miR-605-5p promotes invasion and proliferation by targeting TNFAIP3 in non–small-cell lung cancer
Abstract
Lung cancer is an significant cause of death worldwide, and non–small-cell lung cancer (NSCLC) is the most common type of lung cancer. MicroRNAs (miRNAs) have been identified to play key roles in NSCLC development. Recently, it has been reported that miR-605-5p is a cancer-related miRNA in several types of tumors. In this study, we study the role of miR-605-5p in NSCLC cells. We find that miR-605-5p is upregulated in NSCLC cells. Overexpression of miR-605-5p significantly promotes lung cancer invasion and migration in H460 and H1299 cells. Besides this, miR-605-5p also promotes lung cancer cell carcinoma proliferation and metastasis in vivo. However, downregulation of miR-605-5p inhibits cell invasion and migration by inhibiting lung cancer cell carcinoma proliferation and metastasis. In addition, the luciferase report assay identifies 3′-untranslated region tumor necrosis factor α-induced protein 3 (TNFAIP3) as a target of miR-605-5p. Silencing of TNFAIP3 promotes invasion and proliferation in lung cancer. In addition, the knockdown of TNFAIP3 restores the significant decrease in invasion and proliferation in miR-605-5p-inhibitor–transfected lung cancer cells. In conclusion, miR-605-5p promotes invasion and proliferation by targeting TNFAIP3 in NSCLC, and may provide possible biomarkers for NSCLC therapy.
1 INTRODUCTION
In recent years, the incidence and mortality of lung cancer have increased significantly, and it has become a malignant tumor with the highest mortality in the world and China.1 Among all primary lung cancer cases, non–small-cell lung cancer (NSCLC) accounts for more than 80%.2 In addition to surgery, radiotherapy, chemotherapy, and molecular targeted therapy are also some of the most important treatment methods for lung cancer. Thus, it is important to study the molecular mechanisms of the progression of NSCLC, which may help improve the treatment effectiveness. Accumulating evidence has demonstrated that a large amount of exert critical effects on the progression and development of NSCLC.3-5
MicroRNAs (miRNAs) are single-stranded RNAs 19 to 25 nucleotides in length, which can complementarily bind to the 3′-untranslated region (3′-UTR) of the targeted gene messenger RNA (mRNA) sequence and regulate the target gene expression level at the posttranscriptional level.6 Accumulating evidence has shown the important role of miRNAs in cancer, including NSCLC, and suggests miRNAs to be biomarkers in NSCLC treatment. It was reported that the miR-605-5p expression level is altered in the exosomes derived from colony-stimulating factor (CSF) of young-oneset Alzheimer's disease (YOAD) patients and in a cohort of late-onset Alzheimer's disease (LOAD) patients.7 Besides this, miR-605-5p has been found to be specific to lung adenocarcinoma.8 miR-605-5p may enhance transactivation of p53 by inhibiting MDM2 in lung and breast cancer cell lines.9 Because miR-605-5p has not been studied completely in NSCLC, the aim of this study is to elucidate the underlying mechanism of miR-605-5p in NSCLC.
2 MATERIALS AND METHODS
2.1 Cell culture
NSCLC (H460 and H1299) and HEK-293T cells were obtained from the ATCC. Dulbecco's modified Eagle's medium (Sigma) was added with 1% penicillin (Invitrogen) and 10% fetal bovine serum (FBS) (Thermo Fisher Scientific). Cell culture was done in a 5% CO2 incubator at 37°C.
2.2 Reverse-transcription polymerase chain reaction
Total RNA came from cultured cells from RNAiso Plus and cDNA came from reverse transcription of RNA by using the Prime Script TM RT Master Mix. Quantitative polymerase chain reaction (qPCR) was completed using a SYBR Premix Ex Taq II (TaKaRaBio). The 7500 Fast RT-PCR System (ABI) was also used. Primers were purchased from Nanjing Realgene company (Nanjing Realgene, Nanjing, China).
2.3 Western blot analysis
One hundred to one-hundred and fifty micrograms of protein of each group was added on sodium dodecyl sulfate polyacrylamide gels. Then they were transferred onto polyvinylidene difluoride membranes. After having been blocked for 1 hour, the membranes were probed with specific primary antibodies. The antibodies were as follows: anti-tumor necrosis factor α-induced protein 3 (anti-TNFAIP3) and anti-actin (1:1000; Abcam, Cambridge, UK).
2.4 Migration and invasion assays
We used the transwell assay to evaluate cell invasion and migration. H460 and H1299 cells were transfected with miR-605-5p mimic or anti-miR-605-5p. To study cell migration, 1 × 105 transfected cells were maintained in the upper chamber within 200 μL serum-free media with coated-matrigel (BD Biosciences). Besides this, the upper chamber was loaded with 100 μL of 2% matrigel for the invasion assay, and the lower chamber was filled with 600 μL Roswell Park Memorial Institute (RPMI) 1640. After 24-hour incubation, a cotton swab was used to gently remove the nonmigrated and noninvasive cells, which were on the upper space of the filter. Then, all the cells transferred to the membrane with 0.1% crystal violet solution were fixed and stained. Finally, cell numbers were estimated in eight random fields of views with an inverted microscope.
2.5 Cell proliferation assay
We used a 5-ethynyl-2′-deoxyuridine (EdU) Apollo567 In Vitro Kit (RiboBio, China) and a cell counting kit-8 (CCK8) (Biyuntian, China) to identify the proliferation of NSCLC cells. For EdU assay, 2 × 105 cells were used for each culture dish and experimental steps were performed according to the protocol. Finally, we studied the samples using laser confocal scanning microscopy. For CCK8 assay, 2 × 103 cells were seeded in 96-well plates (Corning) for 12, 24, and 36 hours. Then, each group was added with 10 μL CCK-8 reagent and incubated for another 1 to 4 hours at 37°C. The results were measured at 450 nm.
2.6 Soft agar colony assay
The experiment was performed in six-well plates with 2 mL RPMI 1640 medium containing 5% FBS and 0.6% Bacto agar (Amresco) for each well. About 2 or 4 × 103 H460 or H1299 cells were seeded in 2 mL medium including 5% FBS with 0.35% agar. Then, they were layered onto the base. About 3 weeks later, we could take a picture of the colonies.
2.7 Luciferase reporter assays
The wild-type (wt) or mutant (mut) miR-605-5p binding site in the 3′-UTR of TNFAIP3 was subcloned into luciferase reporter plasmid pmirGLO. The 293T cells were transfected with miR-605-5p (or the miR-control vector) with TNFAIP3 wt 3′-UTR/mut (or the control luciferase reporter plasmid pmirGLO). Luciferase activity was assayed 48 hours later.
2.8 H1299 xenograft mouse model
BALB/c nude mice were bought from the Shanghai Institute of Materia Medica (Shanghai, China). These mice were subcutaneously injected with 100 μL medium containing H1299 cells, which overexpressed miR-605-5p or negative control stably. The subcutaneous tumor tissues were removed 1 week later and then they were implanted into the livers of mice. After 35 days, the mice were killed. We used the following formula to measure volume and size: tumor volume (mm3) = 1/2(length × width2). Formaldehyde solution was used to fix the lungs of mice, and then they were embedded with paraffin. The fixed tissues were serial sectioned, and then they were stained with hematoxylin-eosin to study metastatic lesions. Animal procedures were carried out in accordance with methods approved by the Medical Experimental Animal Care Commission of Tongji Medical College, Huazhong University of Science and Technology.
3 RESULTS
3.1 miR-605-5p is upregulated in NSCLC cells and promotes lung cancer invasion and migration
We first identified the expression of miR-605-5p in five human lung cancer cells lines, including BEAS-2B, H460, H1299, H1650, and PC9 (Figure 1A). We found that the expression of miR-605-5p was significantly upregulated in NSCLC compared to others. To elucidate the biological function of miR-605-5p in NSCLC, H460 and H1299 cells were transfected with miR-605-5p mimic or miR-NC and inhibitor-miR-605-5p mimic or inhibitor-miR-NC for 48 hours. The efficiency of mimic-miR-605-5p and inhibitor-miR-605-5p was confirmed by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) (Figure 1 B-E). We used transwell assay to determine the role of miR-605-5p in NSCLC metastasis. According to the results, miR-605-5p mimic significantly promoted the migration of H460 and H1299 cells, while miR-605-5p inhibitor inhibited H460 and H1299 cell migration (Figure 1F). The effect of miR-605-5p on the invasive capacity of H460 and H1299 cells was similar to the above (Figure 1G). Therefore, miR-605-5p overexpression promotes lung cancer invasion and migration.
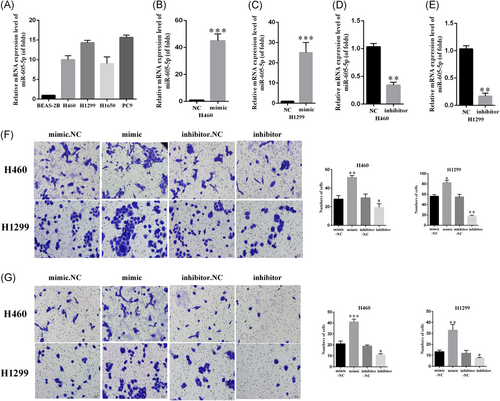
miR-605-5p is upregulated in non–small-cell lung cancer cells and promotes lung cancer invasion and migration. A, Expression levels of miR-605-5p in lung cancer cells. B-E, The efficiency of mimic-miR-605-5p and inhibitor-miR-605-5p was determined by qRT-PCR. F, Transwell assay with matrigel showed that miR-605-5p increased cell migration in lung squamous cell carcinoma. G, miR-605-5p increased cell invasion in lung squamous cell carcinoma. The number of cells were scored. All data are mean + S.D. Differences in colonies were analyzed by t-test. *P < .05, **P < .01, ***P < .001. miR, microRNA; NC, negative control; qRT-PCR, quantitative reverse-transcription polymerase chain reaction
3.2 miR-605-5p promotes lung cancer cell carcinoma proliferation
For estimating the proliferation of H460 and H1299 cells with miR-605-5p mimic or inhibitor-miR-605-5p, CCK-8 and EdU assay were used. The CCK8 assays results implicated miR-605-5p in promoting lung squamous cell carcinoma proliferation (Figure 2A-D). Consistently, EdU assay showed that miR-605-5p promotes lung squamous cell carcinoma proliferation (Figure 2E and 2F). Similar results were obtained in the colony formation that was used to analyze cell viability of H460 and H1299 cells (Figure 2G and 2H). Taken together, these observations suggested that miR-605-5p promotes lung cancer cell carcinoma proliferation.
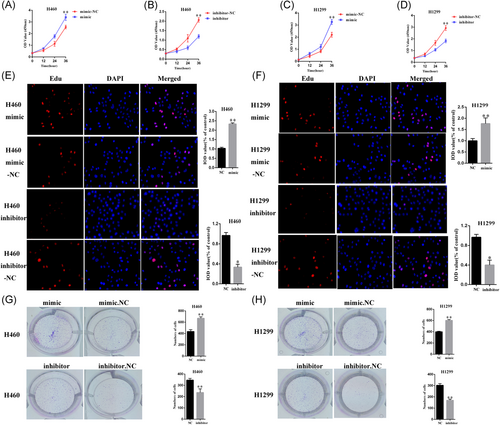
miR-605-5p promotes lung cancer cell carcinoma proliferation. A-D, CCK8 assays indicated that miR-605-5p inhibited lung squamous cell carcinoma proliferation; E-F, Result of EDU assay shows that miR-605-5p inhibited lung squamous cell carcinoma proliferation; G-H, Colony formation was used to analyze cell viability of lung cancer cell. G, The mRNA expression of TNFAIP3 was determined by qRT-PCR. H, The protein expression of TNFAIP3 was determined by Western blot analysis. *P < .05, **P < .01. CCK8, cell counting kit-8; DAPI, 4′,6-diamidino-2-phenylindole; EDU, 5-ethynyl-2′-deoxyuridine; IOD, integrated optical density; mRNA, messenger RNA; NC, negative control; qRT-PCR, quantitative reverse-transcription polymerase chain reaction
3.3 miR-605-5p regulates TNFAIP3 expression by targeting the 3′-UTR of TNFAIP3
Given the profound effect of miR-605-5p on lung cancer proliferation and metastasis, we found the possible targets of miR-605-5p by means of a miRNA target prediction website. The 3′-UTR of TNFAIP3 was identified as a binding site of miR-605-5p (Figure 3G). We also found that overexpression of miR-605-5p inhibits the expression levels of TNFAIP3 at the mRNA and protein levels in H460 and H1299 cells by qRT-PCR (Figure 3A-D) and Western blot analysis (Figure 3E and 3F) assays compared to miR-NC groups. On the other hand, the expression levels of TNFAIP3 significantly increased at the protein and mRNA levels in comparison to the miR-NC groups when miR-605-5p was downregulated in H1299 and H460 cells. Before performing the luciferase reporter assay, the miR-605-5p was cotransfected with TNFAIP 3′-UTR into the H460 and H1299 cells. The relative luciferase activities of wt 3′-UTR TNFAIP3 were inhibited in H460 and H1299 cells with miR-605-5p mimic cotransfection, according to the results (P < .01, respectively, Figure 3H), but not in mut 3′-UTR TNFAIP3 transfection. Besides this, miR-605-5p inhibitor cotransfection increased the relative luciferase activities of wt 3′-UTR TNFAIP3 in H460 and H1299 cells but not in the mutant reporter gene, which suggested that TNFAIP3 is a target of miR-605-5p in NSCLC cells.
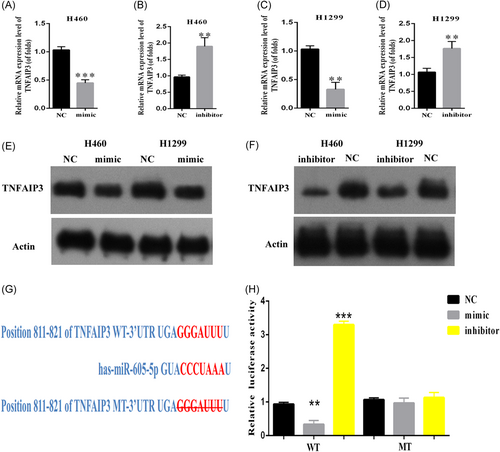
miR-605-5p regulates TNFAIP3 expression by targeting the 3′-UTR of TNFAIP3. A-D, TNFAIP3 was negatively correlated with mir-605-5p measured by qPCR; E-F, TNFAIP3 was negatively correlated with mir-605-5p measured by WB; G, The target gene of miR-605-5p was predicted through a miRNA target prediction website; H, The 293T cells were transfected with miR-605-5p or the miR-control vector and were cotransfected with the control luciferase reporter plasmid pmirGLO or with the reporter plasmid containing the TNFAIP3 wt 3′-UTR or the TNFAIP3 mut 3′-UTR. Luciferase activity was assayed 48 hours later. **P < .01, ***P < .001. 3′-UTR, 3′-untranslated region; miR, microRNA; mRNA, messenger RNA; mut, mutant; NC, negative control; qPCR, quantitative polymerase chain reaction; TNFAIP3, tumor necrosis factor α-induced protein 3; wt, wild type
3.4 Silencing of TNFAIP3 promoted invasion and proliferation in lung cancer
For investigating the effect of TNFAIP3, we knocked down TNFAIP3 by siRNA transfection in H1299 and H460 cells. qRT-PCR assays were used to confirm the transfection efficiency (P < .01, Figure 4A and 4B). For identifying the role of TNFAIP3 in NSCLC metastasis, we performed the transwell assay. The results of the experiment suggested that TNFAIP3 siRNA transfection significantly promoted the invasion of H460 and H1299 cells in comparison to the control group (Figure 4C and 4D). Similar results were also seen while studying the role of TNFAIP3 in the cloning experiment of H460 and H1299 cells (Figure 4E-H). Therefore, silencing of TNFAIP3 can promote invasion and proliferation in lung cancer.
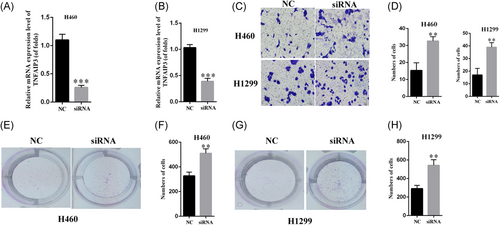
Silencing of TNFAIP3 promoted invasion and proliferation in lung cancer. A-B, The efficiency of silencing is confirmed by RT-PCR assays. C-D, Silencing of TNFAAIP3 promotes the invasion of lung cancer cells. G-H, Silencing of TNFAIP3 promotes the proliferation of lung cancer cells. **P < .01, ***P < .001. NC, negative control; RT-PCR, reverse-transcription polymerase chain reaction; siRNA, small interfering RNA; TNFAIP3, tumor necrosis factor α-induced protein 3
3.5 Down regulation of miR-605-5p inhibited invasion and proliferation by targeting TNFAIP3 (H1299)
As per the previous study, the miR-605-5p inhibitor inhibited H460 and H1299 cell invasion. To test whether this phenotype acts through TNFAIP3, we transfected TNFAIP3 siRNA or si-NC to the miR-605-5p-inhibitor-transfected or miR-605-5p-NC-transfected H1299 cells (Figure 5A and 5B). The invasion experiments showed that the transfection of TNFAIP3 siRNA to miR-605-5p-inhibitor-transfected H1299 cells can promote the invasion in comparison to the control group (miR-605-5p-inhibitor-transfected with si-NC). Consistently, the population wherein TNFAIP3 siRNA was transfected to miR-605-5p-inhibitor NC-transfected H1299 cells was greater than the previous one. Similar results were obtained in colony formation upon analyzing the cell proliferation of H1299 cells (Figure 5C and 5D). These observations suggested that downregulation of miR-605-5p can inhibit invasion and proliferation by targeting TNFAIP3.
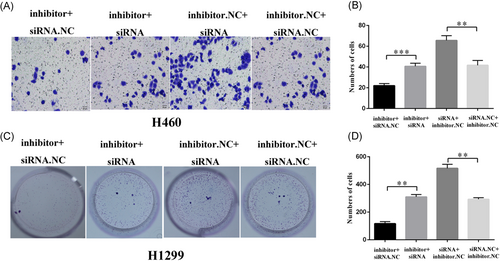
Downregulation of miR-605-5p inhibited invasion and proliferation by targeting TNFAIP3. A-B, Knockdown of TNFAIP3 restored the significant decrease in invasion in miR-605-5p-inhibitor-transfected H460 cells. C-D, Knockdown of TNFAIP3 restored the significant decrease in proliferation in miR-605-5p-inhibitor-transfected H1299 cells. **P < .01, ***P < .001. miR, microRNA; NC, negative control; siRNA, small interfering RNA; TNFAIP3, tumor necrosis factor α-induced protein 3
3.6 Effects of miR-605-5p on lung cancer proliferation and metastasis in vivo
Combined with the above results, we proceeded to investigate the effects of miR-605-5p in an H1299 xenograft mouse model (Figure 6A). The expression of mir-605-5p in the tumor was determined (Figure 6B). The volumes of tumors generated by H1299 cells expressing miR-605-5p inhibitor were smaller than that of the control group in six samples compared with the results in vitro (Figure 6C). In the inhibitor-miR-605-5p mimic overexpression group, the tumor weights were decreased compared with the control group (Figure 6D). For studying the role of miR-605-5p in metastasis lesions, some parts of lungs stained with hematoxylin–eosin were used. (Figure 6E). As shown in the results (Figure 6F), in the control group, the metastasis rate was 55% with 25% in the miR-605-5p inhibitor group of H1299 cells. These data illustrated a critical role for miR-605-5p in lung cancer metastasis and growth in vivo.
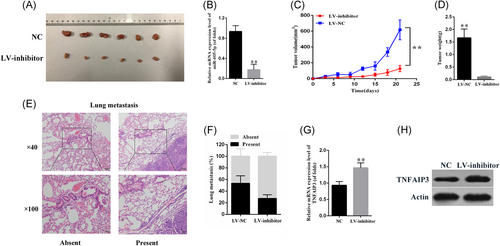
Effects of miR-605-5p on lung cancer proliferation and metastasis in vivo. A, The mouse model was constructed by using H1299 cells infected with miR-605-5p. The size of local liver tumors in these two groups was calculated and compared in the diagrams. Results were represented as mean ± SD (n = 6). B, The expression of mir-605-5p was determined. C, Tumor size. E, Typical pictures for lung metastasis of the H1299 xenograft mouse model. Images were captured at ×40 (up) and ×100 (down). F, The percentage of mice with or without metastatic nodules in the lungs was calculated. **P < .01. miR, microRNA; NC, negative control; TNFAIP3, tumor necrosis factor α-induced protein 3
4 DISCUSSION
In this study, we showed that miR-605-5p promotes invasion and proliferation by targeting TNFAIP3 in NSCLC. Thus, miR-605-5p was a promising marker for treatment of NSCLC.
Abnormal miRNA expression levels can be found in many cancers through regulating tumorigenesis, migration and invasion.10 miRNAs have been found to be potential prognostic markers in NSCLC acting as either oncogenes or tumor suppressors. miR-133a might act as a tumor-suppressor and an independent prognostic and diagnostic biomarker for NSCLC.11 microRNA-758 can inhibit proliferation, invasion, migration and promoted apoptosis of NSCLC by negatively regulating HMGB.12 However, miR-500 and miR-628 promote NSCLC occurrence, migration and invasion by targeting ING1.13 However, the specific role of MiR-605-5p in NSCLC remains unclear. Therefore, we first researched the expression of miR-605-5p in NSCLC. Results showed that the expression of miR-605-5p in NSCLC tissues was higher than other lung cells. Besides this, we showed that ectopic expression of miR-605-5p can promote lung cancer migration, invasion and lung cancer cell carcinoma proliferation while inhibition of miR-605-5p would inhibit migration, invasion and cell proliferation. We also identified miR-605-5p as an oncogene in vivo by means of an xenograft animal model. The organ-specific function of miR-605-5p has not been well studied in the past few years. One group showed that miR-605-5p promoted the P53 stress response, thus inhibiting apoptosis in colorectal carcinoma cells.9 Another study recently showed that miR-605-5p acted as a tumor-suppressor in melanoma by inhibiting INPP4B.14 This may be due to the different cancer types or tissues.
To find the underlying mechanism of miR-605-5p in regulating NSCLC genesis and progression, we predicted the target genes of miR-605-5p by using the bioinformatics analysis website. It showed that TNFAIP3 was a target of miR-605-5p. TNFAIP3 was encoded by the TNFAIP3 gene in humans,15 which was induced by the tumor necrosis factor (TNF). The TNFAIP3 protein is a zinc finger protein,and has been identified to inhibit NF-κB activation and TNF-mediated apoptosis. TNFAIP3 has been identified to interact with TNIP1,16 TRAF1,17, 18 TRAF2,18 TRAF6,18, 19 YWHAB,20 YWHAZ,20, 21 YWHAH,20, 21 IKBKG,22 and TAX1BP1.22 In KRAS(G12V)-transduced BEAS-2B cells, anti-miR-29b constructs increased their sensitivity to apoptosis. It was reported that TNFAIP3 may be a functionally relevant target of miR-29b.23miRNA-214 also can regulate TNFAIP3 in NSCLC.3
In summary, we found that miR-605-5p expression was much higher in NSCLC. miR-605-5p can promote lung cancer invasion and migration. Besides this, miR-605-5p promoted lung cancer cell carcinoma proliferation. Consistently, miR-605-5p played an important role in the promotion of lung cancer growth and metastasis in vivo. We also found there was a negative correlation between TNFAIP3 and miR-605-5p in NSCLC tissues. Further investigation showed that TNFAIP3 3′-UTR was the target of miR-605-5p. Overexpression of miR-605-5p reduces the TNFAIP3 mRNA and protein level in NSCLC cells. In addition, silencing of TNFAIP3 promoted invasion and proliferation in lung cancer. Downregulation of miR-605-5p inhibited invasion and proliferation by targeting TNFAIP3.
From what has been discussed above, we showed that miR-605-5p plays a tumor-suppressor role in NSCLC. It functions mainly by targeting TNFAIP3 expression. These findings suggested a specific mechanism of NSCLC progression and suggest miR-605-5p to be a potential therapeutic target for NSCLC.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
All authors contributed adequately to this study.