MicroRNA-9 overexpression suppresses vulnerable atherosclerotic plaque and enhances vascular remodeling through negative regulation of the p38MAPK pathway via OLR1 in acute coronary syndrome
Abstract
Acute coronary syndrome (ACS) is characterized by atherosclerotic plaque rupture with a high incidence of recurrent ischemic events. Several microRNAs are found to be aberrantly expressed in atherosclerotic plaques. This study aims to investigate the effects of microRNA-9 (miR-9) on vulnerable atherosclerotic plaque and vascular remodeling in ACS and underlying mechanisms. Microarray-based gene expression profiling was used to identify differentially expressed genes related to ACS and regulatory miRNAs. Oxidized low-density lipoprotein (lectin-like) receptor 1 (OLR1) was identified to be aberrantly activated in ACS and regulated by miR-9. OLR1 was verified as a target gene of miR-9 by bioinformatics prediction and dual luciferase reporter gene assay. The atherosclerotic models were induced in ApoE−/− mice, in which the agomir or antagomir of miR-9, or small interfering RNA (siRNA) against OLR1 were separately introduced. Serum lipid levels and expression of vascular remodeling and inflammatory response-related factors were determined, respectively. On the basis of the obtained results, in the atherosclerosis mice treated with the agomir of miR-9 and siRNA against OLR1, the p38-mitogen-activated protein kinase (p38MAPK) pathway was inhibited; levels of triglyceride, total cholesterol, low-density lipoprotein cholesterol, tumor necrosis factor-α, interleukin-6, and vascular endothelial growth factor were reduced, but the high-density lipoprotein cholesterol level was increased, along with decreased vulnerable atherosclerotic plaque area and enhanced vascular remodeling. Taken together, these findings suggested an inhibitory role miR-9 acts in the formation of vulnerable atherosclerotic plaques in ACS mice, along with a promoted vascular remodeling, via a negative feedback regulation of OLR1-mediated p38MAPK pathway.
1 INTRODUCTION
Acute coronary syndrome (ACS), comprising unstable angina and myocardial infarction, is a key contributor to premature mortality, morbidity as well as hospital admissions.1, 2 ACS is featured by decreased blood flow to the heart which can induce myocardial injury and the main clinical manifestations of ACS are chest pain and coronary artery subocclusion.3 A fractured or corroded atherosclerotic plaque is recognized as the common pathophysiology of ACS and patients with ACS also have more active inflammation.4, 5 ACS diagnosis can be based on the imaging modalities (echocardiography, magnetic resonance imaging, and computed tomography) and biomarkers (highly sensitive or ultrasensitive cardiac troponin assays) which allow an early diagnosis of ACS before the performance of targeted therapy.6, 7 Aggressive antiplatelet, antithrombotic, anti-ischemic and lipid-lowering agents combined with lifestyle interventions, modification of the risk factor profile, and long-term medical treatment play important role in the treatment, recurrence reduction and early prevention of ACS.8 Besides, prior investigations have found, under some pathological circumstances, the abnormal expression of microRNAs (miRNAs) in cardiovascular system, which may also be promising to open new approach for the diagnosis and treatment of cardiovascular diseases (CVD).9, 10
As sizable sub-group of small noncoding RNAs, miRNAs play a pivotal role in degrading or inhibiting their target mRNAs translation, thereby modulating target gene expression with potential impacts on multiple biologic processes.11 A previous study has found that upregulated miR-9 can inhibit myocardin expression that is correlated with the development of cardiac systolic, diastolic dysfunction and ultimately heart failure, so administrating miR-9 has implications for cardiac hypertrophy reduction and cardiac improvement.12 As a major endothelial scavenger receptor, the oxidized low-density lipoprotein (lectin-like) receptor 1 (OLR1) gene helps to degrade the oxidized low-density lipoprotein, which is involved in the pathogenesis of atherosclerosis.13 To identify potential target genes of miR-9, we applied the bioinformatics prediction website and dual luciferase reporter gene assay, which have confirmed that OLR1 is the target gene of miR-9. p38-mitogen-activated protein kinase (p38MAPK) system has been recognized as a major pathway in the production of proinflammatory mediators.14 Prior studies have demonstrated that the apoptosis and proinflammatory cytokine expression in human osteoarthritis chondrocytes can be inhibited via the inactivation of the p38MAPK pathway and the P38MAPK pathway is involved in isoproterenol-induced acute myocardial infarction.15, 16 Recently, more and more researchers have recognized the role of miRNA in cardiovascular diseases, but how these miRNAs are involved in the onset and development of cardiovascular diseases remain largely undefined.17 Therefore, we hypothesized that miR-9 could serve as a biomarker for patients with ACS. In this study, we aim to explore the role of miR-9 in atherosclerotic plaque and vascular remodeling in ACS with the underlying mechanism that involves the p38MAPK pathway.
2 MATERIALS AND METHODS
2.1 Ethics statement
Mouse handling and treatment were in accordance with the guidelines issued by the National Institutes of Health (NIH publication No. 85-23) and were approved by the Animal Ethics Committee of Hainan Medical University.
2.2 Microarray analysis
The chip data of ACS GSE19339 was downloaded from the Gene Expression Omnibus (sub-database of National Center for Biotechnology Information [https://www.ncbi.nlm.nih.gov/]). The Affy package (http://www.bioconductor.org/packages/release/bioc/html/affy.html) based on robust multiarray average algorithm18 was used for background correction and standardization of pretreatment for chip data. The limma package of R language (http://master.bioconductor.org/packages/release/bioc/html/limma.html) was performed to identify the differentially expressed genes (DEGs) of ACS. The log2 Fold Change (log2FC) of genes was calculated. After correction, the p value was expressed as adj.p.Val. |log2FC| > 2.0 and adj.p.Val < 0.01 were regarded as obviously differential expression. The genes related to ACS were retrieved in DisGeNET (http://www.disgenet.org/web/DisGeNET/menu/search?4) database. The information about gene interaction was provided by String (https://string-db.org/)19 database to construct the network of interactions between DEGs and genes related to ACS, and the Cytoscape 3.6.0 software20 was used to visualize the network. The Chilibot (http://www.chilibot.net/index.html) was used to extract information among various Biological concept, genes, proteins or drugs and to construct the relationship network with rich contents.21 Moreover, the Chilibot website was performed to further exploit the correlation between DEGs and ACS. Furthermore, the four prediction tools for a miRNA-mRNA relationship, including DIANA (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=microT_CDS/index), RNA22 (https://cm.jefferson.edu/rna22/), TargetScan (http://www.targetscan.org/vert_71/), and miRNA (http://34.236.212.39/microrna/getGeneForm.do) were used to predict the target source miRNA of DEGs. The jvenn (http://jvenn.toulouse.inra.fr/app/example.html) performed to compare and analyze the miRNA predicted results from four prediction tools and Venn diagrams were plotted.22
2.3 Dual-luciferase reporter gene assay
The target gene of miR-9 was analyzed using the biological prediction website (http://www.microRNA.org), and the luciferase reporter method was then applied to verify whether OLR1 was a direct target gene of miR-9. Synthetic OLR1 3′-untranslated region gene fragment was introduced into pcDNA3.1-Luc, a carrier of pcDNA3.1 (Invitrogen, Carlsbad, CA) after transformation, through restriction endonuclease sites of siXhoI and BamH. Complementary sequence mutation sites of seed sequences were designed on the OLR1 wild type. The target fragment was then inserted into the pcDNA3.1-Luc reporter plasmid using T4 DNA ligase after restriction enzyme digestion. Sequenced luciferase reporter plasmids WT, MUT, and miR-9 mimic were cotransfected to HEK-293T cells (Beinuo Biotechnology Co, Ltd, Shanghai, China). Cells were collected and lysed after 48 hours of transfection, and luciferase activity was detected by the fluorescein test kit (K801-200; Biovision, Mountain View, CA). The experiment was repeated three times in each group.
2.4 Animal model establishment and drug delivery
This study included seventy ApoE−/− mice (male and female in equal) with specific-pathogen-free (SPF) grade, aged 6 weeks old and weighed 18 to 20 g, which were provided by the Model Animal Research Center of Nanjing University (Nanjing, China). Another 10 C57BL/6J normal mice (male and female in equal) with the same genetic background serving as the control group with SPF grade, aged 6 weeks old and weighed 18 to 20 g, were purchased from Shanghai Silaike Experimental Animal Co, Ltd (Shanghai, China). All mice were reared in SPF animal house at 19 to 22°C with a humidity of 50%-70%, moderate illumination and good ventilation. The mice were fed with ordinary feedstuff for 1 week, with free access to water. After the mice were acclimatized, maintaining a stable weight, they were assigned into eight groups (n = 10), including the normal group (C57BL/6J mice injected with normal saline and fed with normal diet), the model group (ApoE−/− mice injected with normal saline), the miR-9 agomir negative control (NC) group (ApoE−/− mice injected with miR-9 analogue as NC), the miR-9 agomir group (ApoE−/− mice injected with miR-9 analogue), the miR-9 antagomir NC group (ApoE−/− mice injected with miR-9 antagonist as NC), the miR-9 antagomir group (ApoE−/− mice injected with miR-9 antagonist), the si-OLR1 group (ApoE−/− mice injected with si-OLR1) and the miR-9 antagomir+si-OLR1 group (ApoE−/− mice shot with miR-9 antagonist and si-OLR1). All the ApoE−/− mice were provided with high-fat diets containing 21% fat and 0.15% cholesterol and intraperitoneally injected with 10 μ/d si-OLR1; miR-9 agonist, antagonist and NC substance were dissolved into 0.2 mL normal saline at a dose of 80 mg/kg/d, and were injected into the mice through tail vein once a day, for ten consecutive days.23
2.5 Animal tissue extraction
The mice were fasted for a night, and blood samples collected from orbit were used to detect levels of blood lipids and inflammatory factors. After that, the mice were killed by cervical vertebra dislocation. The thoracic and abdominal cavity of the mice was then opened quickly, and the aortic valve was separated along the aorta. The aortic roots of the mice were used for histological analysis, the crotch of common iliac arteries was taken for the analysis of mRNA and protein, and the mouse right ventricular venous blood was applied for the detection of dendritic cell phenotype.
2.6 Detection of serum lipid levels in mice
Blood samples collected from orbit were stored at room temperature for 1 hour. After centrifugation at 3000 r/min at 4°C for 10 minutes, the upper serum was collected (about 0.4 mL). The levels of triglycerides (TG) (A110-1; Nanjing Jiancheng Bioengineering Institute, Nanjing, China), total cholesterol (TC) (A111-1; Nanjing Jiancheng Bioengineering Institute), high-density lipoprotein cholesterol (HDL-C) (A112-1, Nanjing Jiancheng Bioengineering Institute) and low density lipoprotein cholesterol (LDL-C) (A113-1; Nanjing Jiancheng Bioengineering Institute) were analyzed by an automatic biochemical analyzer (CX7; Hitachi Ltd, Tokyo, Japan).
2.7 Enzyme-linked immunosorbent assay assay
The serum of mice in each group was operated in strict accordance with the instructions of tumor necrosis factor-alpha (TNF-α) enzyme-linked immunosorbent assay (ELISA) Kit (ab100747; Abcam, Cambridge, MA), interleukin-6 (IL-6) ELISA Kit (ab100712; Abcam) and vascular endothelial growth factor (VEGF) ELISA kit (ab10075; Abcam). The absorbance (A) value of each pore (450 nm) was measured within 3 minutes using BioTek Synergy 2, a multifunctional enzyme labeling instrument. The regression equation of a standard curve was set up using standard concentration as abscissa and A value as ordinate. The A value of samples was substituted into the equation, and the target protein concentration of samples was calculated.
2.8 Hematoxylin-eosin staining
Aortic roots of the mice were immersed into the normal saline, and epicardial connective tissue was removed, fixed with 10% formaldehyde solution, and embedded in paraffin. Serial 5 μm aortic root of each group was sliced into pieces. Routine hematoxylin-eosin (HE) staining was as follows: paraffin slices were dewaxed in xylene I (CAS No. 14936-97-1; Shanghai E-research Biotechnology Co, Ltd, Shanghai, China), xylene II (CAS No. 523-67-1; Shanghai Yuduo Biotechnology Co, Ltd, Shanghai, China), 5 minutes each, and were further dewaxed in 100%, 95%, 80%, 75% ethanol for 1 minute respectively. After that, the paraffin slices were washed with distilled water for another 2 minutes, stained with hematoxylin (CAS No. 474-07-7, Qingdao Jisskang Biotechnology Co, Ltd, Qingdao, China) for 5 minutes, rinsed by running water, differentiated by 1% hydrochloric acid ethanol for 30 seconds (inserted number differentiation) and soaked in running water for 15 minutes or warm water for 5 minutes (about 50°C). Next, eosin solution (RY0648; Qingdao Jisskang Biotechnology Co, Ltd) was used to stain the slices for 2 minutes, which were then rinsed by running and observed under a microscope. The above steps could be repeated if the dyeing was poor. After that, the slices were dehydrated by 95%, 100%, 100% methanol 1 minute respectively, cleared by xylene carbolic acid (3:1), xylene I, xylene II (1 minute each), and sealed with a neutral resin after being dried. Lastly, the slices were observed under a microscope with Image-Pro Plus 6 software measuring intima-media thickness and the ratio of atherosclerotic plaque area to lumen area. Cell nucleus was stained dark blue, and the cytoplasm and fibrous tissue were red at different depths.
2.9 Masson staining
Paraffin-embedded sections were dewaxed in xylene I and xylene II respectively for 5 minutes, and then dewaxed in 100%, 95%, 80%, 75% ethanol each for 1 minute, rinsed with distilled water for 2 minutes. Next, 100 μL dye compound solution of Masson (reagent A) was applied to staining for 5 minutes, and slices were sufficiently rinsed by distilled water. Afterward, the slices were stained with 100 μL phosphomolybdic acid (reagent C) for 5 minutes and dried. Aniline blue (reagent D) was used to dye the slices for 5 minutes, which were then washed slightly by distilled water; 100 μL differentiation liquid (reagent B) was added for differentiation for 30 to 60 seconds, which was operated twice. The slices were then dehydrated in 95%, 100%, and 100% alcohol respectively for 30 seconds, and were cleared in xylene I and xylene II, 1 minute each. After that, the slices were dried and sealed with neutral gum. These slices were observed under a microscope: collagen fibers were stained blue, the cell nucleus was stained blue-purple, and muscle and cellulose were stained red after Masson staining. The ratio of collagen area to the vascular wall area indicated collagen content, which was measured by image analysis software, Image plus 6.0.
2.10 RNA isolation and quantitation
Trizol reagent (Invitrogen) was used to extract the total RNA of the aortic vascular tissue samples from each group. RNA was dissolved in ultrapure water treated by diethyl pyrocarbonate. The A value of RNA at 260 and 280 nm was measured by ND-1000 ultraviolet/visible spectrophotometer (NanoDrop Technologies Inc, Wilmington) to identify and determine the quality and concentration of the total RNA. complementary DNA (cDNA) was synthesized with a reverse transcriptase kit. The reverse transcriptase was inactivated by heating the cDNA sample at 80°C for 5 minutes and then cDNA was amplified by polymerase chain reaction (PCR). The reaction system of PCR was 25 μL with U6 as the internal reference for miR-9 and β-actin as the internal reference for other mRNAs. The primer sequences are shown in Table 1. The PCR conditions of miR-9 were as follows: predenaturation for 30 seconds at 95°C, followed by 40 cycles of denaturation for 5 seconds at 95°C, 30 seconds at 60°C. The PCR conditions of OLR1 were predenaturation for 3 minutes at 94°C, followed by 35 cycles of denaturation for 30 seconds at 94°C, 30 seconds at 58°C, 30 seconds at 72°C and extension for 70 minutes at 72°C. The PCR conditions of p38MAPK were as follows: pre-denaturation for 5 minutes at 95°C, followed by 45 cycles of denaturation for 5 seconds at 94°C, 30 seconds at 60°C with extension 1 second every 1°C at 65 to 90°C. The PCR conditions of Bax were at predenaturation at 94°C for 5 minutes, followed by 45 cycles of denaturation for 45 seconds at 94°C, 30 seconds at 58.5°C and extension at 72°C for 7 minutes. The PCR conditions of Fas were predenaturation at 94°C for 5 minutes, followed by 32 cycles of denaturation for 45 seconds at 94°C, 30 seconds at 55°C, 60 seconds at 72°C and extension at 72°C for 7 minutes. The PCR conditions of p53 were predenaturation at 94°C for 4 minutes, followed by 35 cycles of denaturation for 30 seconds at 94°C, 30 seconds at 63°C, 45 seconds at 72°C and extension at 72°C for 7 minutes. Measurement of each sample was repeated three times, taking the average. PCR results were evaluated by the melting curve and CT value (inflection point of amplified power curve) was calculated by the relative quantitative method: ΔCt = CT (target gene) – CT (internal reference), ΔΔCt = ΔCt (experimental group) – ΔCt (control group), with 2–ΔΔCt indicating the relative expression of every target gene.
| Gene | Primer sequence (5′-3′) |
|---|---|
| miR-9 | F: GTGAGGGAAGCGAGTTGT |
| R: CCTCGGTGACCTTGAAGG | |
| OLR1 | F: CCCAGGGAAATGCCTGCTA |
| R: GCTGTGACCTTGAGTTAGGCA | |
| p38MAPK | F: GAAGAGCCTGACCTACGAT |
| R: ACTGCCAAGGAGCATCTA | |
| Bax | F: GGATGCGTCCACCAAGAA |
| R: GGAGGAAGTCCAGTGTCC | |
| Fas | F: ATGCACACTCTGCGATGA |
| R; CAGTGTTCACAGCCAGGA | |
| P53 | F: ATGGAGGAGCCGCAGTCAGAT |
| R: GCAGCGCCTCACAACCTCCGTC | |
| β-actin | F: CGCCACCAGTTCGCCATG |
| R: TACAGCCCGGGGAGCATC | |
| U6 | F: TCCGACGCCGCCATCTCTA |
| R: TATCGCACATTAAGCCTCTA |
- Abbreviations: F, forward; miR-9, microRNA 9; OLR1, oxidized low-density lipoprotein receptor 1; p38MAPK, p38 mitogen-activated protein kinase; R, reverse; RT-qPCR, reverse transcription quantitative polymerase chain reaction
2.11 Western blot assay
Aortic tissue samples of the mice were immediately placed in pre-cooled lysis buffer and were ultrasonically comminuted at 4°C. After centrifugation at 8000 rpm for 15 minutes, the supernatant was collected and assayed using a BCA kit (20201ES76; Yeasen Biotechnology Co, Ltd, Shanghai, China) to determine protein concentration in each sample. Next, 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis was used to separate protein, and the protein was electrically transferred to nitrocellulose membrane after gel electrophoresis (ZY-160FP; Zeye Biotechnology Co, Ltd, Shanghai, China). After that, the membrane was blocked with 3% bovine serum albumin for 1 hour at room temperature, washed with Tris-buffered saline (TBS) three times, 10 minutes each. The membrane was then incubated in the primary antibodies, rabbit-anti-mouse polyclonal antibodies (1:500 dilution), at 4°C overnight, including LOX-1 (ab60178; Abcam, Cambridge, UK), Anti-p38 (phospho T180+Y182), (ab195049, Abcam), Bax (ab32503, Abcam), Fas (ab15285, Abcam), p53 (ab26, Abcam), GAPDH (ab181602, Abcam). The membrane was washed with TBS at 37°C three times, 10 minutes each. The secondary antibody, horse radish peroxidase (HRP) labeled Immunoglobulin G (IgG) goat anti-rabbit polyclonal antibody (1:500 dilution) (ab20272, Abcam), was added with shaking at 37°C for 1 hour with TBS washing the membrane 3 times, 10 minutes each. The membrane was then reacted with electrochemical luminescence (ECL) (ECL808-25; Biomiga, San Diego, CA) for 1 minute at room temperature. After that, reaction liquid was removed and the membrane, covered with fresh-keeping film, was observed under an X-ray machine (36209ES01; Shanghai Qcbio Science and Technology Co, Ltd, Shanghai, China). GAPDH was used as internal reference, and the ratio of the gray value of the target band to the reference band was taken as the relative expression of protein. The experiment was repeated three times in each group.
2.12 Statistical analysis
Statistical analysis was conducted using SPSS 21.0 (IBM Corp Armonk, NY). The measurement data were expressed as mean ± standard deviation. Data among multiple groups were compared using one-way analysis of variance with test for homogeneity of variances. Differences between two groups were compared by Q test. When there was no homogeneous variance, the nonparametric rank test would be used. The α level was set at 0.05, and a level of p-value less than 0.05 was considered statistically significant. The count data were expressed in percentage or rate, and χ2 test was used.
3 RESULTS
3.1 MiR-9 is identified to modulate ACS by regulating OLR1 by microarray analysis
According to the ACS chip data GSE19339, 20 genes with an obvious difference were screened with |log2FC| > 2.0 and adj.p.Val < 0.01 as threshold and the heat map of DEGs were plotted (Figure 1A). The genes related to ACS were retrieved in the DisGeNET database, and the first ten genes (IL6, ITGB3, CRP, MMP9, TNF, TLR4, PAPPA, PON1, TNNI3, and TUBB1) were the known genes related ACS. The DEGs and known genes related to ACS were included into the String database. The interactions among genes were analyzed and the networks of gene interactions were plotted (Figure 1B). In this network, the eight DEGs, OLR1, CCL20, CCL2, CXCL3, CXCL2, FN1, PPARG, and ACKR3 were more closely related to other genes (degree > 5), suggesting that they may be correlated with ACS. Subsequently, the relational network between eight DEGs and ACS were further exploited in the Chilibot (Figure 1C). We found the except for ACKR3 and OLR1, the other DEGs had direct or indirect associations with ACS, and we attached much importance on the effects of OLR1 on ACS. It is known that OLR1 is aberrantly activated in ACS, and a previous study has indicated that OLR1 is related to ACS,24 while the deeper molecular mechanism remains poorly understood. So we first detected the OLR1 expression in the Aortic tissue of normal group (C57BL/6J mice injected with normal saline and fed with normal diet) and atherosclerosis group (ApoE−/− mice injected with normal saline) by reverse transcription quantitative polymerase chain reaction (RT-qPCR) assay. The results showed OLR1 in aortic tissue of model group was significantly higher than the control group (Figure 1D). Then we analyzed the upstream mechanism of OLR1.
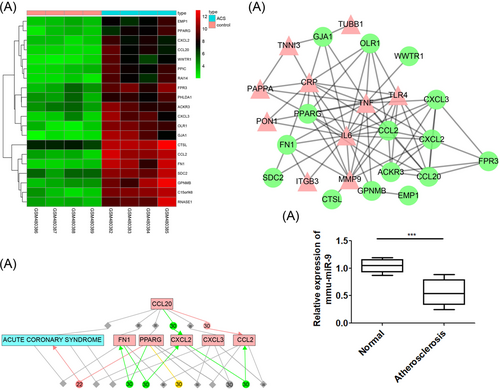
Comprehensive microarray analysis identifies miR-9 modulates ACS by regulating OLR1 via regulation of p38MAPK signaling pathway. Panel A, the heat map of DEGs related to ACS of GSE19339 chip data; the abscissa refers to sample number, and the ordinate refers to names of DEGs. The upper right histogram refers to color gradation. Each block represents the expression level of gene in one sample. The red means high expression, and green means poor expression. Panel B, the network of interactions between DEGs and known genes related to ACS. The pink triangle refers to the known genes related to ACS, and the green circle refers to the DEGs. Panel C, the relational network between DEGs and ACS; Panel D, the OLR1 expression in Aortic tissue of normal group (C57BL/6J mice injected with normal saline and fed with normal diet) and atherosclerosis group (ApoE−/− mice injected with normal saline) was detected by RT-qPCR assay. ACS, acute coronary syndrome; DEGs, differentially expressed genes; miR-9, microRNA-9; OLR1, oxidized low-density lipoprotein (lectin-like) receptor 1; p38MAPK, p38-mitogen-activated protein kinase; RT-qPCR, reverse transcription quantitative polymerase chain reaction
The DIANA, RNA22, TargetScan, and miRNA were adopted to predict the miRNA that could regulate OLR1. There were 84 miRNAs obtained from TargetScan with context++ score less than −0.3 as the threshold, and 92 miRNAs from DIANA with miTG score greater than 0.7 as the threshold. Moreover, there were 725 miRNAs that may regulate OLR1 obtained from RNA22, and 12 miRNAs from microRNA.org with mirSVR score less than −0.7 as the threshold. Through an analysis of four predicted results of miRNA to plot Venn diagrams (Figure 2A), we found that there was only one intersection, mmu-miR-9-5p, indicating that mmu-miR-9-5p was more likely to regulate OLR1. We detected the mmu-miR-9-5p expression in the aortic tissue of normal group and atherosclerosis group by RT-qPCR assay. The results showed OLR1 in aortic tissue of atherosclerosis group was significantly higher than the normal group (Figure 2B). These findings revealed that miR-9 may regulate OLR1 in ACS.

OLR1 is confirmed as a target of miR-9 by the dual luciferase reporter gene assay. Panel A, comparisons of predicted results of miRNAs that regulate OLR1 among DIANA, RNA22, TargetScan, and microRNA, and there is only one intersection, mmu-miR-9-5p. Panel B, the OLR1 expression in the aortic tissue of normal group (C57BL/6J mice injected with normal saline and fed with normal diet) and atherosclerosis group (ApoE−/− mice injected with normal saline) was detected by RT-qPCR assay. Panel C, miR-9 binds to the 3′-UTR of ORL1 using the target prediction program; Panel B, the luciferase activity is decreased after treatment by a combination of miR-9 mimics ORL1-3′-UTR-wt, suggesting that miR-9 regulates ORL1. In panel D, the data are presented as the mean ± standard deviation. *p < 0.05 versus the NC group. The experiment was independently repeated three times. ACS, acute coronary syndrome; DEGs, differentially expressed genes; miR-9, microRNA-9; OLR1, oxidized low-density lipoprotein (lectin-like) receptor 1; RT-qPCR, reverse transcription quantitative polymerase chain reaction; UTR, untranslated region
3.2 miR-9 directly targets OLR1
Initially, a biological prediction website (http://www.microrna.org) was used to analyze target genes of miR-9, and dual-luciferase reporter gene assay was used to verify the targeting relationship between miR-9 and OLR1 (Figure 2C). The result showed that transfection of miR-9 mimics could significantly decrease the activity of luciferase reporter gene (p < 0.05) (Figure 2D). Therefore, ORL1 is a direct target gene of miR-9.
3.3 Upregulated miR-9 inhibits serum lipid levels of TG, TC, LDL-C while enhancing HDL-C level
Next, serum lipid levels of TG, TC, HDL-C, and LDL-C were determined by an automatic biochemical analyzer. The results are shown as follows (Figure 3): compared with the control group, serum levels of TG, TC, and LDL-C in the experimental groups were significantly increased (all p < 0.05), but the HDL-C level was significantly decreased (p < 0.05). There were no significant differences in levels of TG, TC, HDL-C, LDL-C among the atherosclerosis, miR-9 agomir NC, miR-9 antagomir NC and miR-9 antagomir+si-OLR1 groups (all p > 0.05). Compared with the atherosclerosis group, levels of TG, TC, and LDL-C were remarkably lower in the miR-9 agomir group and si-OLR1 group (all p < 0.05), but the HDL-C level was much higher (p < 0.05). On the contrary, levels of TG, TC, and LDL-C were increased significantly in the miR-9 antagomir group (all p < 0.05), while the HDL-C level was decreased remarkably (p < 0.05). There were no significant differences in levels of TG, TC, HDL-C, and LDL-C between the miR-9 agomir group and the si-OLR1 group (all p > 0.05). These results indicated that overexpressed miR-9 could inhibit serum lipid levels of TG, TC, LDL-C while increasing HDL-C level.

Overexpressed miR-9 inhibits serum levels of TG, TC, LDL-C while increasing HDL-C level, determined by an automatic biochemical analyzer. Panel A, the content of TG in mice serum in each group; Panel B, the content of TC in mice serum in each group; Panel C, the content of HDL-C in mice serum in each group; Panel D, the content of LDL-C in mice serum in each group; *p < 0.05 versus the control group, #p < 0.05 versus the atherosclerosis group. The data are presented as mean ± standard deviation, analyzed by one-way ANOVA. n = 10. The experiment was independently repeated three times. ANOVA, analysis of variance; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; miR-9, microRNA 9; NC, negative control; OLR1, oxidized low-density lipoprotein receptor 1; TC, total cholesterol; TG, triglycerides
3.4 Upregulated miR-9 inhibits serum levels of TNF-α, IL-6, and VEGF
In addition, the content of TNF-α, IL-6, and VEGF in mice serum of each group was determined by ELISA, and the results were as follows (Figure 4): compared with the control group, the contents of TNF-α, IL-6, and VEGF in the experimental groups were increased significantly (all p < 0.05), but there were no significant differences among the atherosclerosis, miR-9 agomir NC, miR-9 antagomir NC and miR-9 antagomir+si-OLR1 groups (all p > 0.05). Compared with the atherosclerosis group, the contents of TNF-α, IL-6, and VEGF in the miR-9 agomir group and si-OLR1 group were significantly lower (all p < 0.05), whereas the contents of TNF-α, IL-6, and VEGF were remarkably higher in miR-9 antagomir group (all p < 0.05). There were no differences in the contents of TNF-α, IL-6, and VEGF between the miR-9 agomir group and the si-OLR1 group (all p > 0.05). The results indicated that upregulated miR-9 could inhibit serum levels of TNF-α, IL-6, and VEGF.
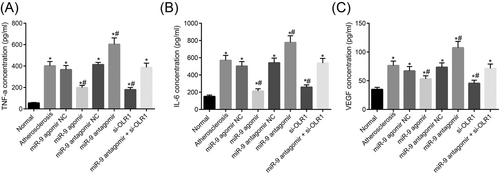
ELISA assay indicates that upregulated miR-9 inhibits serum levels of TNF-α, IL-6, and VEGF. Panel A, the content of TNF-α in mice serum in each group; Panel B, the content of IL-6 in mice serum in each group; Panel C, the content of VEGF in mice serum in each group; *p < 0.05 versus the control group, #p < 0.05 versus the atherosclerosis group. The data are presented as the mean ± standard deviation, analyzed by one-way ANOVA. n = 10. The experiment was independently repeated three times. ANOVA, analysis of variance; IL-6, interleukin-6; miR-9, microRNA 9; NC, negative control; OLR1, oxidized low-density lipoprotein receptor 1; TNF-α, tumor necrosis factor-alpha; VEGF, vascular endothelial growth factor
3.5 Upregulated miR-9 decreases atherosclerotic plaque area and intima-media thickness while enlarging collagen area in mice aorta
Furthermore, the ratio of atherosclerotic plaque area to lumen area and collagen area to vascular wall area were detected by HE and Masson staining with vulnerable plaque formation compared. The collagen content in the fibrous cap of atherosclerotic plaque plays an important role in plaque stability as the collagen content in the fibrous cap of the unstable plaque become less, the probability of plaque instability would increase, thus making plaque breakdown easier. Intima-media thickness was also measured with vascular remodeling compared. The results were as are displayed in Figure 5 as follows: compared with the atherosclerosis group, the ratio of plaque area to lumen area and intima-media thickness in the miR-9 agomir group and si-OLR1 group were decreased significantly (both p < 0.05), and the ratio of collagen area to vascular area was increased remarkably (p < 0.05). On the contrary, the ratio of plaque area to lumen area and intima-media thickness in the miR-9 antagomir group were increased notably (both p < 0.05), and the ratio of collagen area to vascular area was decreased obviously (p < 0.05). There were no marked differences in the ratio of plaque area to lumen area, intima-media thickness and the ratio of collagen area to the vascular area between the miR-9 agomir group and the si-OLR1group (all p > 0.05). There were no differences in the ratio of plaque area to lumen area, intima-media thickness and the ratio of collagen area to vascular area among the atherosclerosis, miR-9 agomir NC group and miR-9 antagomir NC groups (all p > 0.05). Therefore, upregulated miR-9 decreased atherosclerotic plaque area and intima-media thickness while enlarging collagen area in mice aorta.
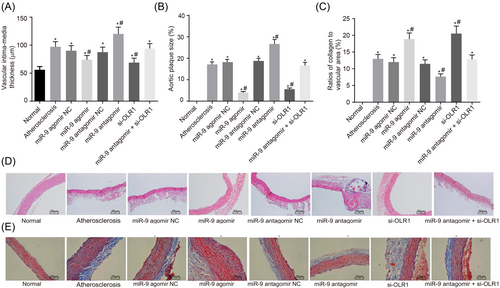
The results of HE and Masson staining show that upregulated miR-9 decreases atherosclerotic plaque area and intima-media thickness while enlarging collagen area in mice aorta. Panel A, intima-media thickness of mice in experimental groups; Panel B, the ratio of plaque area to lumen area of mice in experimental groups; Panel C, the ratio of collagen area to the vascular area of mice in experimental groups; Panel D, HE staining; Panel E, Masson staining. *p < 0.05 versus the control group, #p < 0.05 versus the atherosclerosis group. The data are presented as the mean ± standard deviation, analyzed by one-way ANOVA. The count data were expressed in percentage or rate. n = 10. The experiment was independently repeated three times. ANOVA, analysis of variance; HE, hematoxylin-eosin; miR-9, microRNA 9; NC, negative control; OLR1, oxidized low-density lipoprotein receptor 1
3.6 Upregulated miR-9 inhibits the mRNA expression of OLR1 and the p38MAPK signaling pathway-related genes
RT-qPCR was performed to determine the expressions of miR-9, OLR1, p38MAPK, Bax, and Fas in aortic vascular tissues of every experimental group. The results were as follows (Figure 6): compared with the control group, higher mRNA expression of OLR1, p38MAPK, Bax and Fas was found in the experimental groups (all p < 0.05). There were no significant differences in the mRNA expressions of OLR1, p38MAPK, Bax, and Fas among the atherosclerosis, miR-9 agomir NC and miR-9 antagomir NC groups (all p > 0.05). Compared with the atherosclerosis group, higher miR-9 expression was found in the miR-9 agomir group (p < 0.05), and mRNA levels of OLR1, p38MAPK, Bax, and Fas were decreased significantly (all p < 0.05). Lower miR-9 expression was found in the miR-9 antagomir group (p < 0.05), and mRNA levels of OLR1, p38MAPK, Bax, and Fas were enhanced obviously (p < 0.05). Lower expression of OLR1, p38MAPK, Bax, and Fas was found in the si-OLR1 group (all p < 0.05). Lower miR-9 expression was found in the miR-9 antagomir+si-OLR1 group (p < 0.05), whereas mRNA expressions of OLR1, p38MAPK, Bax, and Fas showed no significant difference (all p > 0.05). The results indicated that upregulated miR-9 could inhibit OLR1 and p38MAPK signaling pathway.
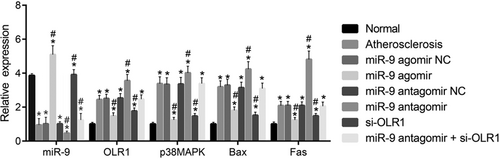
The results of RT-qPCR show that upregulated miR-9 inhibits OLR1 and the p38MAPK signaling pathway. Panel A, mRNA levels of miR-9, OLR1 and p38MAPK in aortic vascular tissues of mice in each group; Panel B, mRNA levels of Bax, Fas, and p53 in aortic vascular tissues of mice in each group. *p < 0.05 versus the control group, #p < 0.05 versus the atherosclerosis group. The data are presented as the mean ± standard deviation, analyzed by one-way ANOVA. n = 10. The experiment was independently repeated three times. ANOVA, analysis of variance; miR-9, microRNA 9; mRNA, messenger RNA; NC, negative control; OLR1, oxidized low-density lipoprotein receptor 1; p38MAPK, p38-mitogen-activated protein kinase; RT-qPCR, reverse transcription quantitative polymerase chain reaction
3.7 Upregulated miR-9 inhibits the protein level of OLR1 and the p38MAPK signaling pathway-related proteins
In addition, several previous studies have demonstrated that the p38MAPK signaling pathway is related to ACS,25-27 and miR-9 could modulate the MAPK signaling pathway.28 These findings revealed that miR-9 could regulate OLR1 and p38MAPK signaling pathway in ACS.
Lastly, Western blot assay was conducted to measure protein levels of OLR1, p38MAPK, Bax, Fas and activated p53, and protein levels were indicated by the ratio of OLR1 to GAPDH, p-p38MAPK to p38MAPK, Bax to GAPDH, Fas to GAPDH and activated p53 to GAPDH. The results are shown in Figure 7: compared with the control group, the protein levels of OLR1, p38MAPK, Bax, Fas and activated p53 in the experimental groups were significantly increased (all p < 0.05). There were no significant differences in protein levels of OLR1, p38MAPK, Bax, Fas, and activated p53 among the atherosclerosis, miR-9 agomir NC, miR-9 antagomir NC and miR-9 antagomir+si-OLR1 groups (all p > 0.05); compared with the atherosclerosis group, protein levels of OLR1, p38MAPK, Bax, Fas, and activated p53 were decreased noticeably in the miR-9 agomir and si-OLR1 groups (all p < 0.05). On the contrary, higher protein levels of OLR1, p38MAPK, Bax, Fas, and activated p53 were found in the miR-9 antagomir group (all p < 0.05). There were no significant differences in protein levels of OLR1, p38MAPK, Bax, Fas, and activated p53 between the miR-9 agomir group and the si-OLR1 group (all p > 0.05). The above results indicated that upregulated miR-9 inhibits the OLR1 and p38MAPK signaling pathway.
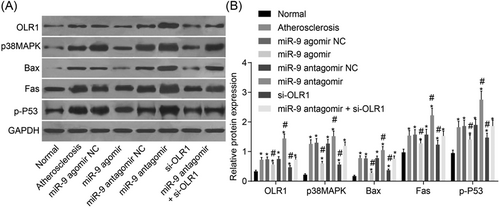
The results of Western blot assay show that upregulated miR-9 inhibits OLR1 and the p38MAPK signaling pathway. Panel A, electrophoresis strips of protein levels of OLR1, p38MAPK, Bax, Fas, and p35 in aortic vascular tissues of mice in each group; Panel B, protein levels of OLR1, p38MAPK, Bax, Fas, and p53 in the aortic vascular tissues of mice in each group; compared with the control group. *p < 0.05 versus the control group, #p < 0.05 verus the atherosclerosis group. The data are presented as mean ± standard deviation, analyzed by one-way ANOVA. n = 10. The experiment was independently repeated three times. ANOVA, analysis of variance; miR-9, microRNA 9; NC, negative control; OLR1, oxidized low-density lipoprotein receptor 1; p38MAPK, p38-mitogen-activated protein kinase
4 DISCUSSION
Currently, precise molecular and cellular triggers that induce ACS still remain vague though several mechanisms regarding the sudden onset and development of ACS have been discovered, of which the most common is vulnerable plaque disruption featuring a large, fibrous cap-covered necrotic core.29 In this study, we aim to discuss whether miR-9 could serve as a biomarker for the treatment and diagnosis of patients with ACS and its effects on OLR1 gene and the p38MAPK pathway. Consequently, this study demonstrates inhibited vulnerable plaque and enhanced vascular remodeling by upregulating miR-9 in ACS via the activation of the OLR1-mediated p38MAPK pathway.
In the current study, remarkably lower serum levels of TG, TC, LDL-C, TNF-α, IL-6, VEGF and noticeably higher HDL-C level were found in miR-9 agomir and si-OLR1, suggesting that overexpression of miR-9 can ameliorate cardiovascular disease by lowering serum lipid profiles, restraining inflammatory responses, and promoting vascular remodeling in atherosclerosis mouse models. Similarly, previous work has demonstrated that miRs are aberrantly expressed in cancer and act functionally as “tumor suppressor genes” or “oncogenes” in tumorigenesis.30 Under the regulation of DNA methylation, miR-9 could play a role in tumor suppression in many cancers.31 Another study has found that miR-9 plays a tumor-suppressive role in nasopharyngeal carcinoma, and that its suppressive functions are realized by inhibiting CXCR4 expression.32 All these previous studies have indicated the importance of miR-9 in disease management. Joseph et al33 have demonstrated that CVD can be prevented through low-density LDL-C reduction with statins, which have a promising prospects for addressing CVD risk reduction. Another work has also pointed out an inverse correlation between HDL-C levels and cardiovascular disease risk through prospective epidemiological studies.34 These studies verify the fact that low-density LDL-C for cardiovascular management is feasible. CVD is widely recognized as a chronic inflammatory disease proceeding in the context of enhanced plasma lipid levels, and some inflammatory mediators play an important role in the CVD management for inflammatory responses are correlated with the pathogenesis of CVD.35-37 As Wang et al38 revealed, miR-9 could alleviate atherosclerosis-related inflammation with the involvement of the JAK1/STAT1 pathway. In line with our results, Chakraborty et al39 have found that overexpressed miR-9 inhibits NF-κB expression, and thereby suppresses inflammation while promoting lymphatic endothelial cells tube formation, the expression of the prolymphangiogenic molecules endothelial nitric oxide synthase and VEGF receptor type 3. Another study has shown that endothelial cell migration and angiogenesis are promoted by tumor-secreted miR-9 by the activation of the JAK-STAT pathway.40 All these studies have elucidated miR-9 could be a therapeutic target for cardiovascular diseases.
In addition, we have found that p38MAPK, Bax, and Fas, levels were reduced after exposure to agonists and si-RNAs, suggesting that upregulated miR-9 inhibits the OLR1 and p38MAPK pathway. A previous study has shown that OLR1 exerts great influence on the degradation of oxidized low density lipoprotein.41 Moreover, gene expression profile of a cell could be greatly affected by post-transcriptional processing of mRNA transcripts.42 Therefore, we have searched for miRs that target ORL1, and the biological prediction website microRNA.org (http://www.microrna.org) shows that OLR1 is a putative target gene of miR-9. In our study, after overexpressing of miR-9 in atherosclerosis mouse models, we confirmed the direct inhibition of OLR1 translation through miR-9 by experiment. As previously reported, overexpression of OLR1 may enhance proliferation, migration, and inhibition of apoptosis by activation of NF-kB target genes, acting as an oncogene.43 According to Ben-Hamo et al,28 metastasis is regulated by hsa-miR-9, which serves as a therapeutic target, and prognosis in glioblastoma multiforme was thus determined with the involvement of the p38MAPK pathway. Consistently, Liu et al44 has demonstrated that, cell motility is obviously enhanced by the overexpression of HPV-induced miR-9 through downregulating a great many gene targets in cell migration. Previous study has also found that miR-9-3 is involved in the apoptotic signaling pathway related to p53, and miR-based therapies are a feasible way for the treatment of breast cancer.45
5 CONCLUSION
Taken as a whole, as shown in Figure 8, our data provide evidence that overexpression of miR-9 can significantly ameliorate cardiovascular disease by inhibiting lipid profiles, reducing inflammatory response and promoting vascular remodeling through the activation of the OLR1-dependent p38MAPK pathway in atherosclerosis mouse models. We suggest that the miR-9 could serve as a potential therapeutic target for ACS. By expanding the sample size, further research on more detailed mechanisms of miR-9 in ACS will be analyzed.
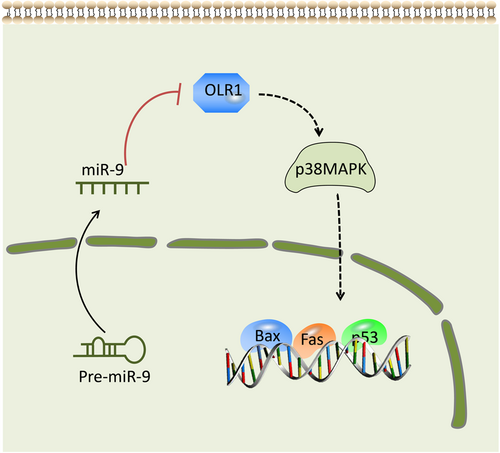
Schematic diagram of the miR-9-OLR1-p38MAPK axis in ACS. The overexpression of miR-9 weakens the ability of OLR1 to enhance the dendritic cell-mediated immune response by reducing Bax, Fas, and p35 levels via a negative feedback regulation of the p38MAPK pathway, which further suppresses vulnerable atherosclerotic plaque and enhances vascular remodeling in ACS. ACS, acute coronary syndrome; miR-9, microRNA 9; OLR1, oxidized low-density lipoprotein receptor 1; p38MAPK, p38-mitogen-activated protein kinase
ACKNOWLEDGMENTS
This study was supported by National Natural Science Foundation of China (No. 81460550), Hainan Province Key R&D Project (No. ZDYF2018154), Hainan Natural Science Foundation (No. 818QN247) and The Foundation for Fostering of Hainan Medical College (No. HY2016-03).
CONFLICTS OF INTEREST
The authors have declared that no conflicts of interest exist.