, ΔFosB protects rat embryo cells from apoptosis and exhibits a significantly higher survival occurring in the mitochondrial pathway dependent on caspase −3 and −9 (Tahara et al., 2003). However, the role of ΔFosB in milk fat synthesis and cell survival remains unknown in GMEC. It has been demonstrated that activation of PPARγ in human epithelial cells inhibits AP-1 DNA-binding activity due to competition for limited amounts of the transcriptional coactivator CREB-binding protein of PPARγ with AP-1 (Step et al., 2014; Subbaramaiah, Lin, Hart, & Dannenberg, 2001). Based upon above facts we hypothesize that pre-treatment of rosiglitazone may increase cellular triacylglycerol (TAG) content and inhibit cell survival, and these effects may be regulated by ΔFosB in GMEC.
2 MATERIALS AND METHODS
2.1 Tissues collection
All dairy goats received humane care as described in the No. 5 proclamation of the Ministry of Agriculture, P.R. China. All experimental procedures with live goats used in this study were approved by the Animal Care and Use Committee of the Northwest A&F University. Five-year-old non-lactating Xinong Saanen dairy goats (n = 6) were slaughtered by exsanguination. Mammary gland, small intestine, lung, kidney, spleen, heart, liver, skeletal muscle, and subcutaneous fat tissues were collected. Tissue samples were snap-frozen in liquid nitrogen and stored at −80 °C for RNA extraction. In addition, mammary gland tissue samples were collected from three healthy Xinong Saanen dairy goats (5-years-old, 4th parity) at peak lactation.
2.2 Luciferase assays
To assess the the effect of rosiglitazone on the degree of PPARγ activation, goat mammary epithelial cells at 80% confluence in 96-well plates were transiently transfected with PPARγ response element×3-Luc reporter plasmid along with a Renilla vector (pRL-TK) as a control. After a 24 hr recovery period in medium, cells were treated with 0, 5, 10, 20, and 40 µmol/L rosiglitazone. Forty-eight hours later, cells were harvested and lysates weremade using reporter lysis buffer (Promega, Madison, WI) according to the manufacturer's instructions. Luciferase activity in the cell extract was determined using luciferase assay buffer and luciferase assay substrate according to the manufacturer's protocol (Promega). As shown in Supplementary Figure S1, treatment with rosiglitazone caused an activation of PPARγ in GMECs. The luciferase levels between the treatment group and the control group (treatment with 0 µmol/L rosiglitazone) were statistically significant (p < 0.05). Data also indicated that the activation of the PPARγ by rosiglitazone reached a peak at 20 µmol/L dose.
2.3 Cell culture and treatment
The GMEC were isolated from Xinong Saanen goats at peak lactation and details on cell culture conditions were described previously (Li, Zheng, Sun, Yu, & Li, 2014). After growth to approximately 80% confluence, the cells were treated with: (a) 20 µM rosiglitazone; (b) 4 mM Ca2+; (c) Ad-ΔFosB or Ad-siΔFosB, respectively; (d) Ad-ΔFosB + rosiglitazone; (e) Ad-ΔFosB + Ca2+; (f) 5 µM SB-3CT; (g) Ad-ΔFosB + SB-3CT. After incubation, the cells were used for the different assays outlined below. Anti-PPARγ, anti-Bcl-2, anti-Bcl-xl, anti-Bax, anti-Cytochrome C, anti-Caspase-3, and anti-β-actin were purchased from (Abcam, Cambridge, MA). ΔFosB overexpression recombinant adenoviruses Ad-ΔFosB and interference expression recombinant adenoviruses Ad-siΔFosB were prepared by our laboratory (Zheng, Li, Li, Ma, & Liu, 2014). Rosiglitazone and SB-3CT were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
2.4 Total RNA extraction and quantitative real-time PCR (qPCR)
Total RNA isolation, cDNA synthesis, and primer design were carried out as described (Shi et al., 2014; Yao et al., 2016). qPCR primers are reported in Supplementary Table S1. We used the 2−ΔΔCt method to analyze relative expression levels.
2.5 Oil red O staining, TAG measurement, and fatty acid analysis
The GMEC were stained for 20 min with Oil Red O solution (0.3% Oil Red O in 60% isopropanol) before fixation in 4% paraformaldehyde for 30 min. For quantification, oil red O staining was dissolved in isopropanol and absorbance was measured at 490 nm. Intracellular TAG content was assayed using a commercial kit (Applygen, Beijing, China). Extraction and analyses of fatty acids (FA) was as previously described (Yao et al., 2016). Total FA were extracted and methylated from approximately 100 mg of GMEC using 2 ml of chloroform or methanol (3:1 vol/vol). Methylated samples were analyzed using a GC-MS with an HP-5 column (Agilent Technologies, Santa Clara, CA). The relative ratios of FA were identified based on percentages of all peak areas.
2.6 Cell cycle assay
Cell cycle analysis was performed using Cell Cycle Testing Kit (Multisciences, Hangzhou, China). The GMEC were grown in six-well plates (1 × 106 cells/well). The cells were harvested and centrifuged at 800g for 5 min. The supernatant was discarded, and the cells were washed once by cold phosphate buffered saline (PBS). GMEC were resuspended using 1 ml of reagent A and 10 µl of reagent B, subsequently blended by vortexing for 10 s and incubated for 30 min, and then analysis was done using a flow cytometry (FACS Canto™ II, BD BioSciences, San Jose, CA).
2.7 Cell proliferation assay
We examined cell proliferation using the Cell Counting Kit-8 (CCK-8) assay (Multisciences) and EdU incorporation assay (Ribobio, Guangzhou, China). For the CCK-8 assay, the cells were plated into 96-well culture plates at a density of 1 × 104 cells/well in 100 µl of culture medium per well, and each treatment group had six independent replicates. After 48 hr of incubation at 37 °C, 10 µl of CCK-8 reagent was added to each well and incubated at 37 °C for 2 hr. The absorbance of each sample at 450 nm wavelength was detected using a microplate reader (Molecular Devices, Sunnyvale, CA). We also assessed cell proliferation using the Cell-Light EdU DNA cell proliferation kit according to the manufacturer's instructions, with three independent replicates per treatment group.
2.8 Measurement of apoptosis using annexin V-FITC/PI staining assays
After incubation, the GMEC from the different treatment groups were washed three times with PBS buffer. Cells were then harvested and resuspended in 1 × binding buffer. Afterwards, cells were treated with Annexin V-FITC (5 µl) and propidium iodide (PI) (Multisciences), and incubated for 10 min at room temperature, after which the cell suspension was used for flow cytometry (FACS Canto™ II, BD BioSciences). Each treatment group had three independent replicates.
2.9 Measurement of apoptosis using hoechst 33342/PI dual staining assays
Cell apoptosis was also assessed with Hoechst 33342 and PI double staining (Solarbio, Beijing, China). In brief, after transfection with Ad-ΔFosB or Ad-siΔFosB for 48 hr, cells were incubated with Hoechst 33342 for 15 min at room temperature. Afterwards, cells were washed and treated with PI for 10 min at room temperature. Fluorescence signal was assessed under a fluorescence microscope (DM5000B, Leica, Bensheim, Germany).
2.10 Western blot analysis
We collected cells from the different treatment groups, pelleted them by centrifugation and lysed them in RIPA buffer (Solarbio) containing 1 mM PMSF (Solarbio). The Mitochondria Isolation Kit (Beyotime Biotechnology, Jiangsu, China) for cultured cells was used to isolate mitochondria and obtained cystoplasmatic protein. The extracts were boiled with 4×SDS loading buffer (150 mM Tris-HCL (pH = 6.8), 12% SDS, 30% glycerol, 0.02% bromophenol blue, and 6% 2-mercaptoethanol) at 98 °C for 10 min and then 20 µg total proteins were loaded and separated on 10% SDS-PAGE gels. After electrophoresis, the proteins were transferred to a 0.2 µm PVDF membrane that was soaked in formaldehyde, and then blocked with 5% skim milk in Tris Saline with Tween (TBST) buffer for about 2 hr at room temperature. The membrane was then incubated overnight with primary antibodies at 4 °C. The PVDF membrane was washed three times with TBST buffer and then incubated with secondary antibody for 2 hr at room temperature. β-actin was used as the internal control with a secondary antibody that was HRP-labeled IgG (Abcam). Finally, antibody reacting bands were detected using ECL luminous fluid (Solarbio).
2.11 Gelatin zymography
The GMEC were treated with indicated concentration of SB-3CT for 24 hr, and cell culture medium was collected and performed as previously described (Kawasaki et al., 2008).
2.12 Measurement of intracellular calcium
Intracellular calcium trafficking of the cytoplasm and mitochondria were detected using Fluo-3 AM and Rhod-2 AM (2 µm, Molecular probes, Life Technologies, Carlsbad, CA), respectively. Cells were treated as described previously (Sawant, Dasgupta, Lavhale, & Sitasawad, 2016; Zheng et al., 2014), and screened by means of flow cytometry and the use of a confocal laser scanning microscope (Leica, Solms, Germany).
2.13 Statistical analyses
The quantitative results are presented as mean ± standard error of the mean (SEM) based on at least three independent experiments. The data for each FA was analyzed as a proportion of the total FA. All data in this study were analyzed by one-way analysis of variance (ANOVA) for p-value calculations using SPSS v19.0 software. p < 0.05 was considered statistically significant differences among means.
3 RESULTS
3.1 Rosiglitazone promotes milk fat synthesis in GMEC
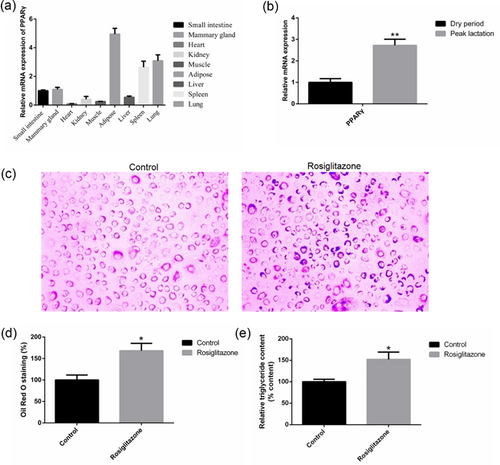
We found that PPARγ was detected and highly expressed in goat mammary gland (Figure 1a). The expression of PPARγ mRNA was greater (2.72-fold) at peak lactation compared with dry period (Figure 1b). The accumulation of lipid droplets after treatment with rosiglitazone increased by 68% (Figures 1c and 1d). Moreover, rosiglitazone had a significant positive effect on TAG content and resulted in an increase by 52%, relative to the control (Figure 1e). These results showed rosiglitazone promotes milk fat synthesis. Previous studies have shown that overexpressing ΔFosB decreases adipogenesis in vivo and in vitro, and decreased the expression of PPARγ (Kveiborg et al., 2004; Rowe et al., 2009; Sabatakos et al., 2000). Therefore, we hypothesized the effect of rosiglitazone on milk fat synthesis may be regulated via overexpression and interference expression of ΔFosB.
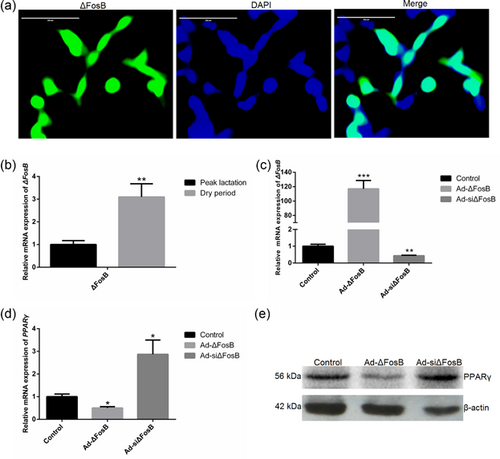
3.2 ΔFosB suppresses cellular lipid droplets accumulation and TAG synthesis
Sub-cellular localization analysis showed that ΔFosB is targeted to the cell nucleus (Figure 2a). Relative to the peak lactation, the expression level of ΔFosB during non-lactation increased 3.1-fold (p < 0.05, Figure 2b). ΔFosB overexpression markedly increased the expression of ΔFosB, and ΔFosB expression reduced significantly in GMEC transfected with interference recombinant adenoviruses Ad-siΔFosB (p < 0.01; Figure 2c). The expression of PPARγ increased significantly in the GMEC transfected with Ad-siΔFosB, and ΔFosB overexpression suppressed the expression of PPARγ (Figures 2d and 2e).
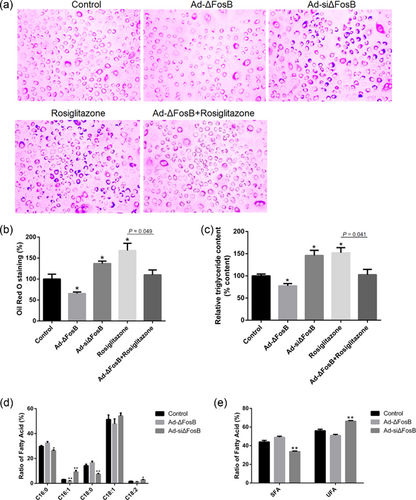
To examine the effect of ΔFosB on rosiglitazone-induced lipid synthesis, the GMEC were transfected with Ad-ΔFosB or Ad-siΔFosB. An inhibition of lipid droplets accumulation and TAG synthesis was seen in the ΔFosB-overexpressing GMEC (Figure 3a–c). By contrast, the accumulation of lipid droplets and content of TAG after infection with Ad-siΔFosB increased by 37% and 46%, respectively (p < 0.05; Figure 3a–c). Moreover, the increased cellular TAG content induced by rosiglitazone was abolished by ΔFosB overexpression in GMEC (Figure 3a–c). The ratios of palmitoleate (C16:1), oleate (C18:1), and linoleate (C18:2) after infection with Ad-siΔFosB adenovirus increased markedly at the expense of palmitate (C16:0) and stearate (C18:0). Specifically, Ad-siΔFosB had a significant positive effect on unsaturated fatty acid (UFA) and resulted in increases of C16:1, C18:1, and C18:2 by 6.1%, 3%, and 1.2%, respectively, relative to the control (Figures 3d and 3e). In contrast, ΔFosB overexpression had a negative effect on C16:1. The saturated FA (SFA) C16:0 and C18:0 decreased markedly in cells transfected with Ad-siΔFosB (Figures 3d and 3e).
3.3 ΔFosB is functional on the key genes related to lipid metabolism
The role of ΔFosB on milk lipid synthesis was confirmed by measuring the mRNA expression of genes encoding proteins associated with fatty acid metabolism in the overexpressing ΔFosB cells compared with GMEC infected with Ad-siΔFosB (Table 1). Relative to the control, qPCR results revealed that overexpression of ΔFosB displayed downregulation of genes responsible for FA de novo synthesis and desaturation (stearoyl-CoA desaturase [SCD] and fatty acid synthase [FASN]), TAG synthesis (fatty acid binding protein 4 [FABP4] and diacylglycerolacyl transferase 1 [DGAT1]), lipid droplet formation (perilipin 2 [PLIN2] and perilipin 3 [PLIN3]), and transcription factor (CCAAT/enhancer binding proteinalpha [C/EBPα] and Liver X receptor α [NR1H3]). But increased the expression of thrombospondin receptor (CD36). In contrast, the GMEC transfected with Ad-siΔFosB exhibited marked upregulation of SCD (1.46-fold; p < 0.05), FASN (1.57-fold; p < 0.05), FABP4 (1.63-fold; p < 0.05), DGAT1 (1.42-fold; p < 0.05), PLIN2 (1.72-fold; p < 0.05), PLIN3 (1.68-fold; p < 0.05), NR1H3 (1.58-fold; p < 0.05), and C/EBPα (1.71-fold; p < 0.05), but downregulated CD36 (0.62-fold; p < 0.05). In addition, ΔFosB had no effection expression of 1-Acylglycerol-3-phosphate O-acyltransferase 6 (AGPAT6; p < 0.05), sterol regulatory element-binding transcription factor 1 (SREBF1; p < 0.05), and lipoprotein lipase (LPL; p < 0.05).
| Gene symbol | Control | Ad-ΔFosB | Ad-siΔFosB |
|---|---|---|---|
| SCD | 1.06 ± 0.11 | 0.72 ± 0.01* | 1.46 ± 0.03* |
| FASN | 0.99 ± 0.04 | 0.58 ± 0.07* | 1.57 ± 0.06* |
| FABP4 | 1.00 ± 0.09 | 0.52 ± 0.08* | 1.63 ± 0.01* |
| AGPAT6 | 1.05 ± 0.04 | 0.89 ± 0.07 | 1.11 ± 0.11 |
| DGAT1 | 1.06 ± 0.05 | 0.47 ± 0.06* | 1.42 ± 0.02* |
| PLIN2 | 0.96 ± 0.06 | 0.63 ± 0.02* | 1.72 ± 0.03* |
| PLIN3 | 0.95 ± 0.03 | 0.57 ± 0.01* | 1.68 ± 0.08* |
| LPL | 1.04 ± 0.09 | 1.12 ± 0.09 | 1.07 ± 0.01 |
| CD36 | 0.96 ± 0.03 | 1.39 ± 0.10* | 0.62 ± 0.06* |
| C/EBPα | 0.98 ± 0.02 | 0.72 ± 0.07* | 1.71 ± 0.01* |
| NR1H3 | 1.08 ± 0.08 | 0.67 ± 0.09* | 1.58 ± 0.02* |
| SREBF1 | 1.00 ± 0.07 | 0.95 ± 0.01 | 1.18 ± 0.03 |
- GMEC were transfected with Ad-ΔFosB or Ad-siΔFosB for 48 hr, and the SCD, FASN, FABP4, AGPAT6, DGAT1, PLIN2, PLIN3, LPL, CD36, C/EBPα, NR1H3, and SREBF1 expression levels were quantified by quantitative real-time PCR. All experiments were performed in triplicate and repeated three times (n = 9). Values are presented as mean ± SEM.
- * p < 0.05 versus control.
3.4 PPARγ mediates anti-apoptotic effects of ΔFosB in GMEC
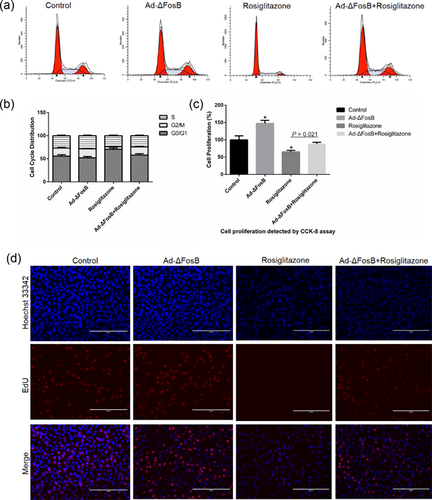
Previous studies have shown that ΔFosB is not only an important regulator of lipid synthesis, but can also regulate cell suvival. To assess whether the protective effects of ΔFosB is depended on PPARγ, we pretreated GMEC with rosiglitazone to analyze the effects of ΔFosB on cell survival in the presence or absence of Ad-ΔFosB or Ad-siΔFosB. The FITC-AnnexinV/PI and Hoechst33342/PI staining assays showed rosiglitazone to induce GMEC apoptosis and this effect was inhibited by ΔFosB overexpression (Figure 4a–c). Cell cycle analysis revealed ΔFosB overexpression increased the number of GMEC in S phase and decreased the proportion of cells in G0/G1 phase (Figures 5a and 5b). Rosiglitazone increased the number of GMEC in G0/G1 phase and this effect was improved by overexpression of ΔFosB. We found rosiglitazone inhibited cell proliferation in GMEC, and this effect was inhibited by ΔFosB overexpression (Figures 5c and sd). Thus, these observations demonstrate that the pro-apoptotic role of rosiglitazone in GMEC could be regulated by ΔFosB.
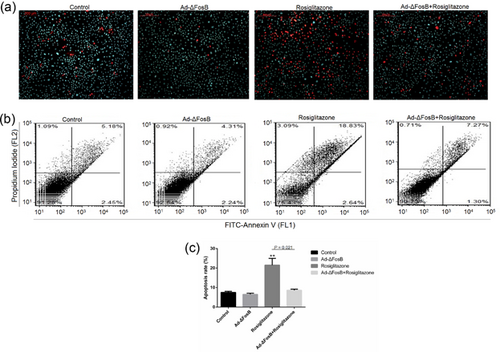
3.5 Overexpression of ΔFosB inhibits mitochondrial calcium overload and the mitochondrial apoptotic pathway
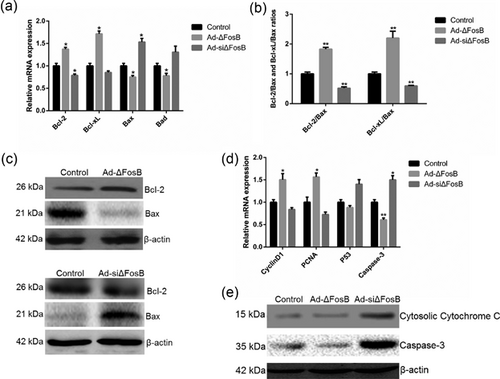
Bcl-2 has been described as anti-apoptotic protein in mitochondrial apoptotic pathway, and plays important roles in regulating apoptosis in mammary epithelial cells and breast cancer cells (Leverson et al., 2015; Schorr, Li, Krajewski, Reed, & Furth, 1999). We asked whether Bcl-2 is involved in the survival-enhancing effects of ΔFosB, and thus quantified the expression of Bcl-2 in GMEC pretreated with Ad-ΔFosB or Ad-siΔFosB using qPCR and Western blot. We found ΔFosB to promote the expression of Bcl-2, and to significantly increase Bcl-xL expression while suppressing the expression of Bax and Bad (Figure 6a–c). We also found ΔFosB to increase the expression of CyclinD1 and PCNA and to inhibit the expression of p53 (Figure 6d). Moreover, ΔFosB could prevent cytochrome c release from the mitochondria into the cytosol in GMEC and decrease the expression of Caspase-3 (Figures 6d and 6e). Taken together, these observations suggest that the anti-apoptotic effect of ΔFosB in GMEC is involved in the mitochondrial apoptotic pathway.
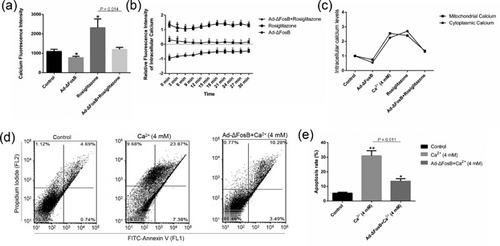
Activation of intracellular Ca2+ triggers cellular dysfunction and death (Li, Wang, et al., 2014; Rovere, Roest, Bultynck, & Parysb, 2016; Zhivotovsky & Orrenius, 2011), and ΔFosB promotes calcium deposition (Kveiborg et al., 2004; Rowe et al., 2009; Sabatakos et al., 2000). Thus we examined cytosolic and mitochondrial calcium levels in GMEC treated with Ad-ΔFosB and (or) rosiglitazone. Overexpression of ΔFosB decreased the mitochondrial calcium fluorescent intensity and stayed at lower levels in GMEC (Figure 7a–c). Rosiglitazone led to a transient increase in the calcium levels and this effect was abolished by ΔFosB overexpression (Figure 7a–c). Moreover, cell apoptosis induced by acute increases in intracellular Ca2+ concentration was abolished by ΔFosB overexpression (Figures 7d and 7e). These results demonstrate ΔFosB protects GMEC from apoptosis by a Ca2+-sensitive mechanism and the subsequent apoptotic events.
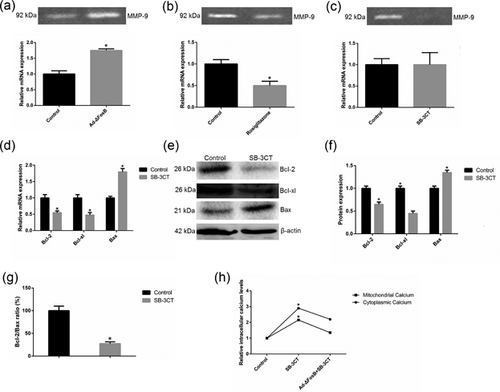
3.6 ΔFosB protects GMEC from apoptosis by regulating MMP-9 expression
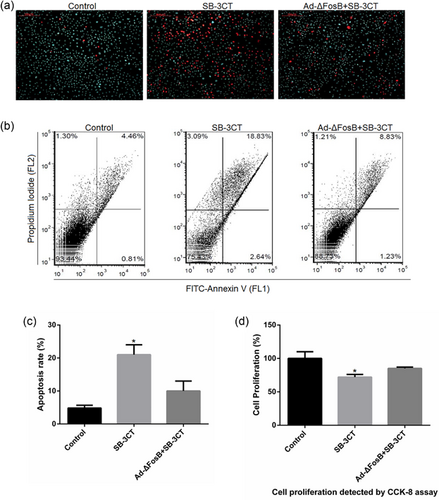
Previous studies have shown that activation of PPARγ by rosiglitazone significantly decreased matrix metalloproteinase-9 (MMP-9) mRNA expression and reduced MMP-9 gelatinolytic activity (Hetzel et al., 2003; Marx et al., 2003), and SB-3CT, an MMP-9 inhibitor, induced apoptosis of mammary epithelial cells (Li et al., 2016). Thus, we intended to detect the expression of MMP-9 in GMEC treated with rosiglitazone and analyze the pro-apoptotic effect of SB-3CT. We found ΔFosB overexpression increased MMP-9 mRNA expression and gelatinolytic activity, and rosiglitazone inhibited MMP-9 gelatinolytic activity (Figures 8a and 8b). SB-3CT significantly reduced MMP-9 gelatinolytic activity but did not affect the mRNA expression (Figure 8c). Then we found SB-3CT suppressed the expression of Bcl-2 and Bcl-xl in mRNA and protein levels, and increased the expression of pro-apoptotic protein Bax (Figure 8d–f). Moreover, SB-3CT markedly decreased Bcl-2/Bax ratio which favors cell inducing apoptosis (Figure 8g). Then we examined intracellular calcium levels in GMEC treated with SB-3CT. SB-3CT increased the cytosolic and mitochondrial calcium levels, and this effect was abolished by ΔFosB overexpression (Figure 8h). The FITC-AnnexinV/PI and Hoechst33342/PI staining assays showed SB-3CT to induce GMEC apoptosis and this effect was inhibited by ΔFosB overexpression (Figure 9a–c). SB-3CT inhibited cell proliferation in GMEC, and this effect was inhibited by ΔFosB overexpression (Figure 9d). Thus, these observations demonstrate that ΔFosB may protect cells from apoptosis by regulating the expression of MMP-9.
4 DISCUSSION
It has been well-established that FASN plays an important role in de novo FA synthesis in human (Chirala, Jayakumar, Gu, & Wakil, 2001), mice (Suburu et al., 2014), bovine (Morris et al., 2007), and goat (Zhu et al., 2015) tissue. The expression of FASN also affects TAG synthesis and accumulation (De Schrijver, Brusselmans, Heyns, Verhoeven, & Swinnen, 2003; Moriyama et al., 2004). The present study showed that ΔFosB inhibited the expression of FASN and suppressed lipid-droplet formation and accumulation of TAG. An unexpected upregulation of CD36, an important gene responsible for FA uptake (Steneberg et al., 2015), was found in GMEC overexpressed ΔFosB, suggesting that the FA synthesis ability may affect FA uptake.
The DGAT1 is involved in TAG synthesis during adipogenesis (Harris et al., 2011), and DGAT1-deficient mices shows few and small lipid droplets in adipocytes (Smith et al., 2000). The secretion of lipid droplets into milk occurs via a process that requires lipid droplet formation-related genes PLIN2 and PLIN3 (Chong, Reigan, Mayle-Combs, Orlicky, & McManaman, 2011; Wolins, Rubin, & Brasaemle, 2001). In the present study, ΔFosB inhibited the expression of DGAT1, FABP4, PLIN2, and PLIN3, and decreased lipid droplets accumulation and TAG content in GMEC, suggesting that ΔFosB may serve as a suppress or of TAG synthesis in the goat mammary gland.
The inhibition of SCD expression with ΔFosB overexpression is consistent with data that ΔFosB increased the unsaturated FA concentration. The SCD protein plays a key role in the synthesis of milk unsaturated fatty acid via catalyst C16:0 and C18:0 to C16:1 and C18:1 (Miyazaki & Ntambi, 2003). The C/EBPα is a key transcription factor involved in adipocyte differentiation (Rosen et al., 2002), and ΔFosB has been shown to decrease the expression of C/EBPα in the multipotential stromal cells (Sabatakos et al., 2000). Our study showed direct evidence that ΔFosB inhibits de novo FA synthesis and TAG synthesis in goat mammary epithelial cells. However, some limitations should be considered, for instance, the protein expression and enzymes activities should be measured, and the mechanisms for how ΔFosB affects milk fat synthesis need to be further studied.
The PPARγ is not only a master regulator of adipogenesis, but also exhibits a role in cellular apoptosis signal transduction (Wrann et al., 2012). Previous studies have shown Bcl-xL overexpression promotes prostate cancer cells survival which can be abolished by activating PPARγ (Shiau et al., 2005). PPARγ activation in breast cancer cells can inhibit cell growth and induce apoptosis through regulating the expression of Caspase-9 and p53 (Bonofiglio et al., 2009). The ΔFosB triggers cell proliferation in mammary epithelial cells and induces a metastatic phenotype of breast cancer cells (Detry, Lamour, Castronovo, & Bellahcene, 2008; Selvamurugan, Kwok, & Partridge, 2004). Here, we indicated that the manipulation of PPARγ expression in GMEC has significant effect on cell proliferation and apoptosis, showing a proapoptotic role of PPARγ in mammary gland. We also showed ΔFosB promote GMEC survival by increasing the expression of Bcl-2. These findings demonstrate that ΔFosB can protect mammary epithelial cells from apoptosis induced by rosiglitazone.
Calcium is a well-known intracellular messenger that modulates many aspects of cell life (Chen et al., 2014; Cruz, Wu, Ulrich, Craig, & Garcia, 2016; Zhivotovsky & Orrenius, 2011). Intracellular Ca2+ accumulation activates the mitochondrial apoptotic pathway with high levels of Bax and activation of Caspase-3 (Greenberg, Lavik, & Distelhorst, 2014; Lam et al., 1994; Rong & Distelhorst, 2008). The ΔFosB is a physiologically important regulator of osteoblast differentiation and increases bone formation and bone mass, leading to osteosclerosis (Kveiborg et al., 2004; Rowe et al., 2009; Sabatakos et al., 2000). Therefore, we speculated that ΔFosB may have an anti-apoptotic effect through regulating intracellular Ca2+ concentration. This notion was supported by the results that ΔFosB overexpression decreased the mitochondrial calcium fluorescent intensity at lower levels, and protected GMEC from apoptosis triggered by calcium accumulation. Previous studies have shown that activation of PPARγ by rosiglitazone significantly decreased MMP-9 mRNA expression and reduced MMP-9 gelatinolytic activity (Hetzel et al., 2003; Marx et al., 2003), and SB-3CT, an MMP-9 inhibitor, induced apoptosis of mammary epithelial cells (Li et al., 2016). We found ΔFosB increased MMP-9 gelatinolytic activity. SB-3CT suppressed the expression of Bcl-2 and increased intracellular calcium levels, and these effects were abolished by ΔFosB overexpression. These findings suggest that ΔFosB may protect cells from rosiglitazone-induced apoptosis via regulating the activity of MMP-9.
5 CONCLUSIONS
In summary, our findings demonstrate that PPARγ agonist rosiglitazone increases cellular TAG content and inhibits cell survival, and these effects were abolished by ΔFosB overexpression. ΔFosB increased MMP-9 gelatinolytic activity, and inhibition of MMP-9 suppressed the expression of Bcl-2 and increased intracellular calcium levels, and these effects were improved by overexpression of ΔFosB. These findings reveal that ΔFosB may protect cells from rosiglitazone-induced apoptosis via regulating the activity of MMP-9.
ACKNOWLEDGMENTS
This work was supported by the National 863 Program of China (Grant no. 2013AA102505), the National Natural Science Foundation of China (Grant no. 31272408), the Program of National Beef Cattle and Yak Industrial Technology System (CARS-38), Special Fund of Xinyang Normal University (No.2017001).
CONFLICTS OF INTEREST
The authors declare no competing financial interest.
AUTHORS’ CONTRIBUTIONS
XW, HL, GZ, and JY gathered samples, conceived the study, performed data analysis, participated in its design, interpreted results, and drafted the manuscript. LL and XW participated in intellectual discussion. YH, XL, and HZ contributed during gathering sample acquisition. HC provided guidance on experimental design and funding. All authors read and approved the final manuscript.