Application of a novel bioreactor for in vivo engineering of pancreas tissue
Abstract
Type 1 diabetes is characterized by autoimmune destruction of pancreatic cells. Organ transplantation is an acceptable treatment for native organ failure. However, it is associated with several problems due to a number of reasons, such as the lack of appropriate donors and immunosuppression. In our present study, a novel model is presented for in vivo recellularization of acellular pancreas by implanting between the host pancreas and the adjacent omental flap. In this study, the pancreases were harvested and cannulated via the common bile duct and then, the scaffolds were acellularized by a detergent-based protocol. After that, the abdomens of 35 rats were opened and the spleen was extracted with the adjacent omentum, and placed outside the abdomen. The acellularized scaffold was stretched over the host pancreas and the omentum was wrapped around it to make a sandwich-like structure, which was then fixed with Chromic Sutures 6–0 and marked with Prolene 4–0 on four sides. All samples were biopsied at 14, 30, 60, 90, and 120 days post-transplantation. The result showed marked recellularization of acellularized pancreas with visible neovascularization and neoβ-cells with minimal inflammatory response. This study provides a new approach to produces a normal-like pancreas by allograft transplantation for pancreas tissue engineering. We observed that in vivo transplantation of acellularized pancreas can promote recellularization, proliferation, and differentiation by blood circulation. These findings support that in vivo studies can contribute to finding faster solutions for the treatment of diabetes.
1 INTRODUCTION
Type 1 diabetes mellitus is generally treated by pharmaceutical interventions and exogenous insulin therapy. Although conventional guidelines for diabetes management could reduce the incidence of diabetic emergencies and complications, complete remission still remains a mystery. Furthermore, unfavorable responses are seen in a large number of patients, and insulin resistance could occur in well-responded diabetic patients, inducing adverse effects such as cardiovascular, renal, and ocular complications (Bloomgarden, 2004). As an alternative therapeutic option, whole-organ transplantation is hampered by conspicuous adverse effects of immunosuppression therapy and inadequacy of donors (London et al., 1994; Secchi et al., 1997). With the advent of the Edmonton Protocol in 2000 (Truong et al., 2004), islet transplantation has been considered as a further therapeutic approach. However, donor deficiency and complications such as portal hypertension, and portal vein embolization have restricted the widespread application of this protocol (Association, 2006; Pepper et al., 2013). Tissue engineering, which is a source of obtainable tissues to replace malfunctioning or degenerative tissues (Griffith & Naughton, 2002), has shown promising results in the regeneration of various tissues such as liver (Baptista et al., 2011), trachea (Kajbafzadeh et al., 2015), bladder (Kajbafzadeh et al., 2016), esophagus (Poghosyan et al., 2016), small intestine (Belchior et al., 2014), and colon (Kajbafzadeh et al., 2014). Tissue engineering appears to be a potential novel alternative therapy for insulin-dependent diabetes mellitus in addition to an immunosuppression-free state after transplantation, which could alleviate inevitable consequences of conventional therapies for diabetes. Islet encapsulation, which is a regenerative technology, mainly consists of packaging islet cells within a semi-permeable membrane that allows insulin, nutrients, and waste products to pass while precluding the immune response (Mirmalek-Sani et al., 2010; Opara et al., 2010; Vaithilingam & Tuch, 2011). Although this technique appears to obviate the requirement of immunosuppression therapy and preserve the islet cells, incompatibility of capsule material and hypoxia due to inadequate revascularization have been major obstacles in clinical studies. Furthermore, biomaterial carriers were utilized as a natural extracellular matrix (ECM) to overcome immune-related complications as well as a phenomenon called anoikis, which is defined as a prompt cellular apoptosis induced by the loss of cellular adhesion (Weber et al., 2008). Despite the fact that biomaterial carriers could provide anchorage and structural requirements, natural ECM appears to have extensive functions in this respect. The ECM is the most important component of the islet microenvironment influencing development, differentiation (Gittes et al., 1996; Hisaoka et al., 1993), proliferation (Hayek et al., 1995), and survival (Hammar et al., 2004; Weber et al., 2008). Since native ECM could provide the requirements for cellular growth, development, and function (Hynes, 2009), most recent studies have focused on the application of natural acellular pancreatic tissue as a bioscaffold for in vitro recellularization. De et al. (2010) were the first researchers to delineate the appropriateness of two-dimensional (2D) bioscaffolds in whole-organ engineering. Furthermore, several research groups have evaluated the capacity of in vitro techniques for whole-organ decellularization and regeneration of pancreatic tissue, including Napierala et al. (2017), who claimed to be the first to provide proof-of-concept regeneration of functional islet cells. Hereby, we demonstrate a novel islet cell repopulation technique and the successful production of acellular bioscaffolds. To the best of our knowledge, this report is the first prosperous attempt in the in vivo regeneration of neo-pancreatic tissue by a novel surgical technique, which could overcome an abundance of existing restrictions such as inadequacy of revascularization and immunological incompatibility. Additionally, transplanted tissues have been extracted to evaluate recellularization and cellular function in a 4-month follow-up period.
2 METHODS
2.1 Animals and experimental groups
Fifty-five Sprague Dawley rats (weighing 200–250 g) were selected as follows: 20 rats as donors to produce whole-pancreas scaffolds and 35 rats as the transplantation group. The transplantation group was divided into six subgroups: checking after i) 2 weeks (n = 6); ii) 1 month (n = 6); iii) 2 months (n = 6); iv) 3 months (n = 6); v) 4 months (n = 6); and vi) sham group as the negative control (n = 5, one rat for each group). The animals were anesthetized by Ketamine/Xylazine (80 mg/kg) for surgical procedures approved by the Tehran University of Medical Sciences ethical committee.
2.2 Acellularization of pancreas
The pancreas was removed and rinsed in PBS (Gibco, Life Technologies), stripped of connective tissue, and cannulated via the common bile duct (Angiocath 26G entered the common bile duct and the entrance of the common bile duct to the duodenum was ligated). The organs were fixed to the cannula using 4–0 Prolene ligatures and fitted to the perfusion system with a peristaltic pump (CESCO Bellofeeder 1300, Germany) set to 10 ml/hr. The protocol consisted of washing with PBS, 0.05% SDS (Merck, Germany) for 6 hr, 0.25% Triton X–100 (Merck) for 12 hr, and PBS/antibiotics for 2 hr at the end.
2.3 Acellularized scaffold investigations
2.3.1 Histopathology evaluation of scaffolds
For the assessment of the scaffolds before transplantation, the samples were fixed in 10% formalin. Then, the 5-μm-thick paraffin-embedded sections were cut and stained with hematoxylin & eosin (H&E), Masson's trichrome stain, reticulin, and Sirius Red to evaluate tissue acellularization and morphology. The slides were imaged using a Nikon E600 microscope (Japan). Each acellularized scaffold was evaluated by a blinded pathologist to compare the stained slides.
2.3.2 Computer tomography (CT) angiography of scaffolds
After acellularization, 2 ml of methylene blue dye (1 mg/ml) was injected to ensure the openness of the vascular network in the pancreas. In addition, CT angiography (BrightSpeed16, flow rate 2 ml/s) was used to take a closer look at the vascular network using by Iodixanol (GE Healthcare) and photos were captured using computer software.
2.3.3 Scanning electron microscopic (SEM) evaluation of scaffolds
The samples were fixed in 2.5% glutaraldehyde (Merck) for 24 hr at room temperature, rinsed in PBS, and dehydrated in ethanol gradient. Samples were dried under a laminar hood for 24 hr, gold-sputtered (physical vapor deposition, Emitec, UK, 30 nm, 180 s), and visualized with SEM (Seron technology, AIS-2100, Korea).
2.3.4 DNA content measurement of scaffolds
We used DAPI staining and fluorescence microscopy for the evaluation of nuclear remnants in the acellular scaffold. DAPI specifically stains double-stranded DNA by binding the A-T rich regions of DNA. Briefly, the samples were fixed in formalin, embedded in paraffin, and 5-μm-thick sections were prepared and fixed on slides. After washing, DAPI staining solution (1 μg/ml) was added and incubated for 10 min and rewashed. The samples were then visualized under a fluorescence microscope with a UV filter. Total DNA in the acellular scaffold and native pancreas (as control) was extracted with a genomic DNA purification kit (Thermo Scientific, Lithuania), and the DNA concentration in the extracted solution was quantified using by Nanodrop (Thermo Scientific Nanodrop 1000) and agarose gel. Total DNA was normalized to the weight in order to quantify the amount of DNA in samples. All tests were done in triplicates.
2.3.5 Estimation of scaffold stiffness
To evaluate the mechanical resistance of the tissue, tensile test was performed with a standard universal testing machine (Hounsfield, load cell: 500 N, UK). The samples were carefully attached to the grips aided by two strips of emery paper. Data were calculated by Excel software.
2.4 MTT assay of acellularized scaffolds
Cell-scaffold biocompatibility was tested to evaluate whether the acellularized scaffolds contained any toxins or not. The cytotoxic activity of acellularized pancreas on human bone marrow mesenchymal stem cells (MSCs) was determined by MTT assay. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) is a yellow tetrazolium salt, which is reduced in active cells to form purple formazan crystals by intracellular NAD(P)H-oxidoreductases in the mitochondria of living cells. The MSCs were cultured in Dulbecco's Modified Eagle's Medium (DMEM) with 10% FBS at 37°C in a CO2 incubator. The cells were added to a 96-well culture plate (1 × 104 cells/well) and incubated overnight at 37°C to be attached. After 24 hr, the acellularized scaffolds (cut into 4 × 4 mm2) were added to the plate and incubated at 37°C for 48 hr. Thereafter, the scaffolds were removed from each well and the culture medium was discarded. MTT (100 μl, 0.5 mg/ml) was added to each well and the plates were incubated for 4 hr at 37°C. Then, MTT was replaced by 100 μl of dimethyl sulfoxide to dissolve the crystals, and the optical density was measured using a plate reader (Anthos 2020, UK) at 540 nm. Each experiment was performed with eight replicates. The optical density of the treated wells was compared with that of the control, which consisted of MSCs (1 × 104 cells/well) without any scaffold and the percentage of cell survival was calculated and plotted.
2.5 Surgical methods
2.5.1 Transplantation of scaffolds into rats
Adult Sprague-Dawley rats were anesthetized with subcutaneous injection of Ketamine/Xylazine (80 mg/kg). The abdomen was shaved and a midline incision was made on it to reach the pancreas adjacent to the spleen, and stomach. The spleen was extracted with the adjacent omentum and placed outside the abdomen. The acellularized pancreas (3 × 3 cm2) was stretched over the host pancreas and the omentum was wrapped around it to make a sandwich-like structure, which was then fixed with Chromic Sutures 6–0 (Ethicon) and marked with Prolene 4–0 (Ethicon). The graft was continuously sutured with 6–0 Polyprolene (Ethicon) and the fascia and skin were closed using sutures 4–0 (Ethicon). The animals were monitored until the end of study.
2.5.2 Post-operative care of animals
Enrofloxacin was injected for 3 days and a normal diet was given to the animals during their monitoring.
2.5.3 Explant of grafted scaffolds
The animals were carefully followed up for 14, 30, 60, 90, and 120 days. The negative control (group VI) was a sham surgery without transplantation. The transplanted grafts were carefully dissected (at the end of desired time for each group), and prepared for H&E staining and immunohistochemistry. A section of the scaffold was frozen at −70°C for the evaluation of gene expression.
2.6 Evaluation of explanted scaffolds
2.6.1 RNA quantification and qPCR of explanted scaffolds
The scaffolds were removed and collected for gene expression analysis. RNA extraction was performed with RNeasy Mini Kit (Qiagen) according to the manufacturer's protocol. RNA content was measured (NanoDrop) and used in a cDNA Reverse Transcription kit (high-capacity cDNA reverse transcriptase kit; Applied Biosystems, Thermo Scientific) following the manufacturer's recommendations. qPCR analysis was performed for Ngn3 (neurogenin-3), Pdx1 (pancreatic and duodenal homeobox 1), and Ins (insulin). The primers are listed in Table 1. The samples were analyzed using a real-time PCR system (Rotoe Gene Q, Qiagen, Germany). The expression rate of each sample was normalized to GAPDH (glyceraldehyde 3-phosphate dehydrogenase) and relative expression was calculated using the ddCt method. qRT-PCR analysis was done in triplicates.
| Sequence | Product size | ||
|---|---|---|---|
| Gene | Forward | Reverse | (bp) |
| GAPDH | CTCTGGTGGACCTCATGGCCTAC | CAGCAACTGAGGGCCTCTCT | 104 |
| NGN3 | CCCTTCTGGCTTTCATTAGTC | GCGGAGTCACCAGGAAGTAT | 200 |
| PDX1 | AAACGCCACACACAAGGAGAA | AGACCTGGCGGTTCACATG | 151 |
| INS1 | TCTTCTACACACCCATGTCCC | GGTGCAGCACTGATCCAC | 149 |
2.6.2 Immunohistochemistry of explanted scaffolds
For fluorescence immunohistochemistry, the samples were blocked for 60 min in 5% goat serum at room temperature (Invitrogen, Life Technologies) in PBS with 0.3% Triton X–100 (Merck) and incubated overnight at 4°C with primary antibody of Ngn3 (Abcam, ab38548), Pdx1 (Abcam, ab47267), and Insulin (Abcam, epr17359). The sections were washed three times in PBS and species-matched secondary antibodies (Abcam) were applied for 1 hr at room temperature. Sections were washed, stained with DAPI (1 min, 25 mg/ml), washed again (three times) in PBS, and then placed on cover slips with Dako Fluorescent Mounting Medium. Images were quantified using blinded specialists.
2.7 Statistical analysis
Statistical analysis was performed by SPSS 21 (IBM SPSS Statistics, IBM Corporation). The paired t-test and independent sample t-test were used to analyze the results, which were expressed as mean ± SD. The level of significance was indicated as p-value < 0.05.
3 RESULTS
3.1 Evaluation of prepared acellularized scaffolds
3.1.1 H&E staining
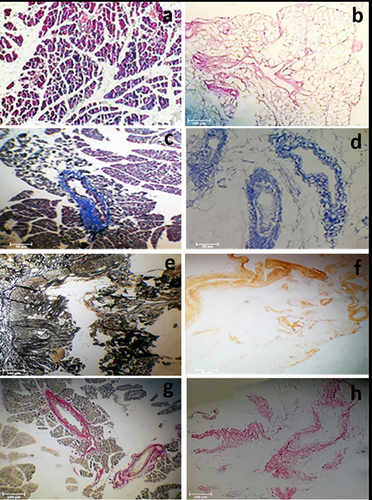
Complete cell removal in the scaffolds after the acellularization procedure was observed, and all cellular and nuclear components were completely removed (Figure 1a,b).
3.1.2 Histologic stainig
Histologic findings showed the remaining ECM in the acellularized scaffolds (Figure 1c–h).
3.1.3 SEM analysis
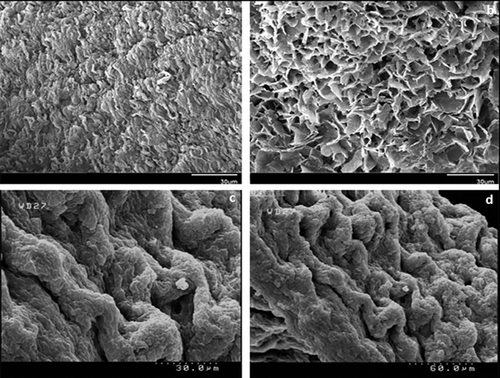
Native and acellularized pancreases showed vacant cells plus similar presence of fibers in the acellular scaffold (Figure 2a,b).
3.1.4 Hydroxyproline content
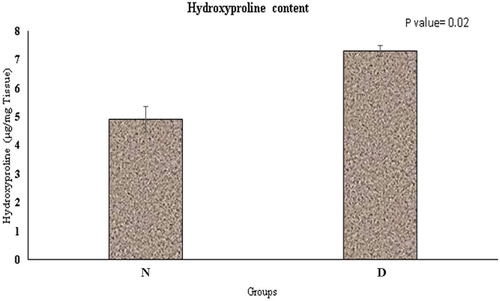
Hydroxyproline content was increased in the acellularized tissue, but did not show statistically significant difference with native tissue (p = 0.02) (Figure 3).
3.1.5 DAPI staining and DNA quantification
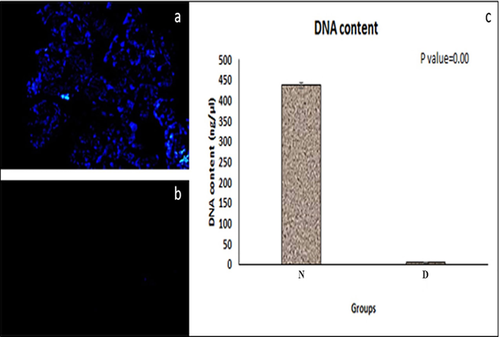
These tests were performed to evaluate the cells in both native and acellularized pancreases and the results showed that acellularized methods could remove well over 97% of the cellular components and nuclear material (Figure 4). These findings confirmed the efficacy of our scaffold for the removal of cell components, which may be responsible for adverse host reactions.
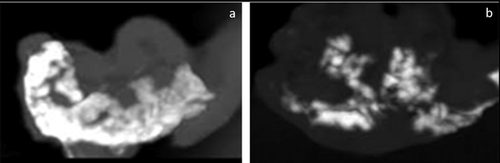
3.1.6 CT angiography
This test demonstrated that the microstructures of ductal networks have been preserved after the acellularization protocol (Figure 5).
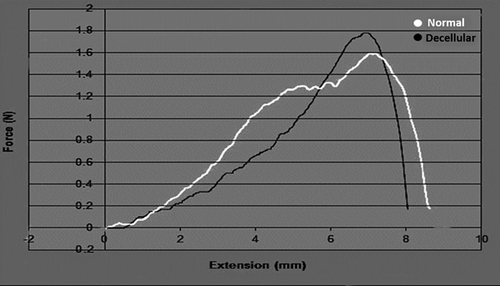
3.1.7 Tensile test
Comparing the acellularized pancreas with the native pancreas, this test indicated that the changes in the biomechanical properties of the matrices were not statistically significant and that the structure was comparable between the two. It revealed that ECM materials of the acellularized scaffolds were preserved, which is indicative of mechanical properties in the tissue (Figure 6).
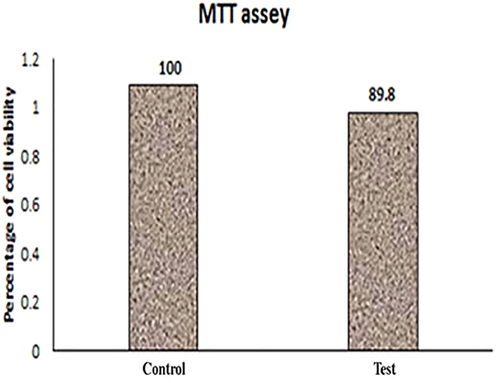
3.2 MTT assay of acellularized tissue
The cell-scaffold biocompatibility experiment showed that bone marrow MSCs were grown in DMEM in the presence of the acellularized scaffold matrix. In turn, the MSCs grew in the acellularized scaffold with a similar proliferation rate as that of the positive control without any evidence of contact inhibition. These results revealed that the acellularized matrix was not cytotoxic for cells (Figure 7).
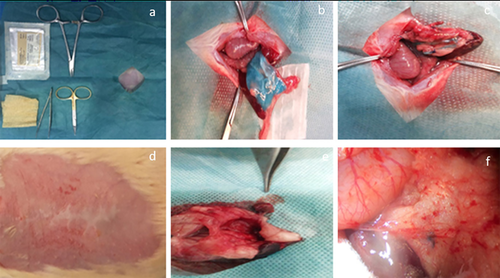
3.3 Graft area analysis
Figure 8 shows the transplantation operation. All animals under this study were subjected to surgical operation and their transplanted acellularized pancreases were removed. The animals survived up to the end of the study period and there was no intraoperative incident. All of the transplanted scaffolds existed within the abdomen between the animal's pancreas and omentum without reactive or infectious changes in the macroscopic view. To evaluate the acellularized pancreas after grafting, the following experiments were conducted.
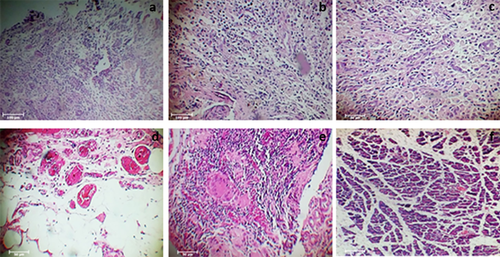
3.3.1 Pathologic findings
H&E staining confirmed the presence of recellularized pancreas in groups IV and V, 90 and 120 days after transplantation, respectively (Figure 9). The content of cell seeding was higher in the biopsy samples obtained 3–4 months after transplantation (Figure 9d,e).
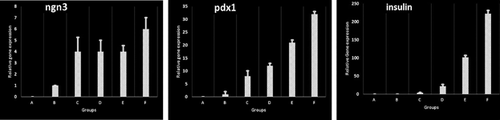
3.3.2 qPCR analysis
The results of quantitative real-time PCR showed that gene expression of Ngn3, Pdx1, and Ins increased in groups IV and V. We also found enhanced expressions of Ngn3 and Pdx1 in group III. Ins expression in group V was significantly higher than that in other groups (p-value = 0.00), although it was lower than that in the control group (p-value < 0.05). The relative gene expression of the samples is shown in Figure 10.
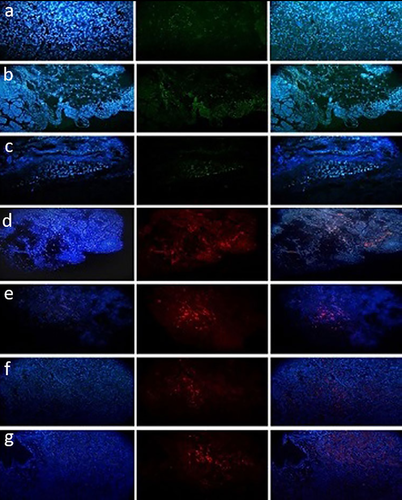
3.3.3 Immunohistochemical findings
The results showed that Ngn3 was positive in all of the biopsies except for those in groups I and II (Figure 11a–c). In addition, Pdx1 and Ins were positive in groups IV and V (Figure 11d–g). An increase in the number of insulin-positive cells was observed over time in group V (4 months). Moreover, immunohistochemical staining for CD31 (PECAM-1) showed that there was angiogenesis in the transplanted scaffold in group V (Figure 12a). In groups I and II, there were inflammatory reactions without any pancreas-positive cells. Moreover, the samples were stained with vimentin to identify mesenchymal-derived cells, which were positive in group V (Figure 12b).
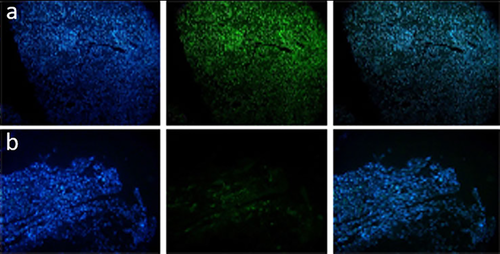
3.3.4 SEM analysis
Cells with regular fibers (like normal tissue) were detected in the transplanted tissue in group V (Figure 3c,d).
4 DISCUSSION
Tissue engineering offers an alternative way of treating insulin-dependent diabetes mellitus by overcoming the limitations of mainstream therapies like islet transplantation, which is restricted by inadequacy of revascularization and loss of cellular adhesion (Bloomgarden, 2004; Hayda et al., 2008; Jansson & Carlsson, 2002; Weber et al., 2008). An ideal scaffold for tissue regeneration consists of functional ECM components, an intact vascular network, preserved biomechanical properties, and minimum obstacles for pancreatic tissue regeneration (Salvatori et al., 2014; Vaithilingam & Tuch, 2011; Weir, 2013). Hereby, we demonstrated that acellularization of the pancreas by perfusion through the pancreatic main duct could lead to the production of a biocompatible three-dimensional (3D) scaffold with relative histological and biomechanical properties of native pancreatic ECM as well as the microstructures of vascular and ductal networks. Furthermore, transplantation of this bioscaffold over the host pancreas with its surrounding omentum showed angiogenesis, recellularization, and gene expression over a 3-month follow-up period.
Various studies have shown that in contrast to other protocols, whole-organ perfusion decellularization could result in salvation of the ECM, a 3D structure, and perfusion networks, besides generation of an acellular matrix (Soto-Gutierrez et al., 2012). The ECM is the most important component of the islet microenvironment and the discomposure of functioning ECM components, especially the basement membrane comprising collagen IV, laminin, and fibronectin, could lead to apoptosis of islet cells (Meredith et al., 1993; Raff, 1992). Preservation of the ECM in the acellularized tissue plays a substantial role in the successful application of these bioscaffolds for grafting purposes. In the current study, H&E staining and histological assessment were used to confirm the acellularization and to evaluate the conservation of ECM components. These assessments demonstrated the absence of cellular components in addition to strong similarities between the ECM in the remaining scaffold and natural tissue. Electron microscopy comparison of intact pancreas tissue with the remaining scaffold confirmed vacant cellular areas and preserved fibers in the scaffold. Hence, we concluded that our acellularization protocol caused minimum conversion of the ECM and protein content of the matrix. Residual DNA fragments in the scaffold have been demonstrated to induce incompatibility and undesirable immunological response (Brown et al., 2009; Keane et al., 2012). Therefore, DAPI staining and DNA quantification were performed to confirm the removal of cellular and nuclear components, showing that our acellularization protocol is capable of disposing more than 97% of cellular and nuclear components. Since various investigations have confirmed changes in the biomechanical properties of the scaffold after the decellularization process (Haag et al., 2012; Williams et al., 2009), the properties of the obtained scaffold were compared with those of natural pancreas tissue via strength testing. The decellularization process caused no statistically significant alteration in the biomechanical properties of the tissue, which was mainly due to the preservation of ECM content. Moreover, CT angiography was employed to evaluate vascular network competency, which has been shown to be an essential delivery vehicle of the acellularization detergent and a potential means for cell seeding of the scaffold (Mirmalek-Sani et al., 2013). Results revealed that the utilized acellularization protocol has no prominent influence on the potency of vasculature.
Although previous investigations, such as one by Goh et al. (2013), have focused on in vitro recellularization of scaffolds, to the best of our knowledge, this is the first study to perform an in vivo recellularization technique in pancreatic tissue. Various studies on in vivo regeneration of certain acellular tissues such as the trachea (Kajbafzadeh et al., 2015) have proposed more efficient recellularization techniques, which could also attenuate immunological reactions. It appears that the establishment of circulating stem cells over the implanted scaffold could result in functional tissue regeneration, pertaining to the activation of cell signaling pathways. Thus, the applied surgical technique is a particular feature of this study. We hypothesized that transplanting the scaffold over the host pancreas could induce islet cell regeneration. In addition, since revascularization of the scaffold appeared to be a challenge, we thought that enclosing the obtained scaffold within a tissue with affluent vascular networks (such as the omentum) could result in angiogenesis induction and consequent promotion of tissue regeneration and maintenance. Considering the in vivo recellularization process, a weaker immunological reaction was expected, which was mainly attributed to tissue regeneration by native cells. In fact, after implantation of the scaffolds, a mild inflammation was present on days 14 and 30 post-operation, which was resolved after 90 days. Inflammatory reactions are expected during the first two weeks of transplantation because of surgical site inflammation and stimulation of the host immune system by transplanted components (Kajbafzadeh et al., 2007). Nonetheless, it was important that no evidence of grafted scaffold rejection was observed in any of the groups.
Histological and immunohistochemical evaluation of the transplanted tissue over 3 months of follow-up revealed signs of recellularization, initiating 30 days following implantation and extending to a peak at 3–4 months from the beginning. Nevertheless, insulin-positive cells were detected in the extracted tissue within 6 months of follow-up. Likewise, qPCR analysis demonstrated the genetic expression of key pancreatic factors from the second month of follow-up. Moreover, immunohistochemical assessments confirmed the occurrence of angiogenesis in the implanted tissue. These results are supportive of the idea that a natural acellular scaffold could be advantageous for the regeneration of pancreatic components in addition to our surgical method for in vivo tissue regeneration and angiogenesis induction in the implanted tissue.
Studies have shown that stem cells (especially MSCs) are present in human blood (Hass et al., 2011; Villaron et al., 2004; Xu & Li, 2014). MSCs are a source of multipotent cells for tissue engineering and several studies have shown that MSCs can migrate and differentiate into various tissues (Hayakawa et al., 2003; Mahmud et al., 2004). Until now, various articles have been published on the generation of insulin-producing cells (IPCs) by MSCs. Jian et al. (2015) successfully generated IPCs from MSC progenitor cells. Seyedi et al. (2015) showed the differentiation of MSCs into IPCs and secretion of insulin in vitro. In turn, MSCs might be one of the possible reasons for IPC production in the scaffold. Furthermore, the 3D ECM environment could assist the morphogenesis of tissues (Lanfer et al., 2009). IPC differentiation is a multistage process, which is specified by changes in cellular morphology and gene expression. Processes such as cell-cell adhesion, adhesion signaling, and cell-matrix interactions are essential for differentiation, and a number of signals are required for the metabolism of cells for vital tissue or cell processes such as proliferation and differentiation (Matta & Mobasheri, 2014). Essentially, stem cells, the ECM, obtainable growth factors, and the physical properties of the 3D scaffold all contribute to tissue regeneration (Daley et al., 2008). Understanding the interaction between cells and the ECM could help in the design of a new approach for therapeutic applications through which organ replacement will probably be achieved. However, it is clear that the acellular scaffold is much more similar to native tissue, and proper employment of this scaffold could be a great aid to patients in need of transplantation.
Further investigations are required to determine the association of host cells in the recellularization of the implanted scaffold, in addition to excluding the participation of remaining donor islet cells in the process, and to evaluate the functional properties of the implanted tissue. Moreover, there has been no comparison with other tissue engineering methods to determine the efficacy of our technique. However, these preparative investigations might pave the way for further applications of acellular pancreatic bioscaffolds in other animals, as well as animals with a specific disease.
5 CONCLUSION
Generally, the current study was focused on bio-imitating the tissue engineering of the pancreas by employing an acellular pancreatic tissue as a non-immunogenic bioscaffold to be used as a novel surgical technique for in vivo tissue regeneration, as well as the unique utilization of the omentum as a bioreactor. Transplantation of an acellular pancreatic scaffold could result in recellularization of the scaffold by native circulating stem cells due to the activation of cell signaling pathways.
ACKNOWLEDGMENT
We are grateful to the research vicar at Tehran University of Medical Sciences for supporting of this study. We wish to thank Pediatric Urology and Regenerative Medicine Research Center, Section of Tissue Engineering and Stem Cells Therapy, Children's Hospital Medical Center for the use of their facilities.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to the publication.




