Identification of a Hematopoietic Cell Dedifferentiation-Inducing Factor
Abstract
It has long been realized that hematopoietic cells may have the capacity to trans-differentiate into non-lymphohematopoietic cells under specific conditions. However, the mechanisms and the factors for hematopoietic cell trans-differentiation remain unknown. In an in vitro culture system, we found that using a conditioned medium from proliferating fibroblasts can induce a subset of hematopoietic cells to become adherent fibroblast-like cells (FLCs). FLCs are not fibroblasts nor other mesenchymal stromal cells, based on their expression of type-1 collagen, and other stromal cell marker genes. To identify the active factors in the conditioned medium, we cultured fibroblasts in a serum-free medium and collected it for further purification. Using the fractions from filter devices of different molecular weight cut-offs, and ammonium sulfate precipitation collected from the medium, we found the active fraction is a protein. We then purified this fraction by using fast protein liquid chromatography (FPLC) and identified it by mass spectrometer as macrophage colony-stimulating factor (M-CSF). The mechanisms of M-CSF-inducing trans-differentiation of hematopoietic cells seem to involve a tyrosine kinase signalling pathway and its known receptor. The FLCs express a number of stem cell markers including SSEA-1 and -3, OCT3/4, NANOG, and SOX2. Spontaneous and induced differentiation experiments confirmed that FLCs can be further differentiated into cell types of three germ layers. These data indicate that hematopoietic cells can be induced by M-CSF to dedifferentiate to multipotent stem cells. This study also provides a simple method to generate multipotent stem cells for clinical applications. J. Cell. Physiol. 231: 1350–1363, 2016. © 2015 Wiley Periodicals, Inc.
Stem cells have provided exciting prospects for cell-based clinical applications. They are undifferentiated cells defined by their abilities to self-renew and differentiate into mature cells. Classically, stem cells can be divided into adult stem cells and embryonic stem cells (pluripotent). Compared to embryonic stem cells, adult stem cells are not pluripotent, but do have many advantages, such as convenience to obtain, diminished ethical issues, and lower tumor risk when used in vivo. Adult stem cells often reside in the different tissues of body and provide the capacity for tissue repair and regeneration during tissue turnover or injury (Bjornson et al., 1999). Normally, many different types of adult stem cells exist. For instance, mesenchymal stem cells (MSCs) are located in bone marrow and adipose tissue can give rise to multiple mesenchymal lineages (Ding et al., 2011). Hematopoietic stem cells (HSCs) in bone marrow contribute to mature cells of various blood lineages (Spangrude et al., 1988). Epithelial stem cells located attached hair follicles can generate keratinocytes (Ohyama et al., 2006). Satellite cells in skeletal muscle are myogenic stem cells responsible for the repair of skeletal muscle (Chen and Goldhamer, 2003). All suggest the importance of adult stem cells in tissue renewal and regeneration. Unfortunately, unlike embryonic stem cells, adult stem cells usually have limited potency and give rise only to the cells within the tissue of residence. In addition, adult stem cells other than HSCs are normally difficult to isolate and grow in vitro. Therefore, the search for convenient sources of adult stem cells with pluripotency or multipotency is of significant importance.
Hematopoietic stem cells (HSCs) are relatively easily accessible sources of adult stem cells. They can be obtained from bone marrow, cord blood, and peripheral blood. It had been postulated that HSCs possess the capacity to trans-differentiate into the non-hematopoietic lineages in vivo. In the past decade, researchers have found that HSCs can directly trans-differentiate into non-lymphohematopoietic cells such as muscle fibers (Gussoni et al., 1999), hepatocytes (Lagasse et al., 2000), microglia and astroglia (Eglitis and Mezey, 1997), epithelial cells of the lung, GI tract and skin (Körbling et al., 2002), and neuronal tissues (Mezey et al., 2000), under conditions of tissue injury.
Although, a significant number of studies (Castro et al., 2002; Terada et al., 2002; Choi et al., 2003; Alvarez-Dolado et al., 2003; Nygren et al., 2004) also report a failure to detect HSC contributions to non-hematopoietic tissues in similar experimental systems, evidence from experiments conducted in vitro seems to strongly support hematopoieic cell trans-differentiation. Recent studies have revealed that hematopoietic cells cultured in vitro are able to trans-differentiate into endothelial cells, osteoblasts, muscle cells, neural cells, and hepatocytes (Rogers et al., 2007; Khurana and Mukhopadhyay, 2008; Khurana et al., 2010; Sellamuthu et al., 2011). Several theories have been suggested to explain the hematopoietic cell trans-differentiation caused by tissue injury (Wagers and Weissman, 2004); among these mechanisms, the most likely is the dedifferentiation of these more mature cells into immature pluripotent or multipotent stem cells. However, no experimental evidence yet exists to support this mechanism.
In this study, we will focus on whether the hematopoietic cells can be dedifferentiated under certain conditions and what factors induce hematopoietic cell dedifferentiation.
Materials and Methods
Cell culture
Mouse dermal fibroblasts and keratinocytes were isolated from skin and cultured as previously described (Ghahary et al., 2004) using Dulbecco's modified Eagle Medium (DMEM) (Invitrogen, Burlington, ON, Canada) containing 10% fetal bovine serum (FBS) (Invitrogen) and CnT-BM.1 medium (Cell N TEC Advance Cell Systems, from Cedariane Laboratories, Burlington, ON, Canada), respectively. Cells in passage 2–8 were used for this study. Conditioned media were collected daily, centrifuged at 3000 g for 10 min and run through a 0.45 μm filter prior to use. To purify and identify a hematopoietic cell dedifferentiation factor, fibroblasts were cultured in a FibroGROTM serum-free medium (Millipore, Etobicoke, ON, Canada) with or without human serum albumin. Macrophage cell line Raw 264.7 (ATCC) was cultured in DMEM containing 10% FBS and antibiotic.
Preparation of mouse splenocytes, peripheral blood mononuclear cells (PBMCs), and bone marrow hematopoietic cells
Splenocytes were prepared as previously described (Fantini et al., 2007). Spleens and blood were collected from euthanized mice: spleens from either normal Balb/C or C57BL/6, or UBC-GFP (Ubiquitin C promoter-Green Fluorescent Protwein)—expressing transgenic mice with a background of C57BL/6 were surgically removed and minced at room temperature in DMEM. The tissue debris was removed by passing the cell suspension through a 40 μm nylon mesh cell strainer. Erythrocytes were lysed by ACK buffer (150 mM NH4Cl, 1.0 mM KCO3, 0.1 mM Na2-EDTA, pH7.2). After washing twice with phosphate buffer saline (PBS), splenocytes were used for culturing. PBMCs were isolated from blood by using Histopaque-1077 reagent (Sigma). Bone marrow was taken from C57BL/6 mice. Tibias and femurs were dissected from 8- to 12-week-old mice, the ends of the bones were cut, and marrow was flushed out with 2 ml of ice-cold DMEM containing 10% FBS. Bone marrow stromal cells and hematopoietic cells were isolated by culture in DMEM containing 10% FBS overnight. Suspension bone marrow hematopoietic cells were collected and used for this study.
Culturing hematopoietic cells in fibroblast conditioned medium (FCM)
Mouse bone marrow hematopoietic cells, splenocytes, or PBMCs were seeded on six-well plates and cultured in either DMEM containing 10% FBS, or an equal mixture of FCM, and fresh DMEM containing 10% FBS. For comparison, splenocytes were also cultured in a mixture of 50% keratinocyte conditioned medium (KCM)—with FBS to 10% added before being cultured with splenocytes—and 50% fresh DMEM containing 10% FBS. After culturing for 3 days, the old medium was removed, cells were washed twice with phosphate buffer saline (PBS), and either fresh DMEM containing 10% FBS or a mixture of media was added. Six days after culturing, adherent cells were detached by 0.25% trypsin-EDTA and cell scrapers. The number of adherent cells was counted using a hemocytometer under a microscope.
Adipose-derived mesenchymal stem cells isolation and culture
Adipose-derived mesenchymal stem cells (AD-MSCs) were isolated from mouse fat by collagenase digestion according to accepted protocol (Crowan et al., 2004) and cultured in DMEM containing 12% FBS. Cells at passage 1–3 were used for this study.
Reverse transcription-polymerase chain reaction (RT-PCR)
We isolated total RNA from cultured cells using a total RNA Purification Kit (Fermentas ThermoScientific, Walthan, MA). The complementary DNA was synthesized by SuperScript II (Invitrogen) using an Oligo (dT) primer (Invitrogen). RT-PCR was performed for 30–40 cycles using Taq DNA polymerase (New England Biolab, Ipswich, MA) or i-TaqTM DNA polymerase (iNtRON Biotechnology, Inc. Kyungki-Do, Korea). The primers used are listed in Table I.
| Gene name | Forward primers | Reverse primers | PCR product size (bp) |
|---|---|---|---|
| CD44 | AGCAGCGGCTCCACCATCGAG | TCGGATCCATGAGTCACAGTG | 184 |
| CD90 | CCAATGAGGATGAGGGCTTA | GCAGGCTCGTGTTTTAGAGG | 198 |
| CD106 | ACACTCTTACCTGTGCGCTGT | ATTTCCCGGTATCTTCAATGG | 304 |
| PDGFR-α | GCCTGGCAAAGAACAACCTC | CAGGTTGGGACCGGCTTAAT | 360 |
| BMPR-1α | TCGTCGTTGTATTACAGGAG | TTACATCCTGGGATTCAACC | 268 |
| GAPDH | GGCATTGCTCTCAATGACAA | TGTGAGGGAGATGCTCAGTG | 201 |
| Brachyury | CTTCAAGGAGCTAACTAACGAGATG | GAGCTTGTTGGTGAGTTTGACTTT | 295 |
| FLT1 | TCAAGGCTATTTAAAAACCTCACTG | TTTCCACAGTCCCTATTTTATTGAA | 403 |
| GFAP | AGAGGAGTGGTATCGGTCTAAGTTT | AGAAAGTCTGTACAGGAATGGTGAT | 395 |
| β3-tubulin | ACAATGAAGCCCTCTACGACAT | AAGTAGCTGCTGTTCTTACTCTGGA | 415 |
| AFP | ATCTGTGTTTCTGGATGAAATTTGT | CATACATGCTCATTTAACATGCTTC | 399 |
| GATA4 | CCAATCTCGATATGTTTGATGACTT | GGCGTCTTAGATTTATTCAGGTTCT | 385 |
Immunofluorescence staining
Primary and secondary antibodies used in this study were listed in Table II. Cells cultured in four-chamber slides or 24-well plates were fixed by 10% formalin for 30 min. Cells were washed three times with PBS after fixation. For intracellular protein staining, cells were treated with PBS containing 0.5% TritonX-100. Cells were then incubated with primary antibodies overnight after blocking with blocking solution containing 5% goat serum and 5% bovine serum albumin for an hour. After washing three times, each time for 5 min, with PBS or PBS-0.05% Tween-20 (PBS-T) at room temperature, cells were incubated with secondary fluorescently conjugated antibodies for another hour. After washing another three times with PBS or PBS-T at room temperature, slides were mounted or the wells were incubated with Vectashield Mounting Medium with DAPI (Vector Laboratories). Images were captured using a Zeiss Axioplan 2 fluorescence microscope and AxioVision image analysis software.
| Antibody name | Catalog number | Source | Use | Concentration |
|---|---|---|---|---|
| Type-1 Pro-collagen | SP1.D8 | Developmental studies hybridoma bank | IF | 1:10 |
| CD45 | Sc-25590 | Santa cruz biotechnologies | IF | 1:200 |
| FC | 1:500 | |||
| α-SMA | ab32575 | Abcam (epitomics) | IF | 1:500 |
| CD11b | 45–0112 | eBioscience | IF | 1:50 |
| CD11c | 11–0114 | eBioscience | IF | 1:50 |
| CD14 | 11–0141 | eBioscience | IF | 1:50 |
| M-CSF | AB-416-NA | R & D system | WB | 1:1000 |
| SSEA-1 (MC480) | 4744 | Cell signalling | IF | 1:200 |
| SSEA-3(MC-631) | MAB1434 | R & D systems | IF | 1:1000 |
| NANOG | 53–5761 | eBioscience | IF | 1:50 |
| SOX2 | 53–9811 | eBioscience | IF | 1:50 |
| OCT3/4 | 53–5841 | eBioscience | IF | 1:50 |
| CD31 | Ab9498 | Abcam | IF | 1:200 |
| Pan-cytokeratin | 41–9003 | eBioscience | IF | 1:100 |
| Nestin | 556309 | BD bioscience | IF | 1:300 |
| GFAP | M0761 | Dakocytomation | IF | 1:1000 |
| Beta 3-tubulin | E7-s | Developmental studies hybridoma bank | IF | 1:2000 |
| Insulin | A0564 | Dakocytomation | IF | 1:750 |
| Glucagon | A0565 | Dakocytomation | IF | 1:750 |
| NF200 | N4142 | Sigma | IF | 1:500 |
Flow cytometry
After dissociation, cells were washed twice with PBS and fixed with 10% formalin for 30 min at room temperature. After washing three times with PBS, non-specific bindings were blocked by incubation with PBS containing 5% goat serum and 5% BSA for 30 min at room temperature. Cells were then incubated with a fluorescence-conjugated CD45 antibody (eBioscience) for 45 min at room temperature. After washing three times with PBS, cells were analyzed by BD accuriTM C6 flow cytometry.
SDS-polyacrylamide gel electrophoresis and western blotting
Proteins were denatured and reduced before fractionation by 10% SDS-polyacrylamide gel electrophoresis (SDS–PAGE). In some cases, proteins were stained with SYPRO ruby Protein Gel Staining Kit (Invitrogen) and visualized under UV light. For western blot technique, proteins were transferred onto PVDF membrane and blocked with PBS-T (0.1% Tween 20) containing 5% skim milk for an hour before incubation with primary antibodies overnight. The membrane was then washed with PBS-T five times and then incubated for an hour with secondary antibodies conjugated to horseradish peroxidase (Bio-Rad). After washing five times with PBS-T, the antigen signal was detected by an enhanced chemiluminescence (ECL) assay kit (Santa Cruz Biotechnologies, Dallas, TX).
Protein purification
Conditioned medium from proliferating fibroblasts (FCM) cultured in FibroGROTM serum-free medium (Millipore) was collected, passed through a 0.20 μM filter, and precipitated by 70% saturated ammonium sulfate solution. The protein pellet was re-suspended in double-distilled water (ddH2O) and further passed through a 50 kDa cut-off centrifugal filter device (Millipore). Next, 0.5 ml of concentrated proteins was loaded onto a Mono Q PC 1.6/5 SMART column (GE Healthcare Lift Science) for fractionation by FPLC. Proteins were eluted using a linear gradient of 0–900 mM of NaCl added to PBS, which begins at fraction 5 and ends at fraction 26. A total of 21 fractions were collected, with a volume of 0.5 ml in each fraction. Finally, 1 μl of each individual fraction was added to 1 ml of cell culture medium for assessment of its ability to induce splenocyte trans-differentiation into FLCs.
Protein identification
A total of 200 μl aliquots of each fraction were buffer exchanged by addition of 1.8 ml of ddH2O and passage through a 10 kDa cut-off centrifugal filter device, resulting in 60 μl samples. These were reduced by the addition of 2 μl of 100 mM dithiothreitol (DTT), incubating 30 min at 60°C followed by 30 min of room temperature alkylation in the dark using 2 μl of 300 mM iodoacetamide. Samples were then incubated overnight at 37°C after the addition of 1 μl of 100 ng/μl trypsin. The resulting peptides were separated using a 75 μm × 100 mm, 1.7 μm BEH130 C18 column with a 3–40% linear acetonitrile gradient containing 0.1% formic acid over 40 min using a Water NanoAcuity LC. The elution from column was directed into the Synapt MS through a 10 μm capillary held at 3.2 kV. The accumulated data were analyzed using ProteinLynx Global Server software (PLGS) set with peptide and fragment mass accuracies of 25 ppm and 0.1 Da, respectively. Uniform carbamido methyl C variable N terminal acetyl and oxidation of methionine (ox-M) were selected as permitted modifications with a maximum protein MW of 250 kDa. Additional AutoMod and DeNovo queries were applied to the subset of proteins identified in the first pass. These search parameters were applied to the mouse species of the Uniprot database (20110727-1.0). A search with similar parameters to the first PLGS pass was also carried out using Mascot databases.
Colony-Forming unit (CFU-F) assays
The colony-forming activity of FLCs was evaluated by culturing in either DMEM containing 2% FBS and 10 ng/ml of M-CSF. About 5 × 103 FLCs at passage 1 were seeded in a 100 mm petri dish. Cells were incubated at 37°C with 5% CO2 incubator. Colonies were stained by Crystal Violet solution (Sigma, Oakville, ON, Canada) after 8 days of culture.
Bisulfite genomic sequencing
The genomic DNA of mouse splenocytes and FLCs at first passage was extracted by phenol/chloroform. Bisulfite treatment was performed by using the CpGenomeTM Turbo Bisulfite modification Kit (Millipore) according to the manufacturer's recommendations. We used PCR primers of OCT3/4 as the previously reported (Takahashi and Yamanaka, 2006). PCR was amplified for 42 cycles by using TaKaRaEpiTaqTM HS (for bisulfite-treated DNA, TaKaRa Bio Inc., Japan). For Nanog, a semi-nested polymerase chain reactino (PCR) was performed by amplified for 42 cycles and 30 cycles, respectively. Ten clones from each group were picked up randomly for DNA sequencing.
Spontaneous differentiation
To induce spontaneous differentiation, FLCs at passage 2 were cultured in DMEM containing 10 ng/ml of M-CSF without medium change for 2 weeks. After 2 weeks, cells either received immunofluorescence staining to identify cell types from three germ layers, or extracted total RNA for RT-PCR.
Fibroblast differentiation
To induce FLCs to differentiate into fibroblasts, FLCs at passage 2 were cultured in DMEM containing 10% FBS and 40 ng/ml of bFGF for 2 weeks, with the medium changed every other day. Differentiated cells were identified by immunofluorescence staining of type-1 pro-collagen (marker for fibroblasts) and α-smooth muscle actin (myofibroblasts).
Neuronal cell differentiation
To induce FLCs to differentiate into neural cells, FLCs were cultured in a medium containing DMEM containing 2% FBS, 3 ng/ml of M-CSF, 0.5 μM retinoic acid, and 50 μg/ml of kynurenine for 10 days. Non-induced (control) cells were cultured in DMEM containing 2% FBS and 3 ng/ml of M-CSF. This medium was changed every 2 days. Neuronal cells were characterized by morphology and by immunofluorescence staining with antibodies specific for neuronal cells.
Insulin-producing cell differentiation
To induce FLCs to differentiate into insulin-producing pancreatic β-cells, cells were cultured in a medium containing 10 mM of nicotinamide, 10 ng/ml of activin A, B27, N2 supplement, and 3 ng/ml of M-CSF for 6–10 days. Insulin-producing β-cells and glucagon-producing α-cells were characterized by double immunofluorescence staining with insulin and glucagon antibodies.
Statistics
All data are expressed as means ± SD. Differences between treatments were determined using a Student's t-test (two-tailed). A P value <0.05 was considered statistically significant.
Results
A conditioned medium of proliferating fibroblasts induces hematopoietic cell trans-differentiation into fibroblast-like cells (FLCs)
Here, we propose that tissue injury may stimulate the production and release of unknown factors from injured tissues, and these factors may cause infiltrated hematopoietic cells to be trans-differentiated. As fibroblasts are always involved in the wound healing process, they may contribute to hematopoietic stem cell trans-differentiation. Therefore, in this study, we felt it reasonable to collect conditioned medium from fibroblasts to test whether it can induce hematopoietic cell or hematopoietic stem cell trans-differentiation. The conditioned media, we collected were from mouse primary dermal fibroblasts at passage 2–8. As shown in Figure S1, almost all cells are expressed type 1 pro-collagen, suggesting these cells are fibroblasts. In some experiments, we collected conditioned media from NIH 3T3 cell line with similar efficacy. Hematopoietic stem cells and hematopoietic cells were obtained from mouse bone marrow non-adherent cells and more pure hematopoietic cells were obtained from splenocytes or PBMCs. As shown in Figure 1A, after 6 days of culture, adherence cells with spindle-shaped fibroblast-like cells are observed in either bone marrow suspension cells or splenocytes or PBMCs cultured in a medium containing 50% fibroblast-conditioned medium (FCM) and 50% fresh medium. These were not observed in the control normal medium (100% fresh medium), suggesting the factors in FCM may induce hematopoietic cell trans-differentiation.
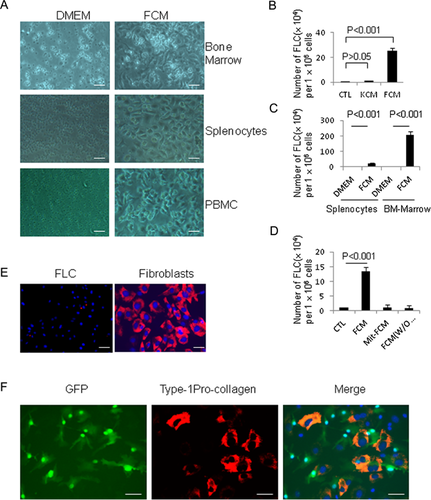
To test specificity for FCM-inducing cell trans-differentiation, keratinocyte conditioned medium (KCM) was also collected, and used to culture mouse splenocytes. These results revealed that only FCM, and not KCM, can induce hematopoietic cells trans-differentiation (Fig. 1B). Furthermore, there is higher number of FLCs in FCM culturing BM-suspension cells compared to that in FCM culturing splennocytes (Fig. 1C). As fibroblasts always become activated and undergo proliferation during the healing process (Martin and Leibovich, 2005), we next examined whether the expression and release of these hematopoietic cell trans-differentiation stimulating factors are related to the status of fibroblast proliferation. We cultured splenocytes in a medium containing 50% fresh medium plus 50% conditioned medium collected from either proliferating fibroblasts (fibroblasts grown in DMEM with 10% FBS) or quiescent fibroblasts (either treated by mitomycin-C to stop cell proliferation, or cultured in the absence of fetal bovine serum). As shown in Figure 1D, the results showed that there was no trans-differentiation of splenocytes into FLCs, in response to culture media obtained from non-proliferating fibroblasts.
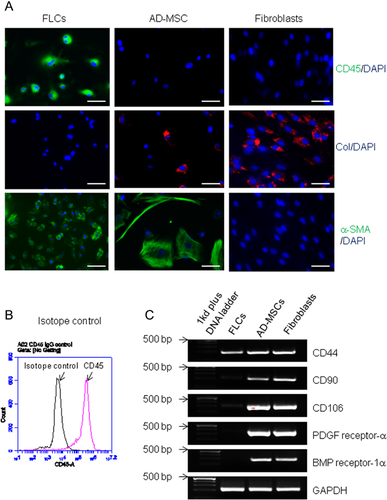
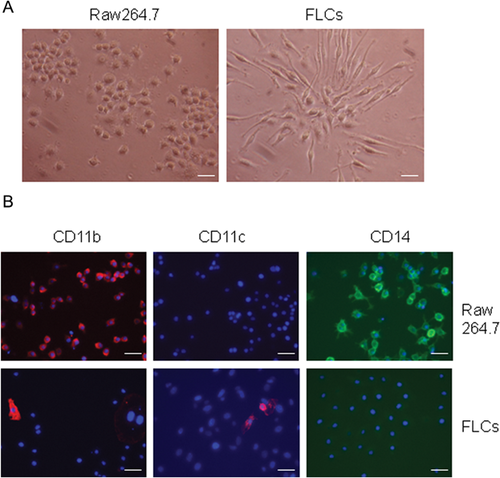
To exclude the possibility that FLCs are tissue fibroblasts from the blood system or contaminated from FCM (although FCM was passed a 0.45 μm filter prior to use), we compared the expression of Type 1 pro-collagen expression in splenocyte-derived FLCs and mouse dermal fibroblasts. As shown in Figure 1E, unlike fibroblasts, FLCs are negative for pro-collagen staining. We further isolated splenocytes from UBC-GFP transgenic mice and cultured in FCM. GFP (Green Fluorescent Protein)–labeled FLCs at passage 2 were then co-cultured with skin fibroblasts. After 2 days, cells were stained with type-1 pro-collagen (a marker for fibroblasts) monoclonal antibody. In contrast with pro-collagen expressed fibroblast (red), we demonstrated that GFP-expressed FLCs (green) are negative for type-1 pro-collagen staining (Fig. 1F), suggesting FLCs are not fibroblasts. Furthermore, to exclude the possibility that FLCs are tissue-derived mesenchymal stem cells, we compared the expression of CD45 (a hematopoietic cell marker), type-1 procollagen (a fibroblast marker) and α-smooth actin (a marker for stromal and stem cells) in FLCs, adipose-derived mesenchymal stem cells (AD-MSCs), and dermal fibroblasts. We found that FLCs are distinguished from AD-MSCs and fibroblasts by the expression of CD45 and α-smooth muscle actin (Fig. 2A and B). RT-PCR also demonstrated FLCs are lower in, or lack entirely, the expression of several mesenchymal stem cell genes such as CD90, CD106, PDGF receptor-α, and BMP receptor-1α, when compared to AD-MSC and fibroblasts (Fig. 2C). To exclude the possibility that FLCs are in fact macrophages or monocytes, we compared FLCs with macrophage cell line Raw264.7 cells in cell morphology and the expression of CD11b, CD11c, and CD14. As shown in Figure 3A, FLCs have different cell morphology than Raw264.7 cells. Raw264.7 cells are positive for CD11b and CD14 while FLCs are CD14-negative and a few cells express CD11c (Fig. 3B), suggesting FLCs are not macrophages or monocytes. Furthermore, splenocytes were divided into adherent cells and suspension cells through 24 h-cultured in a plastic plate (Delirezh et al., 2013). When we cultured these cells with 50% FCM media, we found that most of FLCs with a different morphology with monocytes (adherent cells cultured in DMEM) are grown from suspension cells (Supporting Information Fig. S2A). Differs from cells cultured from adherent-splenocytes in which most cells are CD11b positive and some are CD11c positive, FLCs cultured from suspension cells are CD11b, CD11c negative (Fig. S2B), suggesting FLCs are not monocytes, macrophages, or dendritic cells. Together, these findings clearly indicate that FLCs are a new type of cells derived from hematopoietic cells and induced by FCM.
Fibroblast releasable M-CSF is responsible for the trans-differentiation of hematopoietic cells into FLCs
Knowing the source of this mediating factor sets the stage for conducting a series of experiments to identify this factor from proliferating fibroblast conditioned medium. To achieve this, conditioned medium from fibroblasts cultured in a serum-free FibroGROTM medium was collected and the protein contents were fractionated by using centrifugal filter devices with different cut-off sizes. The active factor was characterized as a protein with a molecular weight of more than 50 kDa, under native and non-reducing conditions (Fig. 4A). This active fraction was perceptible in 50–70% saturated ammonium sulphate (Fig. 4B). For further purification, concentrated conditioned medium (4000-fold concentrated medium through ammonium sulphate precipitation and a 50-kDa cut-off centrifugal filter device) was run through an anion exchange Mono Q column in Fast Protein Liquid Chromatography (FPLC). Twenty-one fractions were collected and evaluated for efficacy of inducing hematopoietic cell trans-differentiation into FLCs. As shown in Figure 4C, two peaks of activity appeared, in fraction 8–11 and 19–21. We selected fractions 19–21 for further protein identification, with the rationale that much less total protein was present in these fractions than in the other peak group (Fig. 4D). Fraction 17, which showed no activity (Fig. 4C), was used as a negative control. In-solution trypsin digestions were carried out for each fraction and protein identification was determined using the data from liquid chromatography and mass spectrometry (MS). Evaluation of the resulting peptides identified a key protein: murine M-CSF (also called CSF-1), which is present in fractions 19–21, and absent in fraction 17 (Fig. S3). We also detected several other proteins including γ-globulin, gelsolin, and thrombospondin-1 in fractions 19–21 and fraction 17. However, hematopoietic cells treated by either recombinant gelsolin or thrombospondin-1 cannot become FLCs (data not shown).
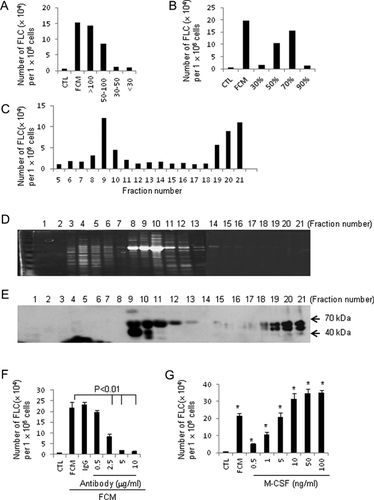
To confirm the MS results, all 21 fractions were subjected to a western blot analysis. Consistent with our original finding, fractions 19–21 (but not fraction 17) revealed double bands (Fig. 4E). The detection of double bands for M-CSF by western blot matches the previous finding indicating that two forms of proteoglycan-M-CSF exist in monocyte-secreted M-CSF (Chang et al., 1998). Importantly, the positive detection of M-CSF in the western blotting for individual fraction matched almost completely with the activity profile (Fig. 4C), also revealing two phases (fractions 9–11 and 19–21).
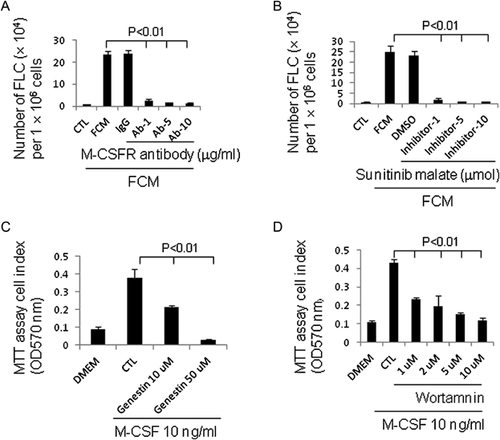
M-CSF is produced by a variety of stromal and tubular epithelial cell types, and its expression is restrictively controlled by cell proliferation state (Harrington et al., 1997). To validate the role of M-CSF in induction of hematopoietic cell trans-differentiation, a neutralizing antibody for M-CSF, and a recombinant M-CSF protein were used. The results revealed that the M-CSF-neutralizing antibody significantly attenuated the effect of FCM on splenocyte trans-differentiation (Fig. 4F), while addition of a recombinant M-CSF markedly induced splenocyte trans-differentiation into FLCs at a concentration as low as 500 pg/ml (Fig. 4G). Together, these observations clearly indicate that FCM-stimulated trans-differentiation of hematopoietic cells into FLCs is mediated by M-CSF.
M-CSF receptor tyrosine kinase signalling pathway is involved in the effect of M-CSF-inducing hematopoietic cell trans-differentiation
M-CSF receptor, a tyrosine kinase receptor encoded by the c-fms proto-oncogene, mediates all known biological effects of M-CSF. To examine whether the effect of M-CSF-inducing hematopoietic cell trans-differentiation is also mediated by this receptor, we treated cells with a functional neutralizing antibody against the M-CSF receptor in the presence of M-CSF. As shown in Figure 5A, the addition of M-CSF receptor antibody completely abrogated the effect of FCM-mediating splenocyte trans-differentiation. We next examined the effect of the receptor signalling pathway on M-CSF-inducing hematopoietic cell trans-differentiation. It has long been known that at least seven tyrosines in the cytoplasmic domain of M-CSF receptor are autophosphorylated upon interaction with M-CSF (Bourette and Rohrschneider, 2000). Sunitinib malate, which targets M-CSF receptor and other receptor tyrosine kinases (Faivre et al., 2007), was used to test whether it can block M-CSF-mediated hematopoietic cell trans-differentiation. The result revealed that the inhibition of receptor tyrosine kinase by sunitinib malate completely blocked the effect of M-CSF-mediated hematopoietic cell trans-differentiation (Fig. 5B). This result was further confirmed by similar results obtained using other tyrosine kinase inhibitors, genistein (Fig. 5C), and Wortmannin (Fig. 5D). These findings demonstrate that M-CSF-mediated trans-differentiation of hematopoietic cells is regulated by its receptor through a receptor tyrosine kinase (RTK) signalling pathway.
Characterization of hematopoietic cell-derived as multipotent stem cells
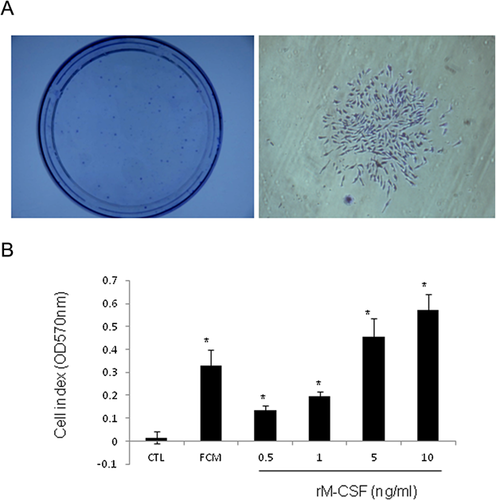
To test whether FLCs are able to self-renew, we performed fibroblastic-colony-forming unit (CFU-F) assay. FLCs at passage 1 were seeded at a density of 5000 cells per 10 ml in a 100 mm petri dish and cultured in a medium containing M-CSF. As shown in Figure 6A, many clones were observed after 8 days of culture. This result indicates FLCs have a similar capacity to stem cells. Further, although FLCs cannot grow in a DMEM medium containing 10% FBS without M-CSF, FLCs at second passage are able to proliferate in a medium containing M-CSF in a dose-dependent manner (Fig. 6B). In fact, cells can be passaged more than 10 times in this condition (data not shown).
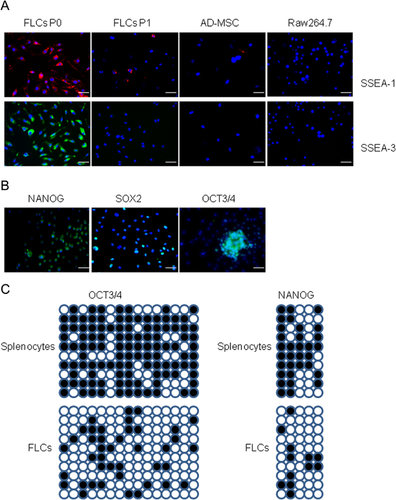
We have demonstrated FLCs are different from mesenchymal stem cells, based on the expression of several mesenchymal stem cell markers (Fig. 2). In addition, previous research has determined FLCs are not likely either multilineage-differentiating stress-enduring (Muse) cells in which CD45 are negative (Kuroda et al., 2010) nor very small embryonic-like (VSEL) cells—which are small in size and are exist in mesenchymal tissues (Kucia et al., 2006). In consideration of previous data showing that hematopoietic stem cells can be trans-differentiated into multiple cell types in vivo, under conditions of tissue injury, we therefore examined whether hematopoietic cell-derived FLCs are a new type of multipotent stem cells, by evaluating the expression of several multipotent stem cell markers such as surface marker stage specific embryonic antigens (SSEA) and transcription factors OCT3/4, NANOG, and SOX2. As shown in Figure 7A, both SSEA-1 and -3 were detected in FLCs at the first passage but not in its progeny cells. FLCs also express transcription factors OCT3/4, NONAG, and SOX2 (Fig. 7B). Evaluating the demethylation of CpG islands in splenocytes and FLCs, we found extensive demethylation at the OCT3/4 and NANOG in the promoter/enhancer region in FLCs (Fig. 7C). All these results indicate FLCs are likely multipotent stem cells.
To further assess the multipotential differentiation capacity of FLCs, spontaneous differentiation was performed: immunofluorescence staining was used to identify the cell types of three germ layers, and RT-PCR was used to detect marker genes of different germ layers. Although, all the marker genes are negative except α-smooth muscle actin in FLCs (data not shown), FLCs are spontaneously differentiated into: (i) Mesodermic cell types as indicated by expressing type-1 pro-collagen (Fibroblasts), α-smooth muscle actin (cell size suggests these cells are likely myofibroblasts), CD31 (endothelial cells) (Fig. 8A, top panels). (ii) Ectodermic cell types as indicated by expressing pan-cytokeratin (epithelial cells), nestin, and GFAP (neural cells and neural precursors (Fig. 8A, middle panels). (iii) Endodermic cell types as indicated by expressing insulin-(pancreatic β cells), glucagon (pancreatic α cells and pancreatic progenitor cells) (Fig. 8A, bottom panels). RT-PCR results revealed that spontaneously-differentiated FLCs express specific gene markers for all three germ layers. These included mesodermal genes (brachyury and FLT1), ectodermic genes (GFAP and β3-tubulin), and endodermic genes (AFP and GATA4) (Fig. 8B).
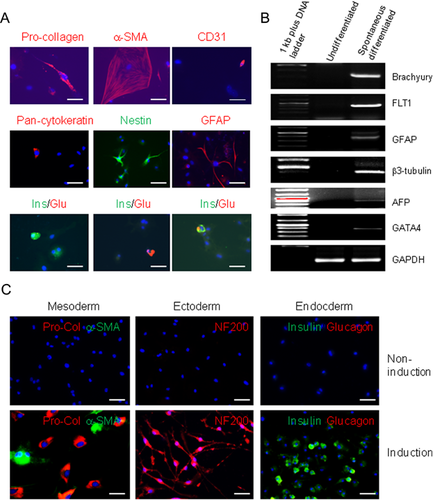
Furthermore, we examined the multipotency of FLCs by inducing differentiation. FLCs showed a capacity to be differentiated into pro-collagen positive fibroblasts (Fig. 8C, left panel), adipocytes (Fig. S4A), osteocytes (Fig. S4B), and blood vessel endothelial cells as indicated by the detection of CD31 positive cells and the formation of blood vessel-like structures (Fig. S4C). FLCs can be also induced to differentiate into neuronal cells as suggested by the neuronal cell-like morphology and the expression of neurofilament 200 (NF200), β3-tubulin, NeuN, transient axonal glycoprotein-1 (TAG-1), and neural growth factor (NGF) receptor P75 positive nerve cells (Fig. 8C, middle panel; Fig. S5). RT-PCR further demonstrated the expression of neuronal cell marker genes (Fig. S6). Culturing cells in a keratinocyte serum-free medium, some FLCs can be differentiated into pan-cytokeratin positive epithelial cells (Fig. S7). FLCs from splenocytes of mouse and rat can be induced to differentiate into insulin-producing pancreatic β-cells (Fig. 8C, right panel; Fig. S8). Together, these data demonstrated that hematopoietic cell-derived FLCs are multipotent stem cells.
Discussion
Although it is still highly controversial, hematopoietic stem cells have been indicated to be trans-differentiated into multiple organ specific cells in vivo by previous studies (Eglitis and Mezey, 1997; Gussoni et al., 1999; Lagasse et al., 2000; Mezey et al., 2000; Körbling et al., 2002). Cell trans-differentiation may be explained by multiple mechanisms (Wagers and Weissman, 2004). One of the mechanisms could be accompanied cell dedifferentiation through reprogramming of the nucleus (Wagers and Weissman, 2004). In this study, we have demonstrated a subpopulation of hematopoietic cells could be dedifferentiated into multipotent stem cells though a proliferating fibroblast releasable factor, M-CSF. M-CSF induces the demethylation of transcription factors in hematopoietic cells. These reprogrammed hematopoietic cells then express multipotent stem cell markers such as SSEA-1, SSEA-3, OCT3/4, NANOG, SOX2, and have the capacity to further differentiate into the cell types of three germ layers.
Hematopoietic cell-derived multipotent stem cells are obviously different from induced pluripotent stem cells (iPSCs) (Takahashi and Yamanaka, 2006). Unlike iPSCs, which have complete DNA demethylation, we found DNA demethylation for OCT3/4 and NANOG genes in hematopoietic cell-derived stem cells are not complete. Further, these stem cells cannot generate teratoma: no teratoma formation was found after 6 weeks in six syngeneic mice which received splenocyte-derived FLCs within the kidney capsules or subcutaneous tissues (data not shown).
These hematopoietic cells-derived multipotent stem cells are also distinct from previously well-characterized endogenous stem cells such as hematopoietic stem cells (Ratajczak, 2008), mesenchymal stem cells (Dominici et al., 2006), endothelial progenitor cells (Asahara et al., 1997), very small embryonic-like cells (Kucia et al., 2006), and multi-lineage differentiating stress enduring cells—Muse cells (Kuroda et al., 2010). It is unlikely that hematopoietic cell-derived multipotent stem cells are hematopoietic stem cells or endothelial progenitor cells: hematopoietic stem cells or endothelial progenitor cells have very limited differentiation capacity, while hematopoietic cell-derived stem cells can be differentiated into cell types of three germ layers. Further, there is no indication that hematopoietic stem cells or endothelial progenitor cells can express SSEAs. Hematopoietic cell-derived multipotent stem cells are also distinct from mesenchymal stromal cells because of their hematopoietic origin (CD45 positive) and also differences in expression of CD106, CD90, PDGF receptor-α and other mesenchymal cell markers.
On the other hand, many common features were found between hematopoietic cell-derived multipotent stem cells and very small embryonic-like cells or Muse cells. They all express stem cell marker SSEAs and transcription factors (OCT3/4, NANOG, SOX2). They have multipotential differentiation capacity. No teratoma formation is induced in vivo when these cells are grafted. However, hematopoietic cell-derived multipotent stem cells can still be distinguished from very small embryonic-like cells and Muse cells. The very small embryonic-like cells are CD45 negative (Kucia et al., 2006) while hematopoietic cell-derived multipotent stem cells are CD45 positive. Muse cells express not only multipotent stem cell markers but also mesenchymal cell markers such as CD105, CD90, and CD29 (Kuroda et al., 2010). However, we found that hematopoietic cell-derived multipotent stem cells express nothing or very low levels of mesenchymal cell markers. Furthermore, hematopoietic cell-derived multipotent stem cells originate from hematopoietic cells, which are present in peripheral blood. Muse cells are only found in mesenchymal tissues such as bone marrow mesenchymal stem cell, adipose tissue, and dermis (Kuroda et al., 2010). All differences noted between hematopoietic cell-derived multipotent stem cells and those previously identified stem cells indicate that hematopoietic cell-derived multipotent stem cells are likely to be a new type of stem cell.
Through a set of protein purification procedures and MS peptide sequencing, we have identified a single factor, M-CSF, from the conditioned medium of proliferating, but not quiescent fibroblasts, as the mediator of dedifferentiation of a subpopulation of hematopoietic cells into multipotent stem cells. M-CSF is produced by a variety of stromal and tubular epithelial cell types, and its expression is restrictively controlled by cell proliferation state (Harrington et al., 1997). Indeed, we found that FCM collected from quiescent fibroblasts cannot effectively induce hematopoietic cells to dedifferentiation in vitro. We further demonstrated that a known M-CSF receptor and receptor tyrosine signal are involved in M-CSF-inducing hematopoietic cell dedifferentiation.
M-CSF is a multifunctional proinflammatory cytokine involved in differentiation, proliferation, and survival of monocyte progenitor cells (Hume and MacDonald, 2012). Natural mutations of M-CSF locus in mouse (op/op) and rat (tl/tl) have been reported as resulting in a reduction of macrophage and osteoclast density in tissues, toothlessness, severe growth retardation, and low fertility (Van Wesenbeeck et al., 2002; Pollard, 2009). These deficiencies can be partially restored by injection of recombinant human M-CSF (Kodama et al., 1991). Although M-CSF is well known in mediating monocyte progenitor survival and proliferation, hematopoietic cell-derived multipotent stem cells are unlikely to be of monocyte lineage such as macrophages. In addition to the difference in cell morphology, hematopoietic cell-derived multipotent stem cells have very low number cells expressing CD11b and CD11c (monocyte and dendritic cell markers). As we used the whole population of hematopoietic cells from splenocytes or BM-suspension cells for our experiments, there may be contaminated monocytes and dendritic cells. Indeed, we further culture splenocyte-suspension cells after removing monocytes and dendritic cells, we found that almost all FLCs are negative for CD11b and CD11c, suggesting FLCs are not from monocyte lineage. The concentrations of M-CSF (as low as 500 pg/ml) needed to induce growth of stem cells and dedifferentiation of hematopoietic cells into stem cells are significantly less than those concentrations (10–100 ng/ml) previously reported to stimulate monocyte and macrophage proliferation (Asakura et al., 1999; Otero et al., 2009). Furthermore, hematopoietic cell-derived stem cells express stem cell markers and have the capacity to be differentiated into cell types of three germ layers. However, no evidence shows monocytes or macrophages can be further differentiated into different lineages across the boundary.
We have observed that a very small population of hematopoietic cells can be seen within 2–3 days when blood cells are cultured in a medium containing M-CSF (data not shown). After cells become adherent fibroblast-like cells, stem cells rapidly proliferate, and can be passed for more than 10 times in the presence of M-CSF. Although, hematopoietic cells obtained from bone marrow, peripheral blood, or spleens are heterogeneous populations and we cannot exclude the possibility that a small subset of multipotent stem cells may in fact exist within the hematopoietic cell population, our preliminary data do indicate the cellular origin of these stem cells are CD4+CD25+ regulatory T cells (our submitted article). Further study is needed to clarify its mechanism of regulatory T cell dedifferentiation into multipotent stem cells induced by M-CSF.
Taken together, our findings indicate that hematopoietic cells can be induced to dedifferentiate into multipotent stem cells induced by M-CSF. Our data strongly support previous observations that hematopoietic cells can be trans-differentiated into non-hematopoietic cells. This study also provides a new method to generate multipotent stem cells, which may ultimately lead to potential applications in regenerative medicine and tissue engineering.
Acknowledgments
This work was supported by Canadian Institute of Health Research (CIHR) research grants (A.G.), and partially supported by BC Spectra Energy, the International Brotherhood of Electrical Workers and Rick Hansen Foundation. We thank Mr. H. Tearlee, Mr. D. Trendall, Dr. C. Zaph, Dr. F. Antignano, and Dr. J. Liu for their kind help in mouse spleen sample preparation and collection of blood samples. Dr. W. Tetzlaff at ICORD kindly provided us with antibodies for neural cell identification.




