Comparison of genome-wide analysis techniques to DNA methylation analysis in human cancer
Abstract
DNA methylation was the first epigenetic modification to be detected in human cancers with specific relation to aberrant gene expression. Herein, DNA methylation analysis explains how epigenetic patterns affect gene expression level. Hypermethylation at tumor suppressor gene loci leads to increased tumorigenesis due to tumor suppressor gene silencing, whereas global hypomethylation of CpG islands (CGIs) is followed by genomic instability and aberrant activation of multiple oncogenes. Therefore, characterization of the genes which silenced or activated epigenetically in human tumor cells can improve our understanding of cancer biology. Different genome-wide methodologies are applied to evaluate methylation status. Various commonly conducted techniques for this evaluation are reviewed in this paper. We provided comparative description of the procedures, advantages, and drawbacks of genome-wide DNA methylation analysis methods and biological applications, to give information on selecting the appropriate method for different methylation studies.
1 INTRODUCTION
DNA methylation as an epigenetic alteration commonly occurs at 5′cytosine (5′C) of pyrimidine cycle and plays a key role in genome regulation and development (Bird, 1985; Holliday & Pugh, 1996; Momparler & Bovenzi, 2000). Apart from its role in physiological functions, aberrant DNA methylation is related to the inappropriate transcriptional silencing of genes (Issa, 2004; Molavi et al., 2013), that provides an advantage for several diseases such as diabetes, heart disease, autoimmune, and aging-related diseases (Egger, Liang, Aparicio, & Jones, 2004; Irier & Jin, 2012; Jayaraman, 2011; Movassagh, Vujic, & Foo, 2011; Robertson, 2005) and many types of cancer including colorectal (Hughes et al., 2013; Samowitz, 2007; Toyota et al., 1999a), lung (Marsit et al., 2006; Shigematsu et al., 2005), liver (Alizadeh et al., 2016; Shen et al., 2002), and breast cancer (Fang et al., 2011). In contrast to normal cells, cancer cells globally exhibit a lower levels of 5-methylcytosine in the genome, but simultaneously a higher levels of methylation in tumor suppressor genes promoters (Jones & Baylin, 2002, 2007). DNA methylation can silence the expression of tumor suppressor genes through mechanisms that may interfere with transcription factor binding sites or through conformational changes in chromatin structure leading to transcriptionally silencing (Callahan et al., 1993; Hamaguchi et al., 2002; Yoshihara, Collado, & Hamaguchi, 2007). Therefore, it is important to know how DNA methylation patterns changes during physiological development and pathological disorders. Given the important role of DNA methylation in the biological phenomena, a close examination of DNA methylation status can be applied to identify tumor markers and therapeutic targets in cancer patients (Jones & Baylin, 2002; Shi, Wang, & Caldwell, 2007). Moreover, reversible epigenetic modifications can be considered as more effective targets for therapeutic purposes than irreversible genetic mutations. Silenced genes with DNA methylation or histone deacetylation can be restored to normal functioning through epigenetic inhibitors (Egger et al., 2004; Heng et al., 2009; Yang, Lay, Han, & Jones, 2010). An accurate determination of methylation patterns is required to clarify their pivotal roles in biological processes. Therefore, better understanding of each methylation detection method and its application would be important to select appropriate effective system to evaluate methylation status. There are a growing number of methylation assays which is commonly applied for the evaluation of DNA methylation. In this review, we generally describe three kinds of common approaches to profiling genome-wide DNA methylation; (i) restriction enzyme-based techniques; (ii) affinity enrichment-based techniques; (iii) bisulfite conversion-based methods and explain the main advantages and drawbacks of these techniques (Table 1).
| Category | Method | Advantages | Drawbacks | Ref. |
|---|---|---|---|---|
Restriction enzyme-based techniques |
RLGS |
● High speed–scanning ability ● Applicable for any organism ● High-throughput ● Identifies thousands CGIs in one reaction with no previous knowledge required about genome sequence ● applied to identify both hyper- and hypomethylation |
● Low sensitivity ● Requires high quality DNA, not suitable for FFPE tissues. ● Requires large quantities of input DNA. ● Ability to detect landmark sites (a small number of CGIs) ● Coverage limited by the RE digestion sites |
Costello et al. (2000), Costello, Hong, Plass, and Smiraglia (2009), Smith, Dean, Konfortova, and Kelsey (2003) |
MCAM |
● Small quantities of genomic DNA required ● Highly sensitive ● Useful to identify new methylation markers ● Requires modest quality input DNA |
● Low resolution in comparison with other restriction enzyme-based techniques that use RE with four base pair recognition site. ● Susceptible to PCR bias |
||
DMH |
●Technically simple method ● High-throughput ● A useful method for identifying differentially methylated DNA in two samples ● Can be identify hypo- and hyper-methylated DNA |
● Need for high bioinformatic analysis for comparing multiple groups ● Requires radioactive labeling ● Requires high quality input DNA ● Susceptible to PCR bias |
||
MSNP |
● Small quantities of genomic DNA required ● Allows identification of allele-specific CpG methylation ● Allows measurement of LOH and CNVsimultaneously ● High quantitative method ● Requires modest quality input DNA |
● Requires radioactive labeling ● Susceptible to PCR bias ● Coverage limited by the RE digestion sites |
||
MTA |
● Investigates promoter-wide hypermethylation of many tumor DNA samples in a single experiment ● Rapid assessment of a single methylated CGI ● A single nylon filter can be applied frequently to probe various CGIs |
● Susceptible to PCR bias ● Coverage limited by the RE digestion sites |
||
MSDK |
● Applicable to identify new differentially methylated CGIs ● Does not need previous knowledge about sequence ● Small quantities of genomic DNA required ● Does not require large scale sequencing, complex primer design and variable bisulfite modification |
● Labor-intensive ● Low-throughput ● More expensive |
||
Affinity enrichment-based techniques |
MeDIP |
● High coverage of gene body methylation, whole genome and non-RefSeq transcripts ● Specific to 5-mC ● Capable for detecting DNA methylation at CGIs and repetitive sequences ● Requires moderate quality input DNA ● A useful technique for extensive mapping of DNA methylation changes in cancer epigenetics |
● Less sensitive in regions with low CpG density ● Coverage limited by the anti-5-mC antibody specificity ● Cannot estimate absolute DNA methylation level ● Need for DNA denaturation |
Keshet et al. (2006), Zhao, Whyte, Hopkins, Kirk, and Prather (2014), Taiwo et al. (2012b) |
MIRA |
● High sensitivity and specificity ● Does not require denaturation ● Capable for detecting DNA methylation at CGIs and repetitive sequences ● Compatible with various microarray and NGS technologies to classify human tumors |
● Less sensitive in regions with low CpG density ● Requires high-quality input DNA ● Coverage limited by the Methyl-CpG-binding domain specificity |
Jung et al. (2015), Rauch et al. (2009), Rauch and Pfeifer (2010) |
|
Bisulfite conversion-based methods |
MSO |
● High-throughput ● Ability to analyze different genes in the same array ● Applicable to rapid screen multiple CpG sites in many gene promoters |
● Limitation for analyzing the closely spaced CpGs that are methylated heterogeneously ● Unable effectively to analysis hundreds of patient samples |
Gitan et al. (2002), Lippman et al. (2005), Ho and Tang (2007) |
IIM assay |
● Cost-effective ● High sensitive method ● Small quantities of genomic DNA required ● Single-base resolution ● High-throughput |
● Requires high-quality input DNA ● Applicable for only human sample ● Coverage is limited only to the designed array ● Degradation of DNA by bisulfite treatment |
||
WGBS |
● Single-base resolution ● More sensitive in regions with low CpG density |
● High cost ● Large quantities of genomic DNA required ● Degradation of DNA by bisulfite treatment |
||
RRBS |
● High sensitivity ● Cost- and time- effective method for genome-scale analysis ● Single-base resolution ● High CpG islands coverage ● Coverage is not limited to restriction sites ● Decrease the redundancy and size of sequencing required |
● Requires high-quality input DNA ● Degradation of DNA by bisulfite treatment ● May exhibit a lack of coverage at intergenic and distal regulatory elements ● Limited to regions in proximity to enzymes recognition sites ● Requires bioinformatic knowledge. ● Limited coverage in regions with low CpG density ● Bias in regions with high CpG density |
Meissner et al. (2008), Gu et al. (2010, 2011b), Smith et al. (2009) |
2 RESTRICTION ENZYME-BASED TECHNIQUES
2.1 Restriction landmark genomic scanning (RLGS)
This method utilizes landmark enzymes (also called methylation-sensitive restriction enzymes) such as NotI or AscI, which have GC-rich recognition sequences to cut DNA into smaller pieces only at unmethylated sites, for detecting DNA polymorphisms due to hypo- or hypermethylation of DNA (Ando & Hayashizaki, 2006). First, restriction landmarks are labeled by a radioisotope and subjected to one-dimensional (1D) electrophoresis. Then, four-cutter enzyme is applied to digest the fractionated DNA, and afterwards the products are exposed to 2D electrophoresis. The resulting spots are visualized as a separated DNA after exposure to x-ray. The intensity of spots allows quantitative estimation and indicates copy numbers, since the labeling with a radioisotope just occurs at landmark sites (Ando & Hayashizaki, 2006; Smiraglia & Plass, 2002). Compared to PCR-mediated approach, the difference between RLGS spots can help to analyze the variations in epigenetic modifications developed by DNA methylation (Figure1a) (Koike, Matsuyama, & Ebisuzaki, 2008). The main limitation of this method is the required DNA quality which needs to have minimal degradation and a minimum amount of mechanical shearing (Rush & Plass, 2002). Therefore, the formalin-fixed paraffin embedded tissue samples cannot be appropriate for RLGS. However, RLGS provides evaluating more than 2,000 CGIs on one agarose gel with no previous knowledge required about genome sequence (Costello et al., 2000; Smiraglia & Plass, 2002).
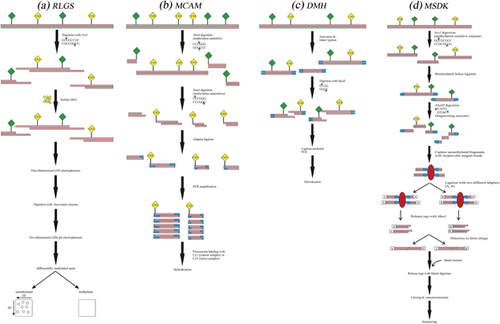
RLGS has been used to quantify the hypermethylation of CpG islands (CGIs) in multiple tumor types including human glioma, lung cancer, cervical cancer, acute myeloid leukemia (AML), and prostate cancer (Li et al., 2016; Ohashi, Ueda, Kawase, Kawakami, & Toda, 2004; Park et al., 2005; Rush et al., 2001; Wang et al., 2008). These experimental studies have found that genome-wide hypermethylation of CGIs is a typical characteristic of cancer cells. RLGS has also been applied to identify both hyper- and hypomethylation of tumor suppressor genes such as SOCS1 (Yoshikawa et al., 2001), BMP3B (Dai et al., 2001), SLC5A8 (Li et al., 2003), TCF21 (Smith et al., 2006), Itga4 (α4-integrin), and Cdkn2a (p16) (Akama et al., 1997). The identified methylation modifications can be incorporated with clinicopathological information to introduce new markers that will increase diagnostic and prognostic accuracy. In human hepatocellular carcinomas, RLGS has been utilized successfully to determine the association between clinical factors (such as postoperative recurrence) and DNA methylation changes. This methodology could predict the overall effect of demethylation of repetitive sequences on postoperative disease-free survival in hepatocellular carcinoma (Itano et al., 2002). Another study has applied this technique to discover a significant relationship between aberrant promoter hypermethylation of RLGS loci and poor overall survival in patients with medulloblastoma (FruÈhwald et al., 2001). These studies suggest RLGS method to determine tumor markers and screening factors in clinical samples for improving molecular diagnosis and treatment.
2.2 Methylated CGI amplification and microarray (MCAM)
Methylated CGI amplification and microarray (MCAM) is a very sensitive and specific two-color array technique which developed by combining an enzyme-based technique, methylated CGI amplification (MCA), with array hybridization (Estécio et al., 2007, 2008). MCAM employs the cutting abilities of SmaI (methylation sensitive) and XmaI (methylation insensitive) enzymes to differentiate between methylated and unmethylated CpGs applying genome-scale detection in DNA samples from cancer tissue biopsies or different cancer cell lines (Chung et al., 2008; Estécio et al., 2007; Shen et al., 2007a; Toyota et al., 1999b). This technique can be implemented with small quantities of genomic DNA for analyzing DNA methylation patterns of CGIs (Estécio, Yan, Huang, & Issa, 2008; Shen et al., 2007b). In this method, after DNA digestion with two different restriction enzymes including SmaI and XmaI with six base pair recognition sites, digested fragments are selectively ligated to the specific oligonucleotide linkers, which enable PCR amplification of methylated CGIs. The control and tumor sample amplicons are then respectively labeled with two different fluorochromes, Cy3 and Cy5, is followed by hybridizing equimolar amounts of labeled amplicons to the selective microarray platform. Eventually, image scanning and data analysis will allow comparison of signal intensities for each control and tumor samples (Figure 1b) (Estécio et al., 2008; Laird, 2010a).
This technique concomitantly decreases genome complexity and elevates specificity by targeting methylated CGIs which can be applied to identify new methylation markers and modified molecular pathways in human cancers (Colyer, Dellett, & Mills, 2012; Estécio et al., 2007). Applying MCAM to examine 15 primary colorectal tumor samples and three cancer cell lines (RKO, Raji, and C8161) revealed a high detection ability to identify hundreds of new methylated genes with 96% specificity and 88% sensitivity, in contrast to bisulfite-PCR as a gold-standard technique (Estécio et al., 2007). In this study, colorectal carcinomas have been classified into three distinctive subgroups by CGI methylator phenotype (CIMP) and microsatellite instability (MSI) status (Estécio et al., 2007). In another study, Omura et al. (2008) indicated that MCAM is a high-throughput experimental method for identifying hundreds of aberrantly methylated genes in pancreatic cancer. They also found that MCAM assay, because of its accuracy and reproducibility in the detection of differential methylation at CGIs in cancer, is preferable to bisulfite sequencing and/or methylation specific PCR (MSP).
2.3 Differential methylation hybridization (DMH)
DMH is a powerful tool for identifying alterations in DNA methylation status frequently observed in human cancers. This technically simple method is based on enrichment of only the hypermethylated DNA fraction and investigation the differences between tumor and control samples on the microarray with immobilized CGIs (Huang et al., 1997). In DMH method, sonicated genomic DNA fragments following adapter ligation are digested with methylation-sensitive endonucleases, BstU1 and HpaII, which recognize unmethylated CGCG and CCGG sequences, respectively. Only methylated fragments that are resistant to the restriction digestions are used as templates for the subsequent ligation-mediated PCR. By contrast, the unmethylated fragments of the genes are digested and no fragments are amplified. PCR products from tumor and normal samples are then labeled with two different fluorescent dyes, Cy5, and Cy3, respectively. The same proportions of the last amplicons from both groups are mixed together and coated on a DMH plate. After rigid hybridization, further rinsing is used to remove the unbound or weak amplicons and subsequent DMH plates are examined with high-resolution fluorescence scanning. The signal intensity value of Cy5 versus Cy3 indicates the methylation status in tumor samples compared to control normal samples (Figure 1c) (Huang, Perry, & Laux, 1999; Sun, Huang, Yan, Huang, & Lin, 2011; Yan et al., 2002; Zuo, Tycko, Liu, Lin, & Huang, 2009).
In a number of cancer studies, including breast, ovarian, and endometrial cancers, as well as small B-cell lymphoma (SBCL), DMH has been used to screen normal and tumor samples to find out whether patterns of distinctive epigenetic modifications can be related to clinicopathological features and outcomes in patients (Ahluwalia et al., 2001; Rahmatpanah et al., 2006; Yan et al., 2000; Zighelboim et al., 2007). For example, using this technology, Ahluwalia et al. (2001) demonstrated that advanced ovarian cancer has epigenetic signatures with high methylation frequency. In addition, they cautiously suggested that there is an association between the amount of methylation in ovarian carcinoma and level of drug response. Huang et al. (1999) developed DMH technique to determine methylation status of 276 CGI loci in the six breast cancer cell lines. DNA hypermethylation was found in 5–14% of these CGIs in all cancer cell lines compared with a normal control. In addition, their DMH data were confirmed independently by traditional Southern hybridization. They also underlined that DMH assay can be upgraded into a high-throughput approach by applying the microarray technologies for screening of methylation in cancer. Another study indicated that, by using expressed CGIs sequence tags, DMH can simultaneously identify both gene-expression silencing and CGIs hypermethylation in breast cancer cell (Shi et al., 2002).
2.4 Methylation-sensitive single nucleotide polymorphism (MSNP)
Between some of the other applications, MSNP is an appropriate technique to explore cancer-related alterations in DNA methylation. At the same time, it can assess DNA copy number aberrations and DNA methylation, leading to uncertainties in understanding the methylation changes and allowing an evaluation of genetic-epigenetic interaction (Schalkwyk et al., 2010; Yuan et al., 2006). Previous studies in cancer epigenetics have mainly dealt with methylation of CGIs, while DNA methylation also takes place in non-island CpGs which may have regulatory roles in gene expression. Thus, the evaluation of DNA methylation status in both non-island CpGs and CGIs seems to be useful. In contrast to CGI array technologies, MSNP arrays in conjunction with gene expression profiling have the potentials to investigate various sites in the genome, including promoter, intragenic, and intergenic regions (Kerkel et al., 2008; Yuan et al., 2006).
In this method, gDNA is initially cleaved by XbaI, with or without the methylation-sensitive restriction endonuclease HpaII, and oligonucleotide linkers are ligated to the restriction fragments. The resulting products are then amplified by ligation-mediated PCR and hybridized to SNP arrays, which include oligonucleotides covering both alleles of thousands SNPs throughout the human genome. HpaII cleaves the unmethylated CCGG sequences and therefore only methylated sequences will be amplified and hybridized to array. The signal intensity value of SNP hybridization obtained from the XbaI/HpaII genomic representations is compared with that from XbaI-only representation which serves as control to analyze DNA copy number (Kerkel et al., 2008; Yuan et al., 2006; Zuo et al., 2009).
MSNP assay has been applied to follow the extent of demethylation as a biochemical response in AML patients receiving decitabine, a demethylating anticancer drug. MSNP technology could identify DNA copy number aberrations, DNA demethylation, and loss of heterozygosity in bone marrow aspirates from these patients. In addition, in this study, the MSNP results were confirmed using southern blotting, bisulfite sequencing and combined bisulfite restriction analysis (COBRA) assay, which allows analyzing the locus-specific modifications in DNA (Yuan et al., 2006).
2.5 Methylation target array (MTA)
MTA, as a powerful method for rapid assessment of a single methylated CGI, can be applied to determine the relationship between clinicopathological characteristics and CGIhypermethylation in cancer (Agrawal, Murphy, & Agrawal, 2007; Chen et al., 2003). The MTA and DMH approaches are complement with each other in that DMH rapidly identifies methylation status of CGIs in cancer genome while MTA rapidly assesses the usage of these CGIs as molecular markers for cancer diagnosis (Szyf, 2005). Similar to the concept of tissue microarray (Kononen et al., 1998), MTA investigates promoter-wide hypermethylation of many tumor DNA samples in a single experiment (Chen et al., 2003). In this method, the experimental DNA samples are initially digested with a four base endonuclease, such as MseI, Bfizl, NlaIII, or Tsp509I. Then, linkers are sticky-end ligated to the resulting fragments, which followed by further treatment with methylation-sensitive restriction enzymes such as BstUI and HpaII, prior to ligation-mediated PCR. Each tumor amplicon is spotted in duplicate onto nylon filter along with matched control and hybridized with a probe for a gene-specific promoter CGI. In each spot, the hybridization intensity is used to score each specimen as methylated or unmethylated (Chen et al., 2003; Szyf, 2005). Chen et al. (2003) applied MTA method to evaluate methylation profiles of ten genes in 93 primary breast cancers, four breast cancer cell lines, and 20 normal breast tissues. They detected stronger hybridization signals, as indicators of aberrant hypermethylation, in tumor samples, but not in normal samples. The hypermethylation of nine known tumor suppressor genes (KL, DAPK1, BRCA1, WT1, uPA, HOXA5, 30ST3B, RASSF1A, and GPC3) was found at the levels of 0%, 9%, 23%, 25%, 28%, 30%, 32%, 58%, and 60%, respectively, in breast cancer cell lines and tumors. One of the major advantages of this method is that a single nylon filter can be applied frequently to probe various CGIs, which provides accelerated analysis of DNA methylation-associated biomarkers to predict clinical outcomes of cancer patients (Chen et al., 2003; Zuo et al., 2009).
2.6 Methylation-specific digital karyotyping (MSDK)
Another restriction enzyme-based technique is MSDK in which gDNA is cleaved by AscI as a mapping enzyme, ligated to biotinylated linker with AscI overhang and digested by NlaIII as a fragmenting enzyme. AscI, which recognizes the palindromic sequence (GGCGCGCC), is a rare-cutting methylation-sensitive restriction endonuclease that cuts genomic DNA at the unmethylated parts, and therefore, unmethylated fragments can be captured with streptavidin magnetic beads and separated from methylated fragments. In the next step, two different adapters containing MmeI restriction enzyme recognition sites are ligated to free NlaIII-digested ends, and fragments then digested with MmeI which yields short sequence tags (17-bp). The resulting library includes a variety of short sequence tags that indicate the methylation patterns of AscI mapping enzyme sites. These tags are then dimerized (ditags) to form concatemers, which can be cloned for sequencing (Figure 1d) (Hu, Yao, & Polyak, 2006; Kahl, 2015; Wang et al., 2002). The advantage of MSDK is that a priori knowledge of the sequence is not required; which makes this method applicable to identify new differentially methylated CpG regions. Also, in this approach, the amount of required input material is quite small (1 μg gDNA), a useful feature for evaluating methylation in clinical samples (Hu et al., 2006).
Using MSDK, Hu et al. (2005) performed the comprehensive analysis of DNA methylation profiles in the stromal cells of breast cancers. By comparing MSDK libraries obtained from myoepithelial and normal epithelial cells, they detected cell type-specific epigenetic differences between normal and tumor tissues. In addition, they conducted cell type-specific MSDK and serial analysis of gene expression (SAGE) to evaluate genome-scale methylation and patterns of gene expression. They showed that epigenetic alterations have an important role in the tumor microenvironment abnormalities in breast cancer. In another study, Steenbergen et al. (2013) implemented MSDK to identify new methylated targets in HPV-induced tumors. Their MSDK data revealed five novel methylation targets, PRDM14, PHACTR3, NKX2-8, LHX1, and FAM19A4 in HPV transformed cell lines. These novel methylated genes can be useful as markers in cervical cancer screening programs. MSDK method has therefore promised to improve our understanding of the cancer genomics and cancer biology.
3 AFFINITY ENRICHMENT-BASED TECHNIQUES
3.1 Antibody enrichment; methylated DNA immuno-precipitation (MeDIP)
MeDIP is an immunocapturing method for the evaluation of methylated DNA which has been developed for specific detection of 5-methylcytosine using an anti-5-methylcytosine antibody. In this technique, following sonication, DNA fragments are denatured by heat treatment and incubated with the anti-5′mC antibody, providing the formation of methylated DNA-antibody complex. Then, secondary antibody-coated magnetic particles are applied to capture the methylated DNA-antibody complex from the solution (Thu et al., 2009, Thu, Pikor, Kennett, Alvarez, & Lam, 2010). Purified DNA sequences can be evaluated by sequencing (MeDIP-seq) or tiling arrays (MeDIP-chip) (Figure 2a) (Weng, Huang, & Yan, 2009). The purified methylated DNA can be applied for whole genome methylation studies, gene expression profiling, locus-specific PCR, and comparative genome hybridization (CGH) arrays (Gazin, Wajapeyee, Gobeil, Virbasius, & Green, 2007; Jacinto, Ballestar, & Esteller, 2008). Given that 60–80 % of the CpGs in human genome are methylated (Taiwo et al., 2012a), most of the methylated CpGs can be explored by MeDIP-seq which is able to detect up to 70% of all CpG dinucleotides in the human genome at a resolution from 100 to 300 bases (Hirst & Marra, 2010). MeDIP-chip is a useful technique for extensive mapping of DNA methylation changes in cancer epigenetics. This method has been applied to identify the genes located in hypermethylated areas of DNA from colon carcinoma (Caco-2) and prostate cancer cells (PC-3) (Keshet et al., 2006). About 200 hypermethylated unique genes have been introduced in a colorectal cancer cell line using combined MeDIP with hybridization on a CGI microarray (Weber et al., 2005). By using MeDIP, Flanagan et al. (2010) analyzed 33 familial breast tumor samples (11 with BRCA1, 8 with BRCA2, and 14 without BRCA1/2 familial mutations) to determine whether tumor DNA-methylation profile would predict mutation status or intrinsic subtype of the breast tumors. They showed that MeDIP methylation profiles might improve the subtype classification beyond mutation analysis, but this approach failed to predict the intrinsic subtypes.
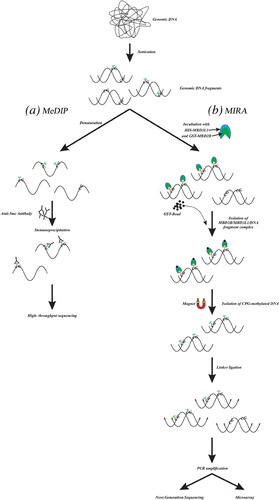
The sensitivity of the anti-5′mC antibody is one of the key elements in MeDIP method. Furthermore, this assay is more sensitive in regions of high CpG density, since DNA sequences with more adjacent methylated CG sites are more effectively captured. However, because of potent hydrogen binding between complementary GC base pairs, CpG density may directly lead to PCR bias and an imprecise estimation of methylation (Jia et al., 2010).
3.2 Methyl-CpG-binding domain (MBD) enrichment; methylated-CGI recovery assay (MIRA)
MIRA method is based upon the fact that MBD2b protein can precisely bind to methylated CpG, and MBD3L1 protein strongly intensifies this interaction (Hendrich & Bird, 1998; Rauch & Pfeifer, 2005; Rauch, Li, Wu, & Pfeifer, 2006, 2006). In this technique, sonicated genomic DNA is incubated with glutathione-S-transferase (GST)-tagged MBD2b and histidine (HIS)-tagged MBD3L1 proteins to generate a heterodimeric complex that firmly binds to the methylated double-stranded gDNA sequences. The CpG-methylated DNA is then isolated and enriched using magnetic glutathione-coated particles, and ligated to adapter oligonucleotides for PCR amplification (Rauch & Pfeifer, 2009). In comparison to antibody-based methods, this technique does not require DNA denaturation and works with double-stranded methylated DNA (Figure 2b) (Jung et al., 2015).
MIRA has been applied in the detection of aberrant DNA methylation in various cancers, including lung cancer (Rauch et al., 2006, Rauch, Wu, Zhong, Riggs, & Pfeifer, 2009), breast cancer (Hill et al., 2010; Tommasi, Karm, Wu, Yen, & Pfeifer, 2009), childhood acute lymphoblastic leukemia (Dunwell et al., 2010), and colorectal cancer (Li et al., 2012). For example, using this method, Tommasi et al. (2009) detected 108 significant CGIs that display aberrant DNA methylation in stage I breast cancer and ductal carcinoma in situ (DCIS), a precursor of invasive and metastatic breast cancer. MIRA based microarray analysis showed that the investigation of DCIS-specific alterations in DNA methylation may be useful to clarify the molecular mechanisms involved in breast cancer development. In lung cancer, MIRA microarray process has revealed a list of methylated genes that need following analysis to find out the methylation frequencies in primary tumors and the biological importance of methylation silencing in cancer progression. In this study, MIRA results also supported the notion that tumor-related DNA methylation changes have non-random patterns and at least some are influenced by specific mechanisms (Rauch et al., 2006). In general, because of simplicity of this method, it can widely be applied to study DNA methylation patterns in a large number of genes that are involved in human cancers. Also, MIRA analysis is compatible with various microarray and high-throughput next generation sequencing (NGS) technologies to classify human tumors according to methylated DNA patterns on a genome-wide scale (Rauch & Pfeifer, 2010).
4 BISULFITE CONVERSION-BASED METHODS
4.1 Methylation-specific oligonucleotide microarray (MSO microarray)
To characterize methylation patterns by MSO microarray, genomic DNA is first treated by sodium bisulfate which chemically deaminates cytosine to uracil without affecting 5-methylcytosine. PCR amplification products of this converted DNA are then hybridized to microarrays (length from about 19–23 nucleotides) that distinguish methylated and unmethylated cytosine (Gitan, Shi, Chen, Yan, & Huang, 2002; Kiviat & Critchlow, 2002). Quantitative comparisons in hybridization which are analyzed by the fluorescent intensity, can show the methylation patterns at specific CpG sites (Gitan et al., 2002). In another version of MSO microarray, gDNA is converted by bisulfite treatment, and then PCR products are hybridized to bead array assays. The information obtained through this technique is restricted to the specific primers bound to the beads. The bead arrays can query the methylation patterns in specific CGIs (Bibikova et al., 2006).
MSO microarray is used to map DNA methylation patterns in tissue samples and cell lines. This method potentially allows generating epigenetic profiles and detecting methylation changes in CGIs (Adorján et al., 2002; Dhingra, Mittal, & Sarma, 2014). Several studies have used this technique to evaluate the methylation status of different genes in human cancers, including non-Hodgkin lymphoma (Yang et al., 2003), breast cancer (Gitan et al., 2002), gastric carcinomas (Hou, Shen, Ji, He, & Lu, 2004a), prostate cancer (Yu et al., 2005), and small B-cell lymphomas (Guo et al., 2005). The MSO assay has been successfully applied to map methylation status of p16 gene CGIs in 18 gastric tumors and corresponding normal tissues. Moreover, this method provided more coverage than MSP approach to measure the frequency of p16 methylation in tumor samples. Interestingly, MSO microarray could determine which CpG dinucleotides (or CpG-rich areas) were immediately methylated in specific tumors (Hou, Shen, Ji, He, & Lu, 2004b). One of the main advantages of MSO array is its ability to analyze different genes in the same array. However, it is important to emphasize that the use of this technique has limitation for analyzing the closely spaced CGIs in the genes that are methylated heterogeneously (Lippman, Gendrel, Colot, & Martienssen, 2005; Zhang et al., 2008).
4.2 Illumina's infinium methylation (IIM) assay
The IIM assay is a bisulfite conversion-based technique for profiling DNA methylation at the genome level and at single CpG site resolution. The ability for genome-wide DNA methylation profiling makes this method very appropriate for understanding the potential roles of DNA methylation and demethylation in disease and normal status (Weisenberger, Van Den Berg, Pan, Berman, & Laird, 2008). In IIM assay, following treatment of gDNA with sodium bisulfite, the differentially converted DNA is amplified on whole genome level. The enriched products are fragmented by restriction enzymes followed by hybridization of purified DNA to the chip. In IIM assay, two types of the query probes are commonly applied to detect the intensity of methylated (cytosine) and unmethylated (thymine) status at the CpG site of interested genes (Du et al., 2010). Methylation status is evaluated by analyzing signal intensities obtained from a single base polymerase extension reaction of two probes (Figure 3a) (Daca-Roszak et al., 2015).
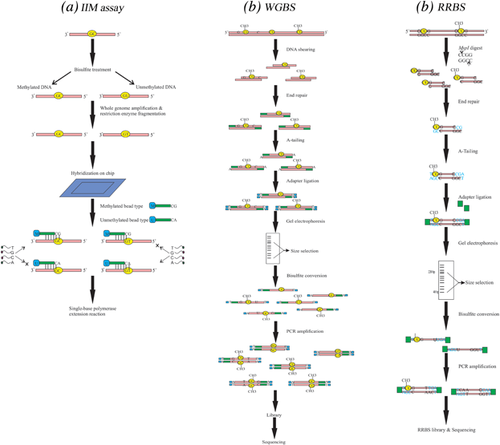
A number of studies have applied IIM assay to detect tumor-specific DNA methylation alterations in various human cancers such as colorectal carcinoma (Jasmine et al., 2012; Naumov et al., 2013), breast cancer (Dedeurwaerder et al., 2011), and renal cell carcinoma (Moran, Arribas, & Esteller, 2016). Naumov et al. (2013) applied this method to study DNA methylation patterns for distinguishing newly occurred methylation in colorectal cancer. IIM method was applied to identify 10,342 hypermethylated and 5,325 hypomethylated CpG sites in CRC that were significantly different from normal tissues. They reported that some of these methylation loci may be employed as genomic markers for specific cancer diagnosis. In another study, IIM assay has also been applied to analyze 27,578 CpG sites from more than 14,000 genes in breast cancer. On the basis of site-specific CpG methylation, the results showed that breast tumors can be classified into subgroups that go beyond the presently known ones, indicating a potential of IIM approach for improving the classification of tumors (Dedeurwaerder et al., 2011).
4.3 Whole-genome bisulfite sequencing (WGBS)
WGBS is considered to be a gold-standard method for detecting DNA methylation because it provides an accurate, quantitative and efficient procedure for conducting single base resolution analysis of methylated cytosines in gDNA; however, the application is limited for large sample size because of expensiveness in performance of this method (Aransay & Trueba, 2016; Fan & Chi, 2016). The main advantage of WGBS is its ability to determine the methylation status of DNA sequences with low CpG density. In addition, this method can be employed to assess methylation sequence context to identify total DNA methylation levels (Yong, Hsu, & Chen, 2016). In this method, following shearing of genomic DNA, a single adenine (A-tailing) is added to the 3′ end of each fragment which is known as end repair step. After ligation of methylated sequencing adapters, in which all the cytosines are methylated to prevent bisulfite- mediated deamination reaction, the ligated DNA fragments are size selected and purified by gel electrophoresis. For library preparation, the selected DNAs are treated by sodium bisulfite followed by PCR amplification process with two primers for the ends of the methylated adapters. Eventually, the resulting library is ready for subsequent cluster generation and sequencing (Figure 3b) (Ji et al., 2014; Urich, Nery, Lister, Schmitz, & Ecker, 2015).
Applying WGBS technique to demonstrate the methylation variation of cancer-specific differentially DNA methylated regions (cDMRs) in breast, lung, thyroid, Wilms’ tumors, and colon cancers showed that variable cDMRs are related to the hypomethylation at CGIs (Hansen et al., 2011). In another study, Lin et al. (2015) conducted WGBS method to clarify the DNA methylation alterations of MCF-7 human breast cancer cells, invasive breast carcinomas and normal breast tissues. They identified differential methylation at kilobase-sized hypomethylated regions (HMRs) and megabase-sized partially methylated domains (PMDs). This study provided further evidence that hypermethylation of the promoter CGIs and the hypomethylation of intergenic and intragenic sequences can be considered as common features of breast cancer cells.
4.4 Reduced-representation bisulfite sequencing (RRBS)
RRBS is an accurate and cost effective method for genome-scale analysis of DNA methylation which integrates restriction endonuclease digestion followed by next generation DNA sequencing (Meissner et al., 2008). In this method, purified genomic DNA is cut with MspI which recognizes and cleaves the 5′-CCGG-3 sites. The MspI digested short fragments are end-repaired, in which the 3′ ends are A-tailed, and ligated to the methylated sequencing adapters. After size selection for fragment lengths between 40-220 bp using gel electrophoresis, bisulfite conversion is carried out and PCR amplification is performed with primers that are complementary to the sequencing adapters. For removing primer contamination from the amplified libraries, a second round of size selection step is implemented and then the RRBS libraries are sequenced (Figure 3c) (Chatterjee, Rodger, Stockwell, Le Mée, & Morison, 2017; Gu et al., 2011a). RRBS has been applied to assess DNA methylation differences between formalin-fixed paraffin-embedded (FFPE) tumor and adjacent normal tissues from the same individuals (Gu et al., 2010). This technique has also been used to analyze differences in CpG site methylation in DNA from chronic phase (CP), accelerated phase (AP), and blast crisis (BC) of chronic myeloid leukemia (CML) patients and control individuals. The RRBS analysis showed a higher numbers of methylated CpGs in BC-CML samples compared with other samples (Heller et al., 2016). In a recent study using RRBS, Ashktorab et al. (2016) reported a list of differentially methylated genes in colorectal neoplasia, tubulovillous adenoma, tubular adenoma, and normal samples. These genes were initially methylated in tumor samples but not in other colonic lesions, and also were involved in major carcinogenic pathways. In summary, RRBS is time- and cost-effective method for rapid identification of the whole genome methylation status in cancer biopsies. Applying this technique with high sensitivity enables us to quantify DNA methylation mapping at a single-base resolution (Smith, Gu, Bock, Gnirke, & Meissner, 2009).
5 CONCLUSION
Human cancer is a complex genetic and epigenetic disease. Unlike non- reversible mutagenic events, reversible epigenetic alterations can be considered as potential diagnostic markers and therapeutic targets for cancer therapy (Herceg & Hainaut, 2007; Valdespino & Valdespino, 2015). One of the most important epigenetic events in human cancer is aberrant DNA methylation which is known to regulate gene-expression (Phillips, 2008). The genome-wide analysis technologies provide a better understanding of how changes in DNA methylation affect tumor growth and invasiveness. In this review, the common approaches for genome-scale DNA methylation mapping were investigated and the procedure and applications of each method were discussed. The currently applied methods are predominantly performed based upon restriction endonuclease digestion, affinity enrichment, and sodium bisulfite conversion, combined with sequencing or array- based methods. The application of each approach depends on the scientific question, power and facility of the method, expected changes in the level of methylation, amount of required DNA, run time and cost (Kurdyukov & Bullock, 2016).
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest regarding the authorship of the present manuscript.




