miRNAs and ovarian cancer: An overview
Abstract
Ovarian cancer (OC) is the sixth most common cancer in women globally. However, even with the advances in detection and therapeutics it still represents the most dangerous gynecologic malignancy in women of the industrialized countries. The discovery of micro-RNAs (miRNA), a small noncoding RNA molecule targeting multiple mRNAs and regulation of gene expression by triggering translation repression and/or RNA degradation, has revealed the existence of a new array for regulation of genes involved in cancer. This review summarizes the current knowledge regarding the role of miRNAs expression in OC. It also provides information about potential clinical relevance of circulating miRNAs for OC diagnosis, prognosis, and therapeutics. The identification of functional targets for miRNAs represents a major obstacle in our understanding of microRNA function in OC, but significant progress is being made. The better understanding of the role of microRNA expression in ovarian cancer may provide new array for the detection, diagnosis, and therapy of the OC.
1 INTRODUCTION
Ovarian cancer is one of the most common gynaecologic malignancies in women worldwide, with each year of about 230,000 new cases and almost 140,000 death per annum (Dahiya & Morin, 2010). Ovarian cancers usually develop in granulosa theca cells or germ cells, moreover >90% of ovarian cancers have an epithelial histology and were assumed to have been arisen from cells that cover ovarian surface or from the line of subsurface of inclusion cysts (Feeley & Wells, 2001). The risk factors for ovarian cancer include family history, age, and persistent ovulation with attributed carriers of mutated BRCA1 hereditarily (Nikitin & Corney, 2008). Inspite of advances in detection and cytotoxic therapies, only 30% of advanced stage cancer patients survive nearly 5 years after initial stage prognosis (Greenlee, Hill-Harmon, Murray, & Thun, 2001), while other 70% patients have high mortality rate due to late stage diagnosis (Iorio et al., 2007). Only, 19% of ovarian cancer patients are diagnosed at its early stage (Iorio et al., 2007). Routine diagnostic procedures, pelvic examination, serum CA125, and transvaginal ultrasonography usually fail to detect early stage of cancer and thus more death rates (Kinose, Sawada, Nakamura, & Kimura, 2014). Primarily ovarian cancer patients have some early and specific symptoms shared in common genitourinary, gastrointestinal, and gynaecological conditions which are not usually proven useful for early diagnosis (Kinose et al., 2014). The basis of this poor prognosis is due to insidious symptomatic nature in early stage, tumour resistance to chemotherapy, and lack of robust and minimal invasive method at its early detection (Iorio et al., 2007). Hence, advanced approaches for detection for early stage of ovarian cancer is necessary for proper medication and treatment timely.
Ovarian carcinomas have four major histological subtypes clear cell, endometrioid, serous, and mucinous, with serous being most frequent (Iorio et al., 2007). Latest data on large scale analysis of ovarian cancer samples suggest acquisition of invasiveness accumulated at mesenchymal subtype of tumours to be associated with transforming growth factor-β (TGF- β), TGF- β is a multifunctional protein which induces epithelial to mesenchymal transition (EMT) that leads to metastasis and is associated with chemotherapy resistance in multiple cancers (Parikh et al., 2014). Recent investigations indicate that these histological types are associated with distinct morphologic and molecular genetic alternations (Bell, 2005). And further investigations are requisite for the molecular mechanisms of ovarian cancer to determine pathway of these subtypes. Over past few years, expression profiling technologies have been greatly developed, expanding the knowledge on clinical implications of cancer at a molecular level to provide potential use for diagnosis, therapy, and drug development. This effort has brought out with new and better markers and therapeutics for diagnosis and curative process of ovarian cancer.
miRNAs are small, endogenously expressed, single stranded RNAs, non-coding RNAs of almost 19–25 nucleotides in length cleaved from 70 to 100 nucleotide hairpin pre-miRNA precursors (Iorio et al., 2007). Recently, many research groups have identified and studied altered expression of miRNAs in ovarian tumorigenesis leading to ovarian cancer. The miRNA are used as novel target and markers for detection, prognosis /diagnosis, and therapy (Bartels & Tsongalis, 2009). It was first discovered in Caenorhabditis elagans (Lee, Feinbaum, & Ambros, 1993), however now it is found to be present and highly conserved in all types of organisms (Wheeler et al., 2009). Bioinformatic analyses predict nearly 1,000 miRNAs in human genome which are capable of regulating almost 60% of human transcriptome (Di Leva & Croce, 2013). The miRNAs are found to be multifunctional due their conservation, high abundance, and tissue specific expression in almost all biological processes- cellular developmental control, regulation, and development of various diseases, including cancer (Di Leva & Croce, 2013). It possesses negative regulation of gene expression at the post transcriptional level in a sequence specific manner, with 3′ untranslated region (3′ UTR) of the target messenger RNA transcripts (Parikh et al., 2014).
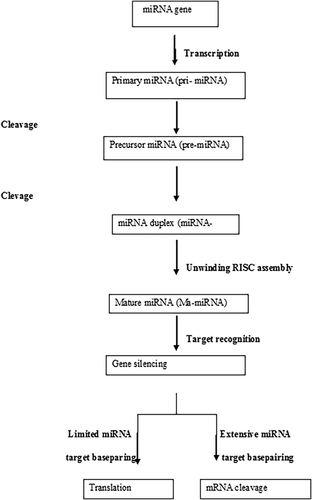
The miRNAs are synthesised in nucleus by enzyme DNA polymerase II as a precursor called primary- miRNA that is processed by two enzymes, Drosha and Pasha, (Figure 1) into a precursor primary miRNA that is carried to the cytoplasm by exportin5 (Bohnsack, Czaplinski, & GÖRLICH, 2004; Cullen, 2004; Zeng & Cullen, 2004). Once the pre-miRNA reaches the cytoplasm, Dicer cleaves it into nearly a 22 nucleotide long functional mature miRNA, which then assembles into a ribonucleoprotein complex known as RNA- induced silencing complex (Pratt & MacRae, 2009). The miRNA downregulates their target by inhibiting mRNA translation and/or promoting mRNA degradation and its mode of repression may depend on the level of complementarity of miRNAs (Dahiya & Morin, 2010). However, miRNAs do not always require perfect complementarity for functional mRNA target interaction, a single miRNA can regulate multiple targets and miRNAs can regulate individual mRNAs (Lewis, Shih, Jones-Rhoades, Bartel, & Burge, 2003). The miRNA genes express its own promoters in intergenic regions, and significant portion of these genes is located within other transcriptional units, (Baskerville & Bartel, 2005), usually in intronic regions and some in exonic regions as well, and these intronic miRNAs can regulate genes involved in pathways of the host mechanism, including complexity of these regulatory genes (Barik, 2008).
1.1 miRNAs in circulation
The miRNAs are circulating continuously in our body secreted from specific cells to the recipient cells (Kosaka et al., 2010; Valadi et al., 2007). The miRNAs circulating in our body were first detected in plasma and serum during 2008 and subsequently in various parts of our body fluids like- urine, breast milk, saliva, and many more (Han et al., 2013). Earlier studies reported that miRNAs present in human plasma are remarkably stable form, protected from endogenous RNase activity (Mitchell et al., 2008). For example, miR-141 could distinguish patients with prostate cancer and normal individuals. Park et al., 2009 reported that miR-125a and miR-200a were present at significant lower quantity in the saliva of oral cancer patients then in normal individuals (Park et al., 2009). Circulating miRNAs were found to be stable even under harsh environment, such as extreme high temperature, pH values, long term storage and many more (Chen, Ba, et al., 2008; Mitchell et al., 2008; Wang et al., 2015). The miRNAs can be either upregulated or downregulated in specificity of any diseases like cancer. This specificity and stability make circulating miRNAs as biomarker, diagnostic tool, and therapeutic targets.
1.2 miRNA in ovarian cancer
The miRNAs are estimated to control over 50% activities of all protein coding genes (Krol, Loedige, & Filipowicz, 2010), and are observed to be involved in regulation of almost all the cellular processes (Huang et al., 2011). Many studies revealed that dysregulation of miRNAs leads to variety of human diseases including cancer (Kinose et al., 2014; Perera & Ray, 2007). The miRNAs involved in oncogenesis can be classified as tumour suppressors or oncogenes, depending on their expression pattern and function (Calin & Croce, 2006). Analysis of miRNAs with high- through put technologies has revealed fingerprints of cancer specific targets in all types of cancers (Calin & Croce, 2006; Croce, 2009). The widespread misexpression of miRNAs leads ultimately to cancer and the different mechanisms responsible for this conditions are: DNA point mutation, epigenenetic mechanism, chromosomal alterations, translational modulation, genetic and epigenetic alterations in transcriptional and post- transcriptional machinery (Di Leva & Croce, 2010). The Drosha and Dicer are essential enzymes for the biogenesis of miRNAs and the low Dicer is significantly associated with final stage of ovarian cancer and low Dorsha with suboptimal surgery (Kinose et al., 2014). An essential aspect of miRNA in ovarian cancer is the fact that miRNAs can be up or downregulated in malignancies with respect to tumour suppressor genes or oncogenes (Esquela-Kerscher & Slack, 2006). The miRNAs present in serum correlate the presence of malignancies and tumours and have also been used as detection tool for cancer (Boeri et al., 2011). On the basis of various reports of expression profiling of miRNAs in ovarian carcinomas detailed data are presented in the Tables 1 and 2.
| Cell types | Upregulated | Reference |
|---|---|---|
| Serous ovarian cancer | miR-16, miR-20a, miR-21, miR-27a | Nam et al. (2008) |
| EOC cell line | miR-26, miR-26b, miR-103, miR-182 | Zhang et al. (2008) |
| StageIII/IV epithelial ovarian cancer | miR-182, miR-135b, miR-200a, miR-195 | Wyman et al. (2009) |
| Ovarian carcinaoma | miR-146, miR-221, miR-508 | Dahiya and Morin (2008) |
| Serous papillary ovarian carcinoma | miR-223, miR-206, miR-195, miR-10b, let-7i | Laios et al. (2008) |
| Cell types | Downregulated | Reference |
|---|---|---|
| Serous ovarian cancer | miR-145, miR125b, miR-100 | Nam et al. (2008) |
| EOC cell line | miR-377, miR-432, miR-124a, miR-436, let 7d | Zhang et al. (2008) |
| StageIII/IV epithelial ovarian cancer | miR-493, miR-210, miR-935, miR-31, miR-22 | Wyman et al. (2009) |
| Ovarian carcinaoma | Let 7f, miR-106b, miR-422a, miR-424, miR-648 | Dahiya and Morin (2008) |
| Serous papillary ovarian carcinoma | miR-125b, miR-31, miR-99a, miR-9, miR-137 | Laios et al. (2008) |
1.3 miRNA target
The miRNA exerts important function with regulation of specific target mRNAs. Various miRNA target locating site can be identified with several algorithms, such as miRanda (John et al., 2004), PicTar (Krek et al., 2005), Target Scan (Lewis et al., 2003), RNA hybrid (Rehmsmeier, Steffen, Höchsmann, & Giegerich, 2004) and DIANA- micro T (Maragkakis et al., 2009). Experimental approaches are necessary for accurate full complement of miRNA target (Creighton, Nagaraja, Hanash, Matzuk, & Gunaratne, 2008; John, Sander, & Marks, 2006), hence, databases with experimentally verified miRNA target are being constructed (Sethupathy, Corda, & Hatzigeorgiou, 2006). In addition to computational methods, various functional approaches can be used for identification of actual target miRNAs. The miRNAs with overexpression or down regulation characteristics are cultured and examined with effects on mRNA and protein level expression (Valadi et al., 2007) profile or proteomics approaches (Dahiya & Morin, 2010). For example, identification of mRNAs regulated by miR-1 and miR-124 (Lim et al., 2005) was performed by overexpressing miRNAs with its observable changes in genetic expression using microarrays. The translational regulation of proteomics analysis can performed with stable isotope labelling by amino acid in cell culture followed by mass spectroscopy for investigation of miR targets in Hela cells (Vinther, Hedegaard, Gardner, Andersen, & Arctander, 2006) with significant overlap of these with targets identified using microarray.
Multiple biochemical methods are also used for identification of miR targets with their corresponding targets. For example, miR124a target is enriched with immunoprecipitation of Ago2 gene (Karginov et al., 2007). Also, synthesis of cDNA clones of known mRNA templates with miR primers allows identification of miRs as well as binding sites for mRNA (Vatolin, Navaratne, & Weil, 2006).
1.4 miRNA expression profiles in ovarian cancer
Several studies using miRNA microarray and cDNA microarrays revealed wide transcriptional changes in ovarian cancer (Zhang et al., 2008). Various miRNA are identical to be downregulated in advanced stages or high grade of ovarian cancer, supporting to the fact of miRs to be involved in malignancy and tumourogenesis (Kinose et al., 2014).
Iorio et al. (2007) reported expression profiles of miRNAs between ovarian cancer tissues/cell lines and normal tissues. Of 29 miRNAs, they identified that only four(miR-14, miR-200a, miR-200b, and miR-200c) were upregulated and rest all the 25 miRNAs including miR-140, miR-145, miR- 199a, and miR-125b-1 were downregulated in the cancer samples, and all the miRNA signatures were different in ovarian cancer histotypes. Vang et al. (2013), reported miRNA expression profiles between primary serous ovarian cancer and their omental metastasis using miRNA qPCR arrays and found 17 miRNAs with differential expression pattern out of which miR-146a and miR-150 were significantly responsible for omental metastases, cisplatin resistance and enhancement of spheroid formation. Calura et al. (2013) reported miRNA profile expression of each EOC histotype at stage1 and reported miRNA markers for clear cell and mucinous histotypes with high expression of miR-30a-5p and miR-30a-3p on clear cell histotype and miR-192 and miR-194 on mucinous histotype. Nam et al. (2008) analysed miRNA profile expression of 20 serous ovarian carcinoma using miRNA microarray and compared with normal samples and reported 11 miRNAs were upregulated (miR-16, miR-20a, miR-21, miR-23a, miR-23b, miR-27a, miR- 93, miR-141, miR-200a, miR-200b, and miR-200c) and 12 downregulated (miR-10b, miR-226a, miR-29a, miR-99a, miR-100, miR-125a, miR-125b, miR-143, miR-145, miR-199a, miR-214, and let-7b).
The Cancer Genome Atlas project has analysed mRNA expression, promoter methylation, miRNA expression and DNA copy number of 489 high grade serous ovarian adenocarcinomas (Network, 2011) and reported TP53 mutations in about 96% tumours, in addition to recurrent somatic mutations in BRCA1, BRCA2, NF1, RBI and CDK12 (Kinose et al., 2014). Eight key miRNAs (miR-25, miR-29c, miR-101, miR-128, miR-141, miR-182, miR-200a, and miR-506) were identified and predicted to target 89% ofits network (Kinose et al., 2014).
1.5 miRNAs alteration in ovarian cancer
miRNA expression may be altered with the changes in copy number of gene with the proximity of CAGRs (Rossi, Sevignani, Nnadi, Siracusa, & Calin, 2008). A more detailed report of cancer alterations was provided with array comparative genomics hybridisation to identify miRNA loci gained/ lost in ovarian cancer, breast cancer, and melanoma (Zhang et al., 2006). Huang et al. (2011), analysed 283 miRNA loci and found 105 (37.1%) significantly alters in their copy number (Zhang et al., 2006). Similar, significant copy changes were observed in 206 (out of 283), that is, 72.8% in breast cancer sample and 23 (out of 283), that is, 85.9% in melanomas sample. ThemiR-15a/ 16-1 locus is lost in 23.9% of ovarian cancer sample and miR-17-92 locus in all tumour samples. Huang et al. (2011), also found 24.8% and 51.5% of ovarian tumour gains copy number of Dicer1 and Argonautee 2 (Ago2)loci (Zhang et al., 2006). Dicer and Ago2 proteins are necessary for efficient miRNA processing and function, short- hairpin-RNA (shRNA)- mediated knockout of Dicer1 and Ago 2 enhances colony formation in soft agar and tumour formation in vivo (Kumar, Lu, Mercer, Golub, & Jacks, 2007). Alterations in Dicer1/ Ago2 expression leads to vast changes in miRNA expression and these are common in cancer via several mechanisms such as germline deletion, mutation, (Calin et al., 2005) or promoter methylation (Saito et al., 2006). Mutation of transcription factor p53 is also one of the common genetic alterations found in EOC, particularly in high grade serous tumours (Corney, Flesken-Nikitin, Choi, & Nikitin, 2008).
1.6 Dysregulation of miRNA
Various studies of miRNAs expression in ovarian cancer to normal ovaries, primary ovaries, primary ovaries surface epithelial cells (OSE) and immortalized ovarian surface epithelial cells (Creighton et al., 2010; Nagaraja et al., 2010; Vaksman et al., 2011), have been performed and these reports have been summarised; with 310 dysregulated miRNAs in ovarian cancers out of which 34 miRNAs were found to be consistently dysregulated in ovarian carcinomas of atleast three independent studies (Tables 1 and 2). Several miRNAs found to regulate growth of ovarian cancers are given in Table 3, including miR-31, miR-34abc, miR-125b, miR-127, and let-7a/b/d/f miR-31 (Zhang et al., 2015).
| Biomarkers | Tumour histology | Sample | Reference |
|---|---|---|---|
| miR-21, miR-141, miR-200a, miR-205 | Serous adenocarcinoma | Exosome (Serum) | Taylor and Gercel-Taylor (2008) |
| miR-30c-1-3p | Serous adenocarcinoma, Endometrioid adenocarcinoma | Whole blood | Häusler et al. (2010) |
| miR-205 | Serous adenocarcinoma, Endometrioid adenocarcinoma and Clear cell adenocarcinoma | Plasma | Zheng et al. (2013) |
| miR-200a, miR-20ob, miR-200c | Serous adenocarcinoma | Serum | Kan et al. (2012) |
| miR-92, miR-93, miR-126 | Serous adenocarcinoma, Endometrioid adenocarcinoma and Clear cell adenocarcinoma | Seum | Resnick et al. (2009) |
1.7 miRNA expression on high grade serous ovarian cancer
Almost 70% of ovarian cancer mortality is due to advanced stage and high grade serous ovarian cancer (Network, 2011). High grade serous cancers are signature with multiple copy of abnormalities, TP53 mutation and epigenetic changes (Zhang et al., 2015). Alterations of BRCA1 and BRCA2 are frequently associated due to this phenomenon (Zhang et al., 2015).
Miles, Seiler, Rodriguez, Rajagopal, and Bhanot (2012) identified 17 dysregulated miRNAs responsible for high grade serous cancer compared to normal ovarian samples, eight upregulated miRNAs (miR-183-3p, miR-15b, miR-15b-3p, miR-590-5p, miR-16, miR18-a, miR-18b, and miR-96) and nine downregulated miRNAs (miR-34c-5p, miR-34b-5p, miR-133a, miR-34c-3p, miR-139-5p, miR-140-3p, miR-145, miR-143-5p, and miR145-3p). Other reports revealed miRNA expression in ovarian cancer and normal ovarian tissues (Iorio et al., 2007; Lee et al., 2009; Vogt et al., 2011), three upregulated (miR-96. miR-15b and miR-16) and five downregulated (miR-140-3p, miR-143-5p, miR-34b-5p, miR-34c-5p, and miR-145) (Zhang et al., 2015).
1.8 Epigenetic alterations in miRNAs
Hypermutation of tumour suppressor miR-34a and miR-34bc confirm ovarian cancer with decreased miR-34 (Calin et al., 2004; Flavin et al., 2009; Vogt et al., 2011). Zhang et al. (2008) used 5-AZA and a histone deacetylase inhibitor 4-phenylbutric acid with five ovarian cancer cell lines and found 16 out of 44 (36.44%) miRNAs were downregulated in advanced stage of ovarian cancer. Iorio et al. (2007), found miR-205, miR-203, and miR-21 overexpressed in ovarian cancers and that level can be further increased with demethylating agent 5-aza-2-deoxycytidine (5-AZA), suggesting regulation with methylation.
1.9 miRNA responsible for BRCA1 and BRCA2 expression
Almost 15% of ovarian cancers are hereditary associated germline mutations of BRCA1 and BRCA2, mismatch repair genes and or rare occasions TP53 (Preston et al., 2013). BRCA1 and BRCA2 affect gene expression profiles and abnormalities in high grade serous ovarian cancer (Lee et al., 2009). The miRNA can downregulate BRCA1 expression, AG to C polymorphisms (rs2910164) in miR-146a precursor leading to mismatch in stem region (Zhang et al., 2015). MiR-146-a binds to 3′ UTR of BRCA1 and BRCA2 mRNAs and can lead to variation in their expression. rs2910164 polymorphisms of miR-146a can affect at initial stage of cancer state (Zhang et al., 2015).
1.10 miRNAs role in TP53
Almost 96% of TP53 mutations are found in high grade serous ovarian cancers which can regulate miRNAs (Zhang et al., 2015). In case, miRNA-34 family upregulates with wild TP53, expression of miRNA 34a decreases by100% and 34b and 34c by 72% of cancers with TP53 mutations (Lee et al., 2009).
1.11 Strategies for miRNA detection in ovarian cancer samples
1.11.1 RNA isolation, reverse transcriptase, and quantification of miRNA expression level
For quantification of endogenous or transfected expression levels total RNA should be isolated using TRI pure reagent (Roche, Indianapolis, IN46250-0414 USA) and 1 μg RNA is used for reverse transcription and quantitative polymerase chain reaction is performed with TaqMan miRNA assays (Dwivedi et al., 2016). Relative miRNAs expression can be calculated using comparative Ct method with U6 as the normalizer (Marcus, Maxwell, Darcy, Hamilton, & McGuire, 2014).
1.11.2 Northern blotting
In northern blot analysis hybridisation is performed at 37°C in ULTRAhyb- Oligo hybridisation buffer (Ambion) for 16 hrs. Membranes are then washed at 37°C twice with 2X Saline- sodium phosphate- EDTA and 0.5% SDS. 5S RNA or EtBr gel staining can be used to normalise and finally blots are stripped in boiling 0.1% SDS for 10 min before rehybridisation (Iorio et al., 2007).
1.11.3 Western blotting
Proteins can be quantified using BCA assay Kit (Thermo Scientific, Grand Island, NY 14071), on SDS-PAGE gel then transferred to Immun- Blot PVDF membrane (Dwivedi et al., 2016). Membranes are blocked using 5% non-fat milk with incubation and finally further proceedings.
1.11.4 Quantitative real time PCR
Single tube taqmann miRNA assays are used to detect and quantify miRNAs using real time PCR instruments. Gene expression level can be quantified using ABI Prism 7,900 HT sequence detection system (Dwivedi et al., 2016). Comparative real time PCR is performed for atleast three times including no template controls. Relative expression can be calculated with comparative Ct method.
1.11.5 miRNA microarray hybridisation and quantification
RNA labelling and hybridisation on miRNA microarray chips can be carried out with probe required. Desired hybridisation signals can be detected either Streptavidin- Alexa 647 conjugate and scanned images (Axonn 4000B, Axon Inc, Scottsdale, AZ) can be quantified using Gene Pix 6.0 software (Axon instruments) (Iorio et al., 2007).
1.11.6 Computational analysis of miRNA in ovarian cancer
Microarray data can be analysed with GenePix Pro. Global median normalisation of ovary microarray data can be performed using BRB Array tools developed by Richard Simon and Amy Peng Lam (Wright & Simon, 2003). The miRNAs which are differentially expressed can be identified using t-test procedure with further significant analysis of microarrays, a method developed by Stanford University Labs (Tusher, Tibshirani, & Chu, 2001). The miRNA signatures can be identified with PAM, which does sample classification from gene expression data, via the “nearest shrunken centroid method” of Tibshirani, Hastie, Narasimhan, and Chu (2002).
1.12 miRNA as potential biomarker
The miRNA can act as an important tool for diagnosis and detection of ovarian cancer. Under-expression of miR-9 has been found to be reported in ovarian cancer patients (Laios et al., 2008). Upregulation of miR-92, miR-21 and miR-15a has been signatory in ovarian cancer. Low expression of miR-31 acts as an indicative feature of early stage of ovarian cancer development (Zhang et al., 2015). Downregulation of miR-34 a/b/c/ miR-449b, miR-503 and miR-507 has been found in final stage of ovarian cancer patients (Corney et al., 2010; Lee et al., 2009, Zhang et al., 2008). Overexpression of miR-200family and under-expression of let-7 family are also found in ovarian cancer patients. Lawrie et. al. (2008), reported serum miRNAs- miR-21, miR-155 and miR-210 were much higher in cancer patients than normal individuals (Lawrie et al., 2008). Chung et. al. (2013), reported let-7b, miR-26a, miR-132 and miR-145 as potential biomarker in ovarian cancer patients (Chung et al., 2013). Zheng et. al. (2013), reported let-7f and miR-205 as biomarker for ovarian cancer detection at early stage of cancer. Detailed description of the list of biomarkers is provided in Table 3.
1.13 miRNAs as diagnostic tool for ovarian cancer
The miRNAs act as a wonderful diagnosis tool for ovarian cancer patients. Patients with least expressed miR-200 genes have poor survival rate (Hu et al., 2009), with cancer development. Also, low let 7a-3 methylation has low survival rate than with high methylation (Dahiya & Morin, 2010). Precursor miR-146a, target for BRCA1 and BRCA2, leads to G to C polymorphisms in ovarian and breast cancer patients (Shen et al., 2008). Ratio of HMGA2 to let-7 acts as important prognosis tool for ovarian carcinoma patients (Shell et al., 2007). The miRNAs in serum sample of patients are useful for detection of cancer biomarkers (Feng, Li, Gentil-Perret, Tostain, & Genin, 2008; Lawrie et al., 2008). Tumour derived exosomes (small lipid vesicles) contain miRNAs with detection and diagnosis markers (Taylor, & Gercel-Taylor, 2008).
1.14 miRNAs as therapeutic targets
He et al., 2014 reported cancer secreted microvesicles contain elevated expression of miR-21, that induces myoblast apoptosis leading to cancer cachexia via Toll like receptor 7 (TLR7) (He et al., 2014). Therapeutic targets for this cachexia can be possibly the inhibition of microvesicles secretion by inhibiting fusion of microvesicles with muscle cells, or blocking the binding of TLR7/8 with miR-21 (Nakamura et al., 2016). Earlier studies reported miR-200 inhibits angiogenesis by targeting interleukin 8 and CXCL1 secreted from tumour epithelial and cancer cells, leading to therapeutic potential of miR-200 in treating ovarian cancer or other oncogenesis (Pecot et al., 2013). Chen, Alvero, et al. (2008) reported miR-199a regulates IKK expression, that modulates inflammatory microenvironment in ovarian cancer. Cittelly et al., 2012 and Cochrane, Spoelstra, Howe, Nordeen, & Richer (2009), reported classIII tubulin (TUBB3) that encodes tubulin isotype found in neural cells, which is the target of miR-200c. The restoration of miR-200c leads to microtubule bindings to chemotherapeutic drugs, vincristine, epothiloneB, paclitaxel that suppressed the action of TUBB3 (Cochrane et al., 2009). Several studies reported involvement of miR-92a in the expression of α5 integrin (Ohyagi-Hara et al., 2013; Sawada et al., 2008). The forced expression on cancer cells by miR-92a highly suppresses peritoneal dissemination in vivo, suggesting targeting of miR-92a as novel and effective gene therapy for ovarian cancer patients (Kinose et al., 2014). Huber et. al. (2012) reported HER2- overexpression leads to cancer as exosomes are released thatbinds to trastuzumab and inhibits anticancer proliferative cell activity (Ciravolo, Huber et al., 2012). Based, on preclinical data Aethlon Medical Inc (CA) developed HER2osome™ as therapeutic target for HER2 positive cancer (Nakamura et al., 2016). Creighton et al, reported expression of miRNAs and mRNAs in serous ovarian cancer, cell lines, and normal ovarian epithelium (Creighton et al., 2010). They found loss of miR-31 leads to defects in TP53 pathway suggesting patients with TP53 deficient activity as therapeutic approach (Creighton et al., 2010; Kinose et al., 2014) (Supplemental S1 and S2).
1.15 Role of miRNAs in ovarian cancer chemotherapy
Multiple cases of primary and recurrent cancers were analysed and found that atleast 60 miRNAs were deregulated more than two fold between primary and recurrent disease (Laios et al., 2008). The most highly deregulated genes, that is, miR-223 upregulated and miR-9 downregulated were found in recurrent versus primary samples (Laios et al., 2008).
Cisplatin acts as the most effective chemotherapeutic agent in ovarian carcinoma with initial response of 40–80% (Dahiya & Morin, 2010). Platinum based combination therapy, with paclitaxel or carboplatin, offers significant efficiency over cisplatin alone and this system is found standard for women with advanced ovarian cancer (McGuire et al., 1996).
miR-214 acts asanti- apoptotic by blocking its expression made with A2780 cells more sensitive to cisplatin induced apoptosis (Yang et al., 2008).
1.16 Current challenges of miRNA based therapeutics
The miRNAs can be used as biomarker in diagnosis and therapeutics. However, there are several challenges in miRNA based therapeutics in relation to efficiency, specificity and safety. The target for miRNA based drug development is stable chemical structure in vivo with high specificity and cell permeability. Chemical modification has been done to enhance oligonucleotide stability to acquire resistance to nucleases. Example, LNA- antimiR-12 was the first drug developed for the treatment of Hepatitis C virus infection (Janssen et al., 2013). The miRNA also activates innate immune system with significant side effects and toxicities (Kinose et al., 2014). The miRNA therapy also acts as target for multiple pathways via imperfect matching with 3′UTRs, that may lead to unwanted silencing of tumour suppressor genes (Kinose et al., 2014).
2 FUTURE PERSPECTIVES
Voluminous information has been acquired in the field of miRNAs in ovarian cancer, yet much is still unknown or if known its exact therapeutic beneficial role is yet to be discovered. In particular, expression profiling of miRNA in ovarian cancer of various stages, grades, drug resistance status and other diagnostic and therapeutic targets are yet to be reported. Once, the required miRNAs and its functional targets are identified, investigation can be done for its clinical perspectives and ultimately novel strategies can be identified for exact detection, prognosis and therapeutics of ovarian cancer.
CONFLICTS OF INTEREST
None.




