Gut microbiota-derived endotoxin enhanced the incidence of cardia bifida during cardiogenesis
Abstract
Cytotoxicity and inflammation-associated toxic responses could be induced by bacterial lipopolysaccharides (LPS) in vitro and in vivo, respectively. However, the mechanism involved in LPS-induced cardiac malformation in prenatal fetus is still unknown. In this study, we demonstrated that LPS was induced in gut microbiota imbalance mice, and next, LPS exposure during gastrulation in the chick embryo increased the incidence of cardia bifida. Gene transfection and tissue transplantation trajectory indicated that LPS exposure restricted the cell migration of cardiac progenitors to primary heart field in gastrula chick embryos. In vitro explant allograft of GFP-labeled anterior primitive streak demonstrated that LPS treatments could inhibit cell migration. A similar observation was also obtained from the cell migration assay of scratch wounds using primary culture of cardiomyocytes or H9c2 cells. In the embryos exposed to LPS, expressions of Nkx2.5 and GATA5 were disturbed. These genes are associated with cardiomyocyte differentiation when heart tube fusion occurs. Furthermore, pHIS3, C-caspase3 immunohistological staining indicated that cell proliferation decreased, cell apoptosis increased in the heart tube of chick embryo. Meanwhile, in vivo, pHIS3 immunohistological staining and Hochest/PI staining also draw the similar conclusions. The LPS exposure also caused the production of excess ROS, which might damage the cardiac precursor cells of developing embryos. At last, we showed that LPS-induced cardia bifida could be partially rescued through the addition of antioxidants. Together, these results reveal that excess ROS generation is involved in the LPS-induced defects in heart tube during chick embryo development.
1 INTRODUCTION
Commensal microbiota colonized in the human host have complicated interaction with bacterial communities that offer significant health benefits to that host. Accumulating data indicate the connection between gut bacteria and practically every aspect of human health (Hay & Zhu, 2016; Zhang et al., 2015). The impact that these bacteria exert on our health are mainly due to their imbalance. In the past decade, extensive clinical and basic evidence has shown that the gut microbiota can bring about intestinal inflammation and tumorigenesis as a consequence of complex interactions between the human host and bacteria (Yang & Jobin, 2014). These intestinal microbes might also exert physiological influences on gut function, immune maintenance, and neurological regulation etc. (Sommer & Backhed, 2013; Trajkovski & Wollheim, 2016). The beneficial role of the microbial community could even be presented in morphogenesis, except for the maintenance of host physiological functions (Sommer & Backhed, 2013). However, the microbes could certainly elicit apathogenic response if there were significant alterations in their compositional balance. It is known that obesity, insulin resistance, and the metabolic syndrome could all lead to alteration of gut microbiota, in which acetate plays a key role through regulation of microbiome-brain-beta cell axis (Perry et al., 2016). Lipopolysaccharide (LPS) is one of the microbial products leading to the possibility that microbiota-derived LPS might interfere with maternal physiology functions and fetal development (Gregory et al., 2016).
LPS is the major component of the outer membrane of Gram-negative bacteria (Alexander & Rietschel, 2001; Raetz & Whitfield, 2002). Of inflammatory-related signaling pathways, Toll-like receptor (TLR) acts as one of the crucial molecules. TLR4 is an innate immune receptor for LPS, which interacts with LPS binding protein to activate TLR4 signaling through the interaction of LPS with TLR4. Subsequently, TLR4 signaling transduction cascade stimulates PI3 K/Akt pathway and NF-κB signaling to cause biological effect (Zhao et al., 2014). It has been reported that maternal LPS exposure can negatively affect the process of gametogenesis, embryo implatation, gastrulation, and neurulation, respectively (Aisemberg et al., 2013; Straley, Togher, Nolan, Kenny, & O'keeffe, 2014; Wang et al., 2014). However, these embryonic phenotypes and the underlying mechanisms caused by maternal LPS are still elusive. In particular, little is known about the cardiac precursor cell migration and cellular changes of cardiac precursors during early heart tube formation in the presence of LPS (Rashidi & Sottile, 2009).
During cardiogenesis primordial heart tube arises predominantly from splanchnic mesoderm cells, which derive from bilateral fields and merge at middle line of carcinogenic region. Spatial-temporal cardiogenesis includes primary heart tube fusion, cardiac looping and accretion, cardiac septation, and coronary vasculogenesis (Martinsen, 2005).
Heart development is a very complicated process, precisely regulated by spatio-temporal gene expressions at different developmental stages. Fibroblast growth factor (FGF)-signaling is required for the migration of pro-cardiac mesoderm cells in Drosophila and chicks (Beiman, Shilo, & Volk, 1996; Yang, Dormann, Munsterberg, & Weijer, 2002). Cardiac precursor cell specification occurs when the cells reach to the anterior lateral plate mesoderm. As an important inducer of myocardium, Bone morphogenetic protein 2 (BMP2) stemmed from anterior endoderm, plays an important role in heart-inducing activity of the chick embryo. Moreover, Nkx2.5, GATA factors, BMP2, myocardin, and Tbx20 are all considered to be the important cardiogenic transcription factors that characterize and induce cardiogenic differentiation (Brand, 2003). Therefore, the early gastrula stage is an extremely high risk period for cardiogenesis since it is sensitive to many harmful external factors (Daft, Johnston, & Sulik, 1986), which could promote the risk of congenital heart disease if there is interference with migration or differentiation of cardiac precursor cells.
In this study, the LPS levels of structural shift of the gut microbiota human and mice have been determined with the results suggesting that microbiota-derived LPS exposure inhibited embryo survival. Using chick embryos, the risk of cardiac bifida was found to be increased during embryonic cardiogenesis. Excess ROS generation might be involved in the mechanism of LPS-induced the phenotypes of cardiac bifida through affecting the vital gene expressions in heart tube formation.
2 MATERIALS AND METHODS
2.1 Experimental animals and embryos
2.1.1 Mice
All processes involving animal treatment in this study were in accordance with the procedures of Ethical Committee for Animal Experimentation, Jinan University. The Kunming mice used in the current study were obtained from the Laboratory Animal Centre of Sun Yat-Sen University (Guangzhou, China). All animals were maintained under environmentally controlled conditions, 23 ± 2°C, with relative humidity of 55 ± 10%, under a 12 hr light/dark cycle, with free access to standard laboratory diet and water. Five-weeks female mice were used to induce gut microbiota imbalance by injecting physiological saline (20 ml/kg body weight), LPS (10 µg/ml, 20 ml/kg body weight; Sigma), lincomycin hydrochloride (LH, 0.3 g/ml, 24 ml/kg for each mouse; Shanghai Macklin Biochemical Co., Ltd., Shanghai, China) over three consecutive days. Two female mice were housed with one normal male mouse overnight in a cage. The day that the vaginal plugs were observed was designated as embryonic day 0.5 (E0.5), while on E13.5 mouse embryos were harvested. All processes involving animal treatment in this study were in accordance with the procedures of Ethical Committee for Animal Experimentation, Jinan University.
2.1.2 Chick embryos
Fertilized chick eggs were obtained from the Avian Farm of the South China Agriculture University. The eggs were incubated until the required HH stage (Hamburger & Hamilton, 1992) in a humidified incubator (Yi heng Instrument, Shanghai, China) at 38°C and 70% humidity. EC (early chick) culture (Chapman, Collignon, Schoenwolf, & Lumsden, 2001) was employed to culture these gastrula chick embryos since it met the experimental requirements. For the LPS-treated embryos, the HH0 chick embryos in EC culture were treated with either 10 μg/ml LPS (Sigma–Aldrich, St. Louis, MO) or simple saline (control) until the experimentally required developmental stage. For the vitamin C-treated embryos, the HH0 chick embryos in EC culture were incubated with 2.86 mM (=0.5 mg/ml) vitamin C (Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China) according with experimental requirements (Li et al., 2014; Wang, Zhang et al., 2015). Briefly, LPS or vitamin C was directly applied to either EC culture or in vitro culture medium to reach the final concentrations 10 μg/ml (LPS) and 2.86 mM vitamin C, respectively. Treated embryos were subsequently incubated for either 18 or 45 hr before being fixed with 4% paraformaldehyde for analysis of morphology and gene expression. For LPS exposure at a later embryonic stage, HH10 chick embryos were exposed 10 μg/ml through injection into windowed eggs in vivo and then further incubated for 5.5 days; the surviving embryos were harvested for further assessment.
2.2 Serum collection and endotoxin determination
Whole blood samples were collected from late negative (LN) and late positive (LP) patients (Liu et al., 2017) or mice. This study was approved by the local ethics committee of The First Affiliated Hospital of Jinan University. All patients provided written informed consent. Serum was separated from the blood by centrifugation at room temperature and stored at −80°C. Serum endotoxin levels were measured according to endotoxin detection kit (Xiamen Horseshoe Crab Reagent Manufactory Co., Ltd., Xiamen, China) by UV spectrophotometry.
2.3 Gene transfection and tissue transplantation
HH3 chick embryos in EC culture were transfected with the GFP gene by electroporation. Briefly, 0.5 ml plasmid DNA (1.5 mg/ml GFP) was microinjected into the space between the vitelline membrane and the epiblast of HH2-3 chick embryos. Electroporation was then implemented with the electroporation parameters previously described (Yang et al., 2002). After electroporation, the embryos were further incubated for 5 hr before the primitive streak tissues were used for transplantation. The labeled GFP+ primitive streak tissue was isolated from the anterior region of the primitive streak and grafted into the same position and developmental stage of an untransfected host chick embryo. Embryos were then incubated in the culture dishes of either both-side uniform sample saline or one-side containing LPS for 6 or 16 hr before being photographed (Figure 5a).
2.4 Explant, primary, and cell culture
2.4.1 Explant culture
HH3 chick embryo primitive streak is divided into six equal size portions, and the anterior primitive streak (cardiac progenitor cells) is deemed to be the second segment of explants from the cranial side (Figure S4a). The explants of anterior primitive streaks were cultured in vitro in culture medium (DMEM-F12 Gibco, Gaithersburg, MD) at 37°C and 5% CO2 (Wang et al., 2013) for 24, 48, or 72 hr. Each treatment was performed in triplicate. Approximately, 95% of the cells in the explant culture were determined to be cardiomyocytes in the control group, which shows the presence of myosin heavy chain using MF20 immunofluorescent staining as previously reported (Li et al., 2015).
2.4.2 Primary culture
Primary cardiomyocyte cultures were established from day 14 chick embryo hearts (DMEM-F12 Gibco).
2.4.3 Cell culture
H9c2 cells was purchased from Guangzhou Jennio Biotech Co., Ltd., Guangzhou, China and cultured in culture medium (DMEM-F12 Gibco).
2.5 Histology
Briefly, 5.5-day-old chick embryos (Control and LPS treated) were fixed in 4% paraformaldehyde at 4°C for 24 hr. The specimens were then dehydrated, cleared in xylene, and embedded in paraffin wax before being serially sectioned at 5 µm using a rotary microtome (Leica, RM2126RT, Wetzlar, Hessen, Germany). The sections were stained with hematoxylin and eosin (H&E) or immunohistochemically.
2.6 In situ hybridization
Whole-mount in situ hybridization of chick embryos was performed according to a standard in situ hybridization protocol (Henrique et al., 1995). Briefly, Digoxigenin-labeled probes were synthesized for VMHC, GATA5 (Kamei et al., 2011; Li et al., 2014), Nkx2.5 (supplied by Dr. Thomas M. Schultheiss). The whole-mount stained embryos were photographed and then frozen sections were prepared on a cryostat microtome (Leica CM1900, Leica, Solms, Germany) at a thickness of 14–18 µm.
2.7 Immunofluorescent staining and F-actin
The chick embryos were harvested after a given time incubation and fixed in 4% PFA overnight at 4°C. Whole-mount embryo immunostaining was performed using the following antibodies: MF20 (1:500, DSHB, Iowa City, IA), pHIS3 (p-histone H3, 1:400, Santa Cruz Biotechnology, Santa Cruz, CA), and C-caspase3 (Cleaved Capase-3, 1:200, Cell Signaling Technology, Danvers, MA). Briefly, the fixed embryos were then incubated with MF20, pHIS3, or C-caspase3 primary antibody at 4°C overnight on a shaker. Following extensive washing, the embryos were incubated with either anti-mouse IgG conjugated to Alexa Fluor 555 or anti-rabbit IgG conjugated to Alexa Fluor 488 overnight at 4°C on a rocker. For F-actin detection, the cultured cells were stained using phalloidin-Alexa-Fluor 488 (1:200, Invitrogen, Waltham, MA) at room temperature for 2 hr. All the embryos were later counterstained with DAPI (1:1000, Invitrogen) at room temperature for 1 hr.
2.8 Western blot
HH10 chick embryos were collected and lysed with CytoBuster™ Protein Extraction Reagent (#71009, Novagen, Italia, Germany). Total protein concentrations were assessed via a BCA quantification kit (BCA01, DingGuo Biotech, Beijing, China). Samples containing identical amounts of protein were fractionated by SDS–PAGE, and then transferred to PVDF membranes (Bio-Rad, Inc., Hercules, CA). Membranes were blocked with 5% Difco™ skim milk (BD) and subsequently incubated with primary and secondary antibodies, then bands of interest protein were visualized using the ECL kit (#34079, Thermo Fisher Scientific, Waltham, MA) and GeneGnome5 (Syngene, Frederick, MD). Gray scale of bands was analyzed using Quantity One software (Bio-Rad). Antibodies: VEGFR2 (Abcam, Cambridge, MA); GATA4 (Abcam); TBX5 (Abcam); GATA6 (Abcam); PCNA (Santa Cruz Biotechnology); c-Caspase3 (Cell Signaling Technology); HRP-conjugated anti-mouse IgG, HRP-conjugated anti-rabbit IgG (Cell Signaling Technology). All primary antibodies were diluted 1000-fold in 5% skim milk, and secondary antibodies were diluted 2000-fold.
2.9 RNA isolation and quantitative PCR
Total RNA was isolated from HH10 chick embryos using a Trizol kit (Invitrogen) according to the manufacturer's instructions. First-strand cDNA was synthesized to a final volume of 20 µl using iScript™ cDNA Synthesis Kit (Bio-Rad). Following reverse transcription, PCR amplification of the cDNA was conducted as described previously (Dugaiczyk et al., 1983; Maroto et al., 1997). SYBR® Green qPCR assay were performed using a Primer Script™ RT reagent kit (Takara Bio, Inc.,Tokyo, Japan). All specific primers used are described in supplementary Figure S8. Reverse transcription and amplification reactions were performed in Bio-Rad S1000™ (Bio-Rad) and ABI 7000 thermal cyclers, respectively. The housekeeping gene GAPDH was run in parallel to confirm that equal amounts of RNA were used in each reaction. The ratio between intensity of the fluorescently stained bands corresponding to genes and GAPDH was calculated to quantify the level of the transcripts for those genes mRNAs.
Fecal samples were collected from ileocecal junction; the samples were stored in a refrigerator with a temperature of −80°C. Total 16SrRNA was isolated from mice fecal using a stool RNA kit (Omega Bio-tek, Norcross, GA) according to the manufacturer's instructions. Following reverse transcription, PCR amplification of the cDNA was performed. The 16S rRNA gene was amplified with 27F and 1492R primers, Bacillus bifidus, Lactobacilli, and Escherichia coli primers used are described in supplementary Figure S8. The ratio between intensity of the fluorescently stained bands corresponding to genes and 16SrRNA was calculated to quantify the level of the transcripts for those genes mRNAs.
2.10 Measurement of SOD, GSH-Pxand MDA
Samples from HH10 chick embryos or primary cardiomyocytes were homogenated and used for measuring the levels of SOD (superoxide dismutase), GSH-Px (glutathione peroxidase), and MDA (malondialdehyde). The protein content of the chick embryos or primary cardiomyocytes was determined using a Coomassie brilliant blue kit (Beijing Dingguo, Beijing, China). The SOD, GSH and MDA activities were determined following the manufacturer's instructions (Nanjing Jiancheng, Nanjing, China), respectively, by UV spectrophotometry.
2.11 Photography
Following immunofluorescent staining or in situ hybridization, whole-mount embryos were photographed using a stereo-fluorescent microscope (Olympus MVX10, Olympus, Tokyo, Japan) and associated Olympus software package Image-Pro Plus 7.0. The embryos were sectioned into 14 μm-thick slices using a cryostat microtome (Leica CM1900), and sections were photographed using an epi-fluorescent microscope (Olympus LX51, Leica DM 4000B) with the CN4000 FISH Olympus software package.
2.12 Data analysis
Statistical analysis for all the experimental data generated was performed using a SPSS 13.0 statistical package program for windows. The data were presented as mean ± SE. Statistical significance were determined using paired T test, independent samples T test or one-way analysis of variance (ANOVA). p < 0.05 was considered to be significant.
3 RESULTS
3.1 The increase in the level of LPS induced by structural shift of the gut microbiota affected the reproduction ability in mouse
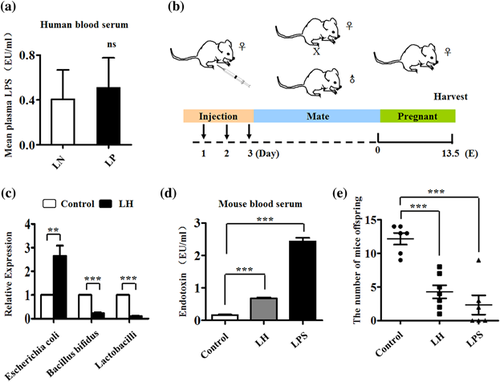
In an earlier study, a significant structural shift of the gut microbiota was reported in Preeclampsia patients, which might be associated with the occurrence and development of the disease (Liu et al., 2017). Therefore the LPS level in serum of LN and LP patients were examined, and it was found that the LPS level is increased in LP (p > 0.05; Figure 1a). When the pregnant mice were treated with physiological saline and LH, which is effective against Gram-positive bacteria (Figure 1b), quantitative real-time PCR revealed that the LH could significantly protect against Bacillus bifidus and Lactobacilli, with the result that there is more Escherichia coli in LH-treated mouse gut compared with their controls (Figure 1c). LPS levels were very significantly increased in both LH- and LPS-treated mice compared with the control (control and LH: p < 0.01; control and LPS: p < 0.001; Figure 1d). The numbers of embryos in E13.5 were observed to be lower in both LH- and LPS-treated mice compared with the control group (control and LH: p < 0.001; control and LPS: p < 0.001; Figure 1e).
3.2 LPS exposure leads to the malformation of early cardiogenesis of chick embryos
Gut microbiota dysbiosis is known to generate LPS (lipopolysaccharides, endotoxin), which could penetrate the placental barrier and impact on embryo development. The effect of LPS on general development of embryos was studied using an early chick embryo experimental model because it can be easily manipulated at the embryonic developing stage (Rashidi & Sottile, 2009). Exposure to 10 µg/ml LPS at gastrula stage caused the reduction of 5.5-day chick embryo size and weight (p < 0.05; Figure S1b,b1,c,c1,d,d1,e). Meanwhile, both survival rate (Figure S1f) and developmental defect rate (Figure S1g) of embryos at this stage increased in comparison to the control group. Two main types of phenotypes in LPS-exposed embryos were observed, a cohort of mid-defects possess relatively normal heart ventricles (Figure S2b) compared to control (Figure S2a), whereas in the severe defect group (Figure S2c) the heart ventricles could not be seen. In the sections of MF20-immunofluorescent stained 5.5-day hearts (Figure S2d–f), both the trabecula (p < 0.001; Figure S2g) and ventricular wall (p < 0.001; Figure S2f) were significantly thicker in LPS-induced heart mid-defect group than in controls (Figure S2d1,d2,e1,e2,f1,f2).
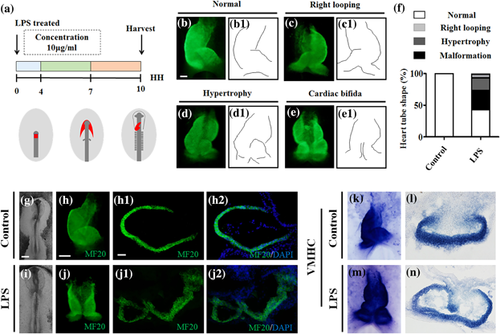
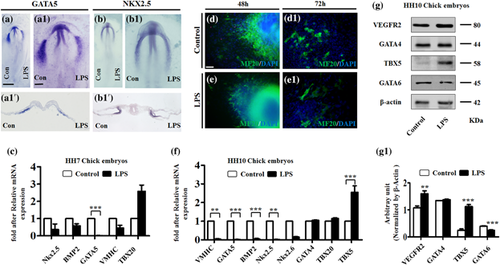
An investigation into whether LPS-induced abnormal cardiogenesis occurred in the stage of heart tube formation was carried out using early gastrula chick embryos (Figure 2), so that whole-mount MF20 immunofluorescent staining were implemented on the HH10 chick embryos to determine the heart tube formation chick embryos in presence/absence of LPS. It is evident that the left C-looping heart tube has been formed in control embryos (Figure 2b,b1), while three kinds of phenotypes including right looping (Figure 2c,c1), hypertrophy (Figure 2d,d1), and cardiac bifida (Figure 2e,e1) were present in differing proportions (Figure 2f) in LPS-treated groups. This observation was confirmed by the transverse sections of MF20 immunofluorescent stained control and LPS-induced cardiac bifida (Figure 2g–j), in which the bifida of heart tube in LPS-treated embryos could be clearly seen (Figure 2j1,j2) while heart tube remains intact in control embryos (Figure 2h1,h2). A similar experimental finding resulted in whole-mount VMHC in situ hybridization (Figures 2k and 2l) and their corresponding transverse sections (Figure 2l–n). All these data indicate that LPS exposure in early gestation indeed could cause malformation of early chick embryo hearts.
3.3 LPS exposure restricted the cell migration of cardiac precursors during gastrula chick embryo development
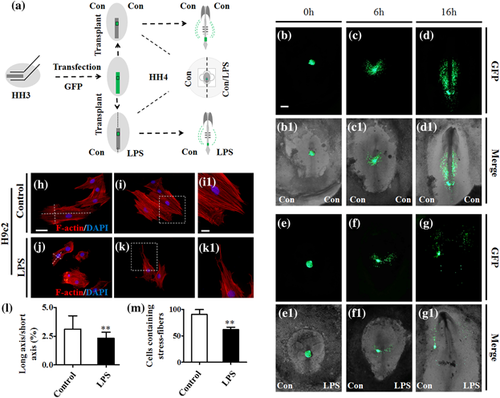
Using a combination of GFP transfection with anterior primitive streak allograft, shown in Figure 3a, the GFP+ cardiac progenitor cell migration was examined in the presence/absence of LPS in EC culture medium. To avoid the diversity of embryo developmental velocities, gastrula chick embryos were employed in home-made 35 mm-diameter culture dishes in which a barrier blocks the medium flow between both sides (Figure 3a). The embryos with the allograft of GFP+ anterior primitive streaks were photographed with fluorescence and bright-field after 0-, 5-, and 16 hr incubations, respectively. The results showed that GFP+ cardiac progenitor cells evenly migrate toward to the site of primary heart field in controls (Figure 3b–d,b1–d1); while in the LPS- exposed half-side embryos, many fewer GFP+ progenitor cells migrate forward in comparison to the saline sample control (Figure 3e–g,e1–g1). F-actin immunofluorescent staining images indicated that fewer cell protrusions were generated in the setting of LPS (Figure 3h–k,i1,k1), which is reflected by the ratios of cell long axis/short axis (p < 0.01; Figure 3l) and cellular stress fibers (p < 0.01; Figure 3m).
In addition, DiI was micro-injected into anterior primitive streak of HH4 gastrula chick embryos, and DiI labeled cardiac progenitor cell migration was studied in the presence/absence of LPS using the above-mentioned strategy (Figure S3a). Two parallel cell migration trajectories of the DiI+ cardiac progenitor cells were found after 15 hr incubation in control embryos (Figure S3b–d,b1–d1); but very few DiI+ cardiac progenitor cells could be seen in the LPS-exposed side of embryo compared to their controls (Figure S3e–g,e1–g1).
To confirm the in vivo results, explant cultures of anterior primitive streak (Figure S4b–b2,c–c2) and H9c2 cell cultures (Figure S4e–e2,f–f2) were studied in vitro in the presence of LPS. After 24- and 48 hr incubations, areas of the cell extensions sent forth from the explants of anterior primitive streaks in vitro culture were measured, and it could be demonstrated that fewer cell extensions occurred in the presence of LPS than in controls (Figure S4d). Moreover, in “scratch wound” migration assays of H9c2 (a permanent cell line derived from rat cardiac tissue) cells, the “gap closure” was shown to be much slower in LPS-treated cells than in untreated controls, based on the photographs after 12- and 24 hr incubations (p < 0.001; Figure S4g). Taken together, this is suggestive that LPS exposure at gastrula chick embryo indeed could repress the cell migration of cardiac progenitor cells toward to primary heart field.
3.4 LPS exposure influenced the differentiation of cardiac precursors during gastrula chick embryo development
The gene expressions related to heart tube formation were determined in presence of LPS by whole-mount in situ hybridization, immunofluorescent staining, Western blot, and quantitative PCR (Figure 4). The GATA5 and Nkx2.5 whole-mount in situ hybridization showed that both GATA5 and Nkx2.5 expressions in the LPS-treated side of embryos were dramatically reduced in comparison to opposite control sides (Figure 4a,a1,b,b1), which could also be clearly seen in their transverse sections (Figure 4a1’–b1’). Using MF20 immunofluorescent staining, fewer MF20+ cells were evident in the monolayer cells emanating from the in vitro cultured explant of anterior primitive streak in LPS-treated group compared to control (Figure 4d,d1,e,e1). Western blotting was employed to determine the expressions of early heart tube formation relevant genes in HH10 chick embryos exposed to LPS, and demonstrated that the expressions of VEGFR2 (p < 0.01) and TBX5 (p < 0.001) were up-regulated to some extent while GATA6 (p < 0.001) was down-regulated by LPS exposure. No change was found in GATA4 (Figure 4g,g1). Moreover, quantitative PCR data showed more gene expression alterations induced by LPS exposure, which are the down-regulated Nkx2.5, BMP2, GATA5, VMHC, and up-regulated TBX20 in both HH7 and HH10 chick embryos exposed to LPS; while Nkx2.6 were down-regulated and TBX20 were up-regulated in HH10 chick embryos. No change was seen in GATA4 expression reduced at mRNA level in HH10 chick embryos exposed to LPS (Figures 4c and 4f). These data imply that LPS exposure certainly altered crucial gene expressions for heart tube formation during early embryo development which, in turn, interfere with the differentiation of cardiac progenitor cells.
3.5 LPS exposure suppressed cell proliferation and enhanced cell apoptosis during gastrula chick embryo development
The LPS-induced reduction of cardiac progenitor cells could also be due to the inhibition of in vivo cell proliferation and apoptosis following the exposure to LPS. Whole-mount co-immunofluorescent staining of MF20 and pHIS3 was carried out in gastrula chick embryos exposed to simple saline (control, Figures 5a and 5c) and LPS (Figures 5b and 5d). The assessment of pHIS3+ cell numbers in MF20+ heart tube sections showed that there were less pHIS3+ cells in LPS-treated heart tubes than in controls (p < 0.01; Figure 5c1–c3,d1–d3,e). Meanwhile, western blot data showed that PCNA expression at the protein level was also significantly down-regulated in the LPS-treated group (p < 0.001; Figures 5k and 5l). Furthermore, immunofluorescent staining against pHIS3 was implemented on the in vitro cultured H9c2 cells, and showed the similar tendency, with less pHIS3+ cells being found in LPS-treated group compared to the control groups (p < 0.01; Figure S5a–a2,b–b2,c).

The whole-mount co-immunofluorescent staining of MF20 and C-caspase3 in gastrula chick embryos was implemented to detect cell apoptosis in the heart tubes exposed to LPS (Figure 5f–i). Counting C-caspase3+ cells on their corresponding transverse sections of heart tubes showed that there were more c-Caspase3+ cells in LPS-treated group than in controls (p < 0.01; Figure 5h1–h3,i1–i3,j). This was confirmed by Western blot data about C-caspase3 protein expression in HH10 chick embryos (p < 0.001; Figures 5m and 5n). Likewise, more PI (propidium iodide)-positive cells were evident in LPS-treated H9c2 in vitro cultured cells than in controls (p < 0.001; Figure S5d–d2,e–e2,f), It is suggested that LPS exposure is able to suppress proliferation and promote apoptosis of cardiac progenitor cells.
3.6 The excessive ROS is responsible for LPS exposure-induced malformation of heart tubes during gastrula chick embryo development
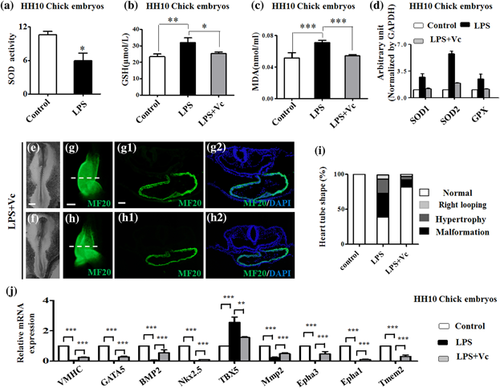
Superoxide dismutase (SOD), glutathione (GSH), and malondialdehyde (MDA) activity assay kits were used to determine the activities of SOD, GSH, and MDA in HH10 chick embryos exposed to either simple saline (control) or LPS (Figure 6a–c). The results showed that SOD activity reduced in presence of LPS (p < 0.05; Figure 6a). The activities of both GSH (control and LPS: p < 0.01; LPS and LPS + vitamin C: p < 0.05) and MDA (control and LPS: p < 0.001; LPS and LPS + vitamin C: p < 0.001) increased in the presence of LPS but fell back again after addition of vitamin C (Figures 6b and 6c). The quantitative PCR data showed that the expressions of SOD1, SOD2, and GPX at the mRNA level were up-regulated by LPS treatment, but addition of vitamin C again showed a normalizing effect (Figure 6d). This adverse effect of vitamin C against LPS-induced abnormity was also seen in MF20 immunofluorescent-stained heart tube formation, which appeared to be normal as control (Figure 6e–h,g1,g2,h1,h2) and abnormity rate dramatically decreased following addition of vitamin C (Figure 6i). Furthermore, the expressions of the key genes related to heart tube formation were determined and showed that LPS- exposure led to the expression reduction of VMHC, GATA5, BMP2, Nkx2.5, TBX5, Mmp2, Epha3, Epha1, and Tmem2 in HH10 chick embryos exposed to LPS, but that all of their expressions were recovered to some degree after addition of vitamin C (Figure 6j).
Next, photographs (Figure S6a–c) and determinations of ROS activity (Figure S6d) were taken after 24 hr incubations in the primary culture of chick cardiac muscle cells exposed to the combined application of 228 µM (=40 µg/ml) vitamin C and 10 µg/ml LPS. The result showed that LPS exposure induced the increase of ROS activity but showed a significant drop when the after application of vitamin C and LPS together (control and LPS: p < 0.001; LPS and LPS + vitamin C: p < 0.001; Figure S6d). In addition, when 228 µM vitamin C was added into the cultured H9c2 cells in presence of LPS, LPS treatment allowed the reduction of pHIS3+ cell numbers to recover to some extent (control and LPS: p < 0.01; Figure S6e–n).
All these data showed that LPS could promote the cell apoptosis of cardiac progenitor cells. Here, using flow cytometry, it has been demonstrated that LPS-exposure increased cell apoptosis, but application of vitamin C and LPS together distinctly suppressed the increase of LPS-induced cell apoptosis in the cultured chick cardiac muscle cells (control and LPS: p < 0.001; LPS and LPS + vitamin C: p < 0.001; Figure S7a–d). Similar experiments were carried out with cultured H9c2 cells immunofluorescent stained with PI, the results also showing that the combinations of vitamin C and LPS obviously repressed the increase of LPS-induced PI+ H9c2 cells (control and LPS: p < 0.001; LPS and LPS + vitamin C: p < 0.001; Figure S7e–n). Vitamin C, as an anti-oxidant, could reduce the various LPS-induced the alterations of cell proliferation and apoptosis, further indicating that excessive ROS production might elevate the percentage of abnormal heart tube formation induced by LPS exposure.
4 DISCUSSION
It has been know that while many congenial diseases are induced by external environmental harmful factors, such as folate deficiency and teratogens, exposure in the first weeks of human gestation may cause severe congenital heart defects (Linask, 2013). However, since most pregnancies are not planned, people might not undertake any precautionary protective actions until they are sure about their pregnancies. The reason that birth defects occur frequently in nervous and cardiovascular systems is because they develop at the earliest stage of embryogenesis. Maternal LPS could come from intestinal microbiota imbalance or from the infection of pregnant women at various stages of gestation. It has been confirmed in this work that the high level of LPS occurs in LP patients (Figure 1a) when data from the mouse model revealed the relationship between intestinal microbiota imbalance and embryonic survival rate. When mice were treated with LH, the LPS level was increased and embryonic survival rate decreased (Figure 1b–e). All the above results suggested the possibility that LPS could be a potential teratogen in both human and mouse. LPS directly applied to pregnant mice yielded the similar phenotype which led to investigation of the potential mechanism by which LPS affects survival of embryos. LPS exposure will enhance the risk of embryonic heart malformation for the affected offspring, as shown by in vitro experiments of embryonic cardiomyocytes (Panaro et al., 2010). Once the mechanism of LPS-induced cardiac birth defects is understood, rational precautionary measures could be effectively implemented.
In this study, avian models are employed because of the advantages they offer for extrapolating the effect of LPS on embryo development to human pregnancy. Using early gastrulating chick model, LPS exposure at gastrula stage was found to significantly reduce embryo weight and cause the death and abnormal developments of some embryos (Figures S1 and S2). Meanwhile, LPS also increased the incidence of chick heart tube malformations, including right looping, hypertrophy, and cardiac bifida, which was clearly displayed by MF20- and VMHC- labeled heart tube in whole-mount and transverse sections (Figure 2). Blad, Welin, Kjellmer, Rosen, and Mallard (2008) reported that preterm lambs were more sensitive to LPS exposure than were more mature foetuses in terms of myocardial/cardiovascular responses (electrocardiography and heart rate), suggesting that foetal cardiac functions could also be impacted by LPS exposure. The mechanism of LPS exposure-induced malformation of heart tubes could be complex. It is well known that cardiac precursors in first heart field derive from anterior primitive streak cells of gastrulating embryos. These cardiac precursors migrate toward to the site of heart tube formation, which is assuredly an essential initial step for normal heart tube formation (Yang et al., 2002). Using in vivo allograft or DiI labeling of anterior primitive streaks of gastrula chick embryos in presence of LPS, it was demonstrated that LPS exposure could significantly inhibit the migratory ability of cardiac precursors toward the first heart field (Figures 3 and S3). The reduction of cardiac precursor migratory ability was also confirmed by the following observations: a) fewer cells emigrated from in vitro cultured anterior primitive streak explants and b) decreasing healing velocity in wound scratch assay of H9c2 cells in the setting of LPS (Figure S4). Similar experimental data indicated that the migratory ability of cardiac precursor cells was negatively influenced in gastrula chick embryos exposed to LPS.
The morphogenesis of heart tubes depends on normal spatiotemporal gene expressions of cardiomyocyte differentiation relevance such as GATA5, Nkx2.5 etc. (Wei et al., ). Whole-mount in situ hybridization and immunofluorescent staining showed that LPS exposure dramatically restricted the expression of GATA5 and Nkx2.5 at cardiac crescent and of MF20 in extended cells from in vitro cultured anterior primitive streaks (Figure 4). Not only these genes, but also the expressions of VEGFR2, GATA4, TbX5, GATA6 etc., were disturbed by LPS exposure at early gastrula stage of embryos (Figure 4). As zinc-finger transcription factors, the GATA family perform vital functions in heart formation since bilateral heart tubes (cardia bifida) and fewer cardiomyocytes could occur in GATA4 knock-out mice. GATA4, GATA5 deficiencies also leads to cardia bifida, while the over-expression of GATA5 results in ectopic Nkx2.5 expression with ectopic beating cells in fish embryos (Brand, 2003). Both the published literature and the results of the work reported here indicate that LPS induces the disorder of crucial gene expressions on controlling cardiomyocyte differentiation during heart tube formation. This could then partially contribute to the above-mentioned phenotypes.
Van Den Berg et al. (2009) reported that there are proliferating growth centers in the caudal coelomic walls, and that these play an important role on elongating the heart tube and provide structural support for early heart formation. In this study, using embryonic heart tube and primary cardiomyocytes, cell proliferation in cardiac progenitor cells were demonstrated to be inhibited by LPS exposure (Figures 5 and S5). Ghatpande, Ghatpande, Zile, and Evans (2000) found that either addition of retinoic acid or anterior endoderm transplantation is able to reduced cell apoptosis and GATA4 expression, which maintains normal heart tube morphogenesis), suggesting the importance of cell apoptosis during cardiogenesis. Both the results of in vivo and in vitro studies described here indicate that cell apoptosis of cardiac precursors are raised following the exposure to LPS during gestation (Figures 5 and S5), which would be associated with subsequent heart tube malformation.
Earlier work revealed that many external factors, including high glucose and ethanol, could be hazardous factors in causing malformation of embryonic heart tube (Li et al., 2015; Wang, Huang et al., 2015), in which excessive ROS generation was observed, thereby implying that ROS plays a vital role in the pathological process. In this study, LPS exposure was observed to reduce the activities of SOD and increase the activities of GSH and MDA in embryo heart tubes and primary culture of chick cardiomyocytes (Figure 6). The combination of vitamin C and LPS could to an extent reduced LPS-induced apoptosis increase and proliferation reduction implying that excessive ROS production mediated LPS exposure-induced malformation of heart tubes (Figures S6 and S7).
In other words, excess production of O2 radicals induced by LPS exposure could, in turn, initiate cell apoptosis in embryonic cells, and so, impair normal embryo development. This is similar to the discoveries of ethanol-induced embryonic dysplasia through stimulating ROS generation (Bailey & Cunningham, 1998; Brown & Brown, 2012; Gonzalez, Pariente, & Salido, 2007). In LPS-induced adult diseases, accumulating evidence suggests that ROS play an important role in cardiac- and hepatic dysfunctions during various inflammasomes and endotoxemia (Yu et al., 2016; Zhao et al., 2016).
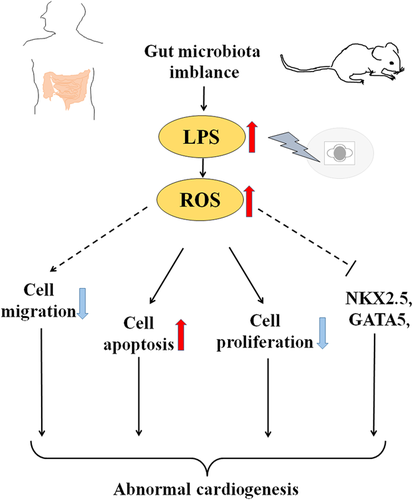
In summary, it has been demonstrated that LPS was induced in gut microbiota imbalance in mice, and observed that LPS exposure during early chick embryo development increased the risk of various malformations of heart tubes including cardia bifida. LPS exposure at gastrula stage can inhibit cell proliferation and enhance cell apoptosis of cardiac precursors. In the context of LPS, excessive ROS is produced during heart tube formation, and both cell migration and differentiation-related gene expressions (Nkx2.5, GATA5 etc.) of cardiac progenitor cells were interfered with. As a consequence, the malformation of heart tube such as cardia bifida resulted, which is schematically summarized in Figure 7. There is no doubt that studies in this field illuminate the opportunities for congenital heart disease prevention and treatment. However, further experimentation is needed to explore the precise molecular biological mechanisms.
ACKNOWLEDGMENTS
This study was supported by NSFC grant (31771331, 81571436, 31401230), Science and Technology Planning Project of Guangdong Province (2014A020221091, 2017A020214015, 2016B030229002, 2014A020213008, 2017A050506029), Science and Technology Program of Guangzhou (201710010054, 201510010073), Guangdong Natural Science Foundation (2016A030311044), The Fundamental Research Funds for the Central Universities (21617466) and Research Grant of Key Laboratory of Regenerative Medicine, Ministry of Education, Jinan University (No. ZSYX-M-00001 & ZSYX-T-00001).
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.