Combinatorial approach of cannabidiol and active-targeted-mediated photodynamic therapy in malignant melanoma treatment
Abstract
Malignant melanoma (MM) continues to claim millions of lives around the world due to its limited therapeutic alternatives. Photodynamic therapy (PDT) has gained popularity in cancer treatment due it increased potency and low off-target toxicity. Studies have pointed out that the heterogeneity of MM tumours reduces the efficacy of current therapeutic approaches, including PDT, leading to high chances of recurrences post-treatment. Accumulating evidence suggests that cannabidiol (CBD), a non-psychoactive derivative of cannabis, can synergise with various anticancer agents to increase their efficacy. However, CBD demonstrates low bioavailability, which is attributed to factors relating to poor water compatibility, poor absorption and rapid metabolism. Nanotechnology offers tools that address these issues and enhance the biological efficiency and targeted specificity of anticancer agents. Herein, we highlighted the standard therapeutic modalities of MM and their pitfalls, as well as pointed out the need for further investigation into PDT combination therapy with CBD.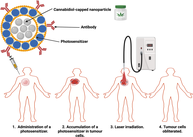
1 INTRODUCTION
Cancer is a group of maladies characterised by alterations in the mechanisms that promote normal cell growth, differentiation and the eradication of transformed cells [1]. It is responsible for alarming mortality rates and grim life expectancy globally [2]. Recent data indicates that over 19 million new cancer cases were reported, which led to roughly 10 million mortalities in 2020 [3]. These alarming incidence rates of cancer worldwide that lead to millions of fatalities annually translate to a dire need for developing efficacious regimens for treating various cancers [4]. Cancer development is attributable to various factors, such as exposure to carcinogens in the environment, internal stress and inheritance [3, 4]. The aforementioned factors vary from patient to patient and hinge on the type of malignancy and geographical location [3].
Skin cancer is the most common type of cancer and has been on the rise in recent years [5]. Skin cancers are distinguished and named based on the cell from which they are derived as well as their clinical hallmarks [6]. They are typically classified into melanoma and non-melanoma skin cancer (NMSC). NMSC entails a group of skin cancers that emerge from cells besides melanocytes, which are cells that produce melanin [6]. The two most common types of NMSC are basal cell carcinoma and squamous cell carcinoma, which all originate from keratinocytes [7]. Melanoma is the most abominable form of skin cancer acquired from mutated melanocytes. In contrast to NMSC, the chances of melanoma generation are closely related to prolonged sun exposure during the teenage years, particularly the occurrence of sunburns between the ages of 15–20 [8]. Fitzpatrick skin type, chemical exposure, tanning bed use, human papillomavirus, family history, melanocytic nevi, and immunosuppressive status are additional risk factors linked to the development of cutaneous malignancies [9].
Melanoma staging criteria hinge on their degree of invasion and dissemination to lymph nodes and other adjacent normal tissues [10]. Stage I and II melanomas exhibit no lymph node infiltration or metastatic properties and are differentiated based on their likelihood of recurrence [10]. Stage IV melanoma, also referred to as metastatic melanoma, is a severe phase of skin cancer whereby cancer cells have acquired metastatic properties and have disseminated from their initial site to other parts of the body. Therefore, early diagnosis remains imperative for a better prognosis because advanced stages are unresponsive, resulting in alarming mortality rates [11]. Studies have shown that, despite recent advances in cancer therapeutic approaches, the incidence of skin cancer is still rising at an alarming rate [12]. Hence, the grim prognosis and mortality rates of malignant melanoma (MM) remain a cause for concern in healthcare worldwide [13]. Therefore, it remains imperative to investigate non-invasive yet efficacious cancer therapies such as cannabidiol-derived nanoparticles (NPs) and photodynamic therapy (PDT) administered as combination treatments to eradicate any drug-resistant MM cells and prevent tumour recurrence post-treatment.
2 CONVENTIONAL THERAPEUTIC APPROACHES FOR MM
Conventional interventions for melanoma entail a wide range of therapeutic options, and the choice of treatment relies on factors such as the severity of the melanoma, its location and the overall health of the patient [11]. Surgery is the quintessential treatment for early-stage melanoma, which is based on the removal of the tumorous tissue along with a margin of normal tissue adjacent to it. In cases where the sentinel lymph nodes are involved, the lymph nodes in the area are sometimes surgically removed [14]. Although chemotherapeutics are less effective for melanomas, chemotherapy is typically used in the treatment of advanced melanoma. Moreover, chemotherapy combinations have been investigated to enhance clinical outcomes but have rarely yielded improved patients' overall survival rates [11]. This is attributed to their inability to trigger apoptosis, which is essentially responsible for chemotherapy drug failure in melanoma [14]. Despite being superseded by additional alternatives, chemotherapy remains indispensable in the palliative treatment of advanced, refractory and secondary melanoma tumours [11, 15].
Radiotherapy is another treatment modality for melanoma that exploits high-energy x-rays or other forms of radiation to target and obliterate cancer cells [16]. This therapy is ladened with unwanted side effects such as dermatological complications, nausea, fatigue as well as off-target toxicity [17]. Based on the stage and condition of the patient, radiation therapy may be complemented with chemotherapy, but their combined effect often causes harsher side effects [18]. Recent advances in immunotherapy and targeted therapy have prolonged patients' life spans. Targeting CTLA-4 or PD-1/PD-L1 holds great promise for the eradication of melanoma and other malignancies. Unfortunately, the aforementioned therapies are not entirely efficient for all patients [19]. Still, treatment failure and undesirable immunological reactions significantly hamper clinical outcomes [20]. Therefore, it remains imperative to develop new therapies to overcome issues pertaining to conventional modalities and enhance the prognosis of melanoma [21, 22]. Recent studies have highlighted that PDT in combination with nanotechnology has paved new avenues for alleviating drug resistance and improving the overall treatment outcomes of melanoma [23].
3 PHOTODYNAMIC THERAPY
PDT represents an underutilised phototherapeutic technique that incorporates two essentials to exert robust oxidative stress in targeted tissues, intended for the obliteration of solid tumours [24]. One of the essentials is a specific wavelength of light, while another one is a photosensitizer (PS), an agent that transforms light energy into chemical activity. Following light absorption, the PS converts into a singlet excited state and, eventually, further turns into a fairly stable energetic state referred to as the excited triplet state via intersystem crossing [25]. The activated PS directly interacts with cellular molecules, such as lipids, to yield cytotoxic free radicals that annihilate cellular structures (type I reaction). In a Type II reaction, the activated PS transfers energy to molecular oxygen to form highly reactive singlet oxygen [26]. The events are illustrated in Figure 1.
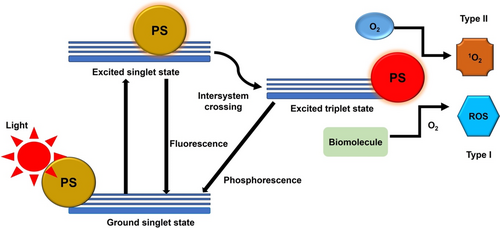
PDT can trigger several modes of cell death, depending on factors such as the type of PS, light intensity and PS accumulation in the specific cellular organelle. Apoptosis is a regulated mode of cell death epitomised by coordinated and systematic destruction of cells [27]. It is often the most desired mode of cell death in cancer treatment. During PDT treatment, the high affinity of PSs for the mitochondria and endoplasmic reticulum can exert oxidative stress and ultimately apoptotic cell death [27]. Therefore, PDT can elicit apoptosis in melanoma cells and counteract their proliferation and metastatic potential [13]. Necrosis is a more austere and uncontrolled form of cell death mechanism as compared to apoptosis. It is normally inflicted by high doses of PDT, which causes cell swelling, organelle mutilation, and the release of cellular contents into the surrounding environment, promoting inflammation [28].
Autophagy is a multifaceted process in which damaged or redundant cellular contents are degraded and recycled [29]. It may contribute to cell rejuvenation or cell death, depending on the context. In response to PDT-induced stress, cells may evoke an autophagic cell survival mechanism, making autophagy an undesirable cell death mode. However, excessive autophagy can promote cell death [13]. Lastly, immunogenic cell death (ICD) is a type of cell death that elicits immunogenic reactions against damaged cells. PDT-induced ICD can evoke the release of dangerous stimuli as well as the infiltration of immune cells, facilitating the anticancer immune response [30].
3.1 PSs for MM
PSs are specialised photoactive chemical compounds that are employed in PDT and other light-based treatment modalities [31]. They are capable of absorbing specific wavelengths of light and transferring that energy to adjacent molecules. This process yields reactive oxygen species (ROS) or other cytotoxic molecules, which can selectively eradicate cancer cells and spare healthy tissues [32]. The ideal PS for PDT applications should be endowed with several key properties to boost its efficiency in selective targeting and obliteration of cancerous cells [26]. In view of these, a PS should exhibit a high affinity for diseased tissues. This selective uptake allows for the intensification of the PDT effect on the desired area while mitigating damage to healthy adjacent tissue. It should effectively absorb light within the therapeutic window (600–800 nm) to allow for a sufficient amount of energy to activate and generate ROS [33]. A PS should be non-toxic in the absence of light exposure and should not elicit any immune system responses but exhibit a repaid clearance from normal tissues [33]. Furthermore, a desirable PS should allow for modification with targeting agents and also remain stable when subjected to physiological conditions [34]. Lastly, it should exhibit good tissue penetration in order to treat deep-seated target cells [26].
To date, PSs used in PDT can be classified based on their historical development, chemical structure and origin [35, 36]. First-generation PSs include hematoporphyrin derivatives (HpD) [37]. These PSs presented several drawbacks pertaining to poor localization specificity, poor water compatibility, unwanted skin hypersensitivity and superficial tumour penetration. These issues led to the development of second-generation PSs, which are derived from curcumin, chlorins, bacteriochlorins, phthalocyanine, methylene blue (MB) derivatives, benzoporphyrins and many others. These are pure, single-molecule compounds with a high singlet oxygen quantum yield and strong absorption in the visible-near-infrared region (NIR) of the electromagnetic spectrum [37, 38].
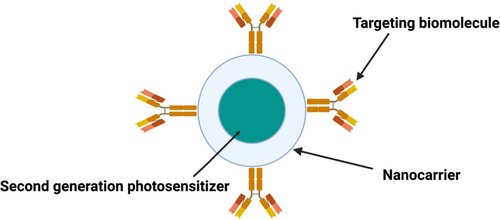
Despite all their positive attributes, second-generation PSs intrinsically exhibit poor water compatibility and off-target phototoxicity effects, which stifle their approval for clinical applications [34]. To overcome these hurdles, second-generation PSs are superseded by third-generation PSs, which are composed of second-generation PSs modified with NPs or targeting molecules such as peptides, antibodies, folic acid and carbohydrates (Figure 2) [39]. Table 1 summarises various PSs that have shown anticancer effects against melanoma cells.
| PS | Cell culture model | Generation | Reference | |
|---|---|---|---|---|
| Photofrin II | In vitro | 1st | Melanoma cells incubated with 15 and 30 μg/mL and exposed to a 632.8 nm laser with a power intensity of 10 mW/cm2 revealed 71.9% and 90% apoptotic cell populations, respectively | [40] |
| 5-aminolevulinic acid (ALA) | In vitro In vitro In vitro In vivo |
2nd | A375 and A431 melanoma cells treated with 0.6 mM ALA and exposed to 643 nm with a power intensity of 14.5 mW/cm2 demonstrated 90% and 61.5% apoptotic rates, respectively | [41] |
| Chlorin e6 (Ce6) | Ce6 at a concentration of 1.2 μM and 650 nm laser irradiation at a power intensity of 18 mW/cm2 reduced the cell viability of melanoma cells to 22.5% | [42] | ||
| Methylene blue (MB) | 2 μg/mL MB and 660 nm laser at a power output of 100 mW/cm2 decreased cell viability via induction of necrosis and apoptosis | [43] | ||
| Bacteriochlorin | 1 μM and 730 nm at 120 J/cm2 could kill 98% of B16F10 cells |
[44] | ||
| Si(i.v.)-naphthalocyanine (isoBO-SiNc) | isoBO-SiNc with photoactivation with 1064 nm at 520 J/cm2 led to a 16 day delay of tumour growth | [45] | ||
| Zinc octacarboxypthalocyanine (ZnPcOC) | 30 μM ZnPcoC and a 685 nm laser with a fluence of 2.5 J/cm2 obliterated melanoma cells via an apoptotic pathway activated by ROS | [46] | ||
| Aluminium chloride phthalocyanine (ClAlPc) modified with solid lipid nanoparticles (SLN) | In vitro In vitro and in vivo |
3rd | 0.75 μg/mL CIAIPc-SLN activated with 670 nm at power output of 0.30 mW showed an outstanding phototoxic effect on melanoma compared to free CIAIPc | [47] |
| Indocyanine green (ICG) modified with chitosan-coated liposomes | 40 μM ICG-chitosan-coated liposomes photoactivated with a 785 nm laser at a power intensity of 100 mW/cm2 showed a significantly increased cellular uptake and inhibitory effect when compared to free ICG | [48] | ||
| Verteporfin (Ver) modified with silica nanoparticles (MSNs) | In vitro and in vivo In vitro |
0.1–10 μg/mL Ver-MSNs irradiated with 693 nm significantly inhibited the growth of melanoma cells in vitro, whereas 5 μg/mL Ver-MSNs reduced tumour mass by 50% in vivo | [49] | |
| Ferrous chlorophyllin (Fe-CHL) modified with PLGA nanoparticles and cRGDyk peptide | 12 mM Fe-CHL-PLGA-cRGDyk stimulated with a 652 nm laser at a power output of 120 mW/cm2 demonstrated excellent bioavailability and photocytotoxicity compared to free Fe-CHL | [32] | ||
| Methylene blue (MB) enhanced with naproxen amides (NAPs) | In vitro | 5 μM MB-NAPs photoactivated with a 660 nm laser at 0.1 mW/cm2 exerted significant inhibitory and antiproliferative effects on melanoma cells expressing the melanocortin-1 (MC1) receptor, leaving a staggery of 4% live cells. | [50] |
3.2 Shortcomings of photodynamic therapy in malignant melanoma treatment
Several lines of evidence have extolled the efficiency of PDT in the treatment of certain types of cancer, but it has limitations when it comes to treating melanoma [51]. Hypoxic melanoma tissues continue to pose a formidable obstacle to the PDT treatment of melanoma because oxygen is an essential component of the process [52]. Light is another fundamental indispensable for PDT initiation, and therefore it should efficiently evade biological checkpoints without any restrictions and activate a PS retained in cancer cells. Biological tissues fail to absorb high wavelengths of light ranging from 650 to 800 nm, so this region is known as an optical therapeutic window [12]. The dynamics, however, differ in melanoma because of its high melanin content. Studies suggest that melanin accelerates melanoma unresponsiveness to PDT by counteracting light from reaching targeted tumour regions [53, 54]. Melanin is able to absorb wavelengths of light ranging from 400 to 750, which makes it a photoprotectant that occupies almost the entire therapeutic window region [12]. Furthermore, studies have highlighted that melanin attenuates oxidation stress by donating electrons to stabilise free radicals and prevent cellular destruction, making it an antioxidant and ROS scavenger [55].
Another challenge faced by PDT in melanoma treatment is the ability of melanosomes to shield melanocytes and melanoma cells from anticancer agents. Melanosomes absorb toxins and prevent them from reaching the cytoplasm [56, 57]. Therefore, these melanosomes remain a cause for concern for PDT and contribute to the high resistance of pigmented melanoma compared to non-pigmented melanoma [58]. The ATP-binding cassette (ABC) transporters found on both the cell membrane and the membrane of subcellular organelles play a key role in transporting diverse molecules across biological membranes and are implicated in multidrug resistance since they facilitate the efflux of anticancer drug [59]. For example, δ-aminolevulinic acid (ALA), a commonly used PS in clinical settings, promotes the production of the PS protoporphyrin (PPIX). PPIX is a precursor to heme, synthesised in cells and essential for typical cellular function. Nevertheless, an overabundance of PPIX can be detrimental and phototoxic to cells. The ABCG2 transporter heme efflux system plays a crucial role in pumping out the excess PPIX. However, in addition to regulating cellular functions, ABCG2 mediates the cancer cell's resistance to PDT and other drugs [60]. Lastly, other challenges of PDT in melanoma treatment include common limitations pertaining to PS, poor water solubility and bioavailability, off-target toxicity, premature degradation by biological barriers, as well as the inability to treat deep-rooted tumours, which all drive PDT resistance [61]. Nanotechnology and combination therapy have attracted a great deal of interest in cancer treatment due to their ability to address concerns relating to therapy resistance, poor drug solubility and delivery [62].
4 NANOTECHNOLOGY
Nanotechnology is the design and application of technologies on a scale of 1–100 nm. Nanomedicine refers to the application of nanotechnology in the paradigm of medicine to revolutionise healthcare systems. NPs are endowed with exceptional physical, chemical or biological features that provide tools for engineering modalities for the diagnosis and treatment of life-threatening maladies [63]. NPs are intrinsically hydrophilic, and this property allows them to improve the water compatibility of anticancer agents, including PSs, which ultimately improves their cellular uptake. They allow multiple drug loadings due to their huge surface area-to-volume ratio. Since NPs are small in dimensions, they effortlessly penetrate tumour tissues via enhanced permeability and retention effect (EPR) [64].
This phenomenon is attributed to the unique characteristics of tumour vasculature, whereby tumour blood vessels are impaired and have abnormal structures, including irregular diameters and leaky junctions between endothelial cells lining vessels. Furthermore, NPs coordinate the release of drugs and prevent their premature release and off-target toxicity. NPs are able to evade enzymatic degradation due to their ability to simulate biological matter, and therefore, the modification of PSs with NPs helps them to bypass biological barriers in vivo, which improves their half-life [65]. The ability of NPs to load various drugs and targeting agents for multiple functions, along with their high biocompatibility and flexible surface chemistry, make them an ideal candidate for PDT applications [63]. Table 2 summarises NPs used as drug nanocarrier platforms and therapeutic agents.
| Nanocarrier | Excitation | Application | Advantages | References |
|---|---|---|---|---|
| Gold nanoparticles (AuNPs) | 520 nm | Drug delivery, imaging, photothermal/photodynamic therapy bioimaging and biosensing | Biocompatible, remarkable stability, easy surface modifications, tunable optical properties and strong absorption in NIR region | [66, 67] |
| Silver nanoparticle (AgNPs) | 239–314 nm | Drug delivery, biosensors, food preservation, cosmetics, antimicrobial, anti-inflammatory and anti-diabetic agent | Excellent optical properties, remarkable activities ease of synthesis, high loading capacity outstanding stability and catalytic | [68-70] |
| Upconversion nanoparticles (UPNPs) | 980 nm | Bioimaging and drug delivery | Biocompatible, great optical, photothermal theranostic and mechanical properties, deeper penetration depth and high stable under biological conditions | [71] |
| Mesoporous silica nanoparticles (MSNs) | 804–815 nm | Drug and gene delivery, biomedical imaging and biosensing | Great biocompatibility, easy surface functionalization, chemical stability and tunable size | [68, 72, 73] |
| Liposomes | - | Drug delivery | Biodegradable, biocompatible, mimic immunogenic compounds, non-toxic and can encapsulate hydrophobic and hydrophilic agents | [74] |
| Micelles | 420 nm | Drug delivery, skin and eye treatment | Biocompatible, biodegradable, ability to solubilise hydrophobic drugs and coordinates drug release | [75, 76] |
| Nanorods | 700–800 nm | Drug delivery, electronic sensing devises and biomedical imaging | Excellent optical properties, remarkable biosafety and biocompatibility | [77] |
| Quantum dots | 800 nm | Drug delivery, cancer therapy, live imaging and diagnosis | Strong light absorption and emission, great photostability, tunable optical properties and increased tumour accumulation | [78] |
| Poly(lactic acid) nanoparticles (PLA NP) | 202–204 nm | Drug delivery | Remarkable safety profile, excellent biocompatibility and biodegradability, negligible immunogenicity and cytotoxicity | [79] |
- Abbreviation: NIR, near-infrared region.
5 CANNABIDIOL AND MELANOMA
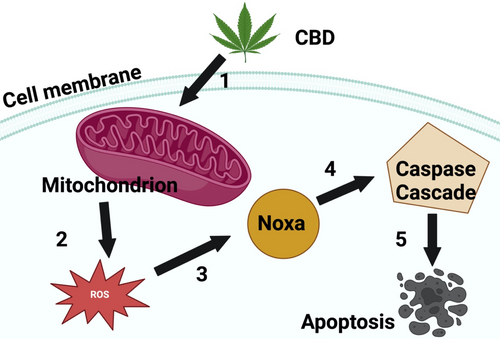
Cannabidiol (CBD) is an abundant non-psychoactive cannabinoid from Cannabis sativa plants [80]. It has attracted a great deal of attention in research due to its broad therapeutic properties and minimal side effects [81]. Studies have stated that CBD is potent against numerous disorders such as neurodegenerative condition, inflammation, autoimmune disorders and cancer [82]. More importantly, CBD exerted antiproliferative effects via induction of autophagy and apoptosis and also has the ability to counteract angiogenic metastatic properties [83]. Studies by Simmerman et al. investigated the effects of CBD on murine melanoma model (B16F10) [84]. Simmerman et al. revealed that CBD prolonged the survival time and significantly reduced tumour size when compared to untreated control mice. Studies by Burch et al 2021 reported that CBD significantly inhibited melanoma cell growth in vitro following treatment with 0.2 and 0.04 mg/mL [85]. Jeong and colleagues screened the anticancer effects of CBD on colorectal cancer cells in vitro [86]. Jeong and coworkers revealed that CBD obliterated cancer cells via the induction of Noxa and ROS. Their research demonstrated that CBD stimulated Noxa by causing oxidative stress to the mitochondria and endoplasmic reticulum. The induction of Noxa subsequently activated the caspase cascade, ultimately resulting in an apoptotic cell death mechanism (Figure 3). Jeong and colleagues concluded that CBD holds great promise as a potent anticancer agent. Studies performed by Nkune and colleagues screened the therapeutic efficacy of the combined therapy of CBD and PDT on melanoma and colon cancer cells [87, 88]. Their findings demonstrated that combination therapy was superior in the eradication of cancer cells compared to single treatments.
5.1 Green synthesised CBD metallic NPs
Green nanotechnology is a domain of green technology that incorporates the concepts of green chemistry and green engineering, where the word ‘green’ symbolises the use of plant extracts [89]. It aims to harness the potential of nanomaterials and processes while mitigating their unwanted impacts on the environment and human health [68]. Green nanotechnology incorporates the principles of sustainability and green chemistry with nanoscale science and engineering to tackle various societal and environmental issues [90]. Plants are used in nanoparticle formulations because they are readily available and contain a wide range of metabolites such as vitamins, antioxidants and nucleotide. For example, gold nanoparticles (AuNPs) have garnered significant attention due to their highly modifiable size, shape and surface chemistry.
A number of copper (Cu) and copper oxide (CuO) NPs have also been derived from medicinal plants. Furthermore, prominent metal oxide NPs such as titanium dioxide and zinc oxide have also been derived from several plant extracts [89]. In recent years, CBD has been intensely investigated due to its lack of psychoactive effects and wide range of pharmacological properties. However, poor stability, erratic pharmacokinetics, poor solubility and bioavailability of CBD limit its therapeutic efficacy [91]. Therefore, NPs derived from CBD can potentially enhance its bioavailability and tumour selectivity. This is attributed to the fact that NPs are small in size and have a large surface area-to-volume ratio, which allows for better absorption and distribution within the body [92]. CBD-derived NPs confer a stable delivery system for the compound, protecting it from degradation and prolonging its half-life in vivo. Studies conducted by Josiah and co-workers synthesised AuNPs and silver nanoparticles (AgNPs) from CBD and tested their safety profiles on human keratinocytes. The NPs were spherically shaped and monodispersed, with average diameters of 8.4 and 4.8 nm for Au-CBD and Ag-CBD, respectively. Josiah and colleagues noted that both NPs had negligible effects on keratinocytes, suggesting that they can be used for a wide range of applications, including drug delivery and pharmaceutical purposes [93].
5.2 Synthesis and characterization of green synthesised metallic NPs
NPs are miniscule particles with sizes ranging from 1 to 100 nm, and their unique properties, such as their small size and large surface area, have garnered attention in various applications such as electronics, optics, anticancer and antimicrobial activities, medicine, pollution remediation, anticancer and antimicrobial activities [94]. There are various techniques used to synthesise NPs. Chemical reduction is a widely used technique for synthesising NPs that relies on reducing agents such as sodium hydroxide or borohydride to transform metal ions into NPs. This technique is typically used to generate inorganic NPs such as silver and gold [95]. Another technique used in the synthesis of NPs is sol–gel, which involves combining a metal salt with a solvent and the gel. The solvent is eliminated through evaporation, sparing the gel. The gel is then subjected to heating to solidify and yield NPs [96].
Microemulsion is a technique that requires hydrophilic compounds, surfactants and oil-soluble compounds. The contents form small droplets composed of metal ions. Upon exposure to heat, metal ions precipitate out of the solution, resulting in the formation of NPs [97]. The solvothermal/hydrothermal technique is based on heating metal ions solubilised in water or in an organic solvent subjected to high pressure. The high pressure and heating allow for metal ions to be precipitated out of solution to form NPs [98]. Sonochemical/electrochemical technique employs ultrasound or an electric current to convert metal salts into NPs [99]. Moreover, there are physical techniques such as sputtering, laser ablation and milling, whereby compounds are reduced to NPs after being subjected to the mechanical action of the instrument used [100]. After the formation of NPs, vast characterisation methods are used to validate their characteristics in terms of size, shape, crystallinity and chemical makeup. Quintessential methods used in nanotechnology include transmission electron microscopy, Fourier-transform infrared spectroscopy, x-ray diffraction, and scanning electron microscopy [101].
6 THE ANTICANCER EFFECTS OF GREEN SYNTHESISED METALLIC NPs
Cannabinoids are typically known to be hydrophobic in nature, with poor water compatibility, but highly soluble in organic solvents. This poor water compatibility significantly hampers the bioavailability of cannabinoids and poses a threat to their therapeutic efficacy. Nanonisation is a facile and efficient technique to enhance the dissolution and cellular uptake rates of these drugs [102]. Green synthesised NPs, such as AgNPs, have shown significant cytotoxic effects on mammalian cells via diverse mechanisms, such as the generation of ROS, destruction of the cell membrane, which is attributed to the direct interaction with AgNPs, loss of DNA replication, impairment of cellular-dependent energy processes due to free silver ion uptake [103], and induction of apoptosis [104].
Recent studies by Korkmaz et al. synthesised CeO2 metal oxide NPs from the leaves of C. sativa L. These green synthesised NPs exhibited significant antiproliferative effects on breast cancer cells with negligible effects on normal cells [105]. Chang and colleagues synthesised AuNPs from C. sativa leaf extract and tested their efficacy on leukaemia cell lines. The study revealed that the NPs exhibited favourable inhibitory effects against lymphoblastic leukaemia and acute T-cell leukaemia cells without any detrimental effects on normal cells, suggesting a potent chemotherapeutic effect [106]. Another study by Yaseen and colleagues investigated the anticancer effects of palladium NPs derived from C. sativa leaf extract on lung cancer cells. The NPs proved to have potent effects on lung cancer cells, suggesting a capable anticarcinogenic agent [107]. Various NPs derived from different medicinal plants have exhibited anticancer effects on melanoma cells (Table 3).
| Plant | Nanoparticle | Effects | Reference |
|---|---|---|---|
| Siberain ginseng (SG) | Gold nanoparticles (AuNPs) | SG-AuNPs induced apoptosis in in vitro-cultured B16 murine melanoma cells | [108] |
| Lemongrass | Gold nanoparticles (AuNPs) | AuNPs demonstrated both pro-apoptotic and antiproliferative effects towards in vitro-cultured melanoma cells (A375 cell line) | [109] |
| Madhuca longifolia | Gold nanoparticles (AuNP) | The biosynthesised AuNPs exhibited anti-melanoma bioefficacy against two melanoma cells lines (B16F10 and A375) via production of cytotoxic ROS, release of nitric oxide and stimulation caspase-3 activities | [110] |
| Trapa natans | Silver nanoparticles (AgNPs) | Green synthesised AgNPs exhibited significant cytotoxicity against human skin cells (A431 cell line) | [111] |
| Plectranthus ambionicus | Silver nanoparticles (AgNPs) | The biosynthesised AgNPs showed potent inhibitory effects on B16F10 murine melanoma cells | [112] |
| Aloe vera | Silver nanoparticles (AgNPs) | AgNPs showed 100% inhibition against mouse melanoma cells (F10B16) | [113] |
| Moringa oleifera | Silver nanoparticles (AgNPs) | AgNPs significantly inhibited melanoma (A375) cells in a dose-dependent manner | [114] |
| Padina tetrastromatica | Silver nanoparticles (AgNPs) | Green synthesised AgNPs exhibited potent cytotoxicity against in vitro-cultured B16F10 and significant tumour regression in vivo models | [115] |
7 ENCAPSULATION AND FUNCTIONALISATION OF GREEN SYNTHESISED METALLIC NPs
Encapsulation is one of the most widely used drug delivery approaches used by researchers in drug screening and development. This method involves enclosing drugs within a shell-like protective layer aimed at protection, controlled release or functionalisation, as well as preventing off-target toxicity of the delivered drugs [116]. Although, various NPs mentioned in Table 2 are endowed with essential pharmaceutical properties ranging from physicochemical properties, NP size and medical applications such as drugs carrier systems, bioimaging, diagnostic and therapeutic. However, the target-selectivity, biocompatibility, pharmacokinetic behaviour and toxicity of most nanomaterials highlighted in Table 2 are still ambiguous.
In recent years, liposomes have garnered significant attention in drug delivery quest because they are highly biocompatible and biodegradable with negligible of toxicity on normal tissues. Liposomes are round-shaped lipid vesicles made up of one or more lipid bilayers surrounding an aqueous core. Their highly modifiable surface chemistry allows for functionalisation with targeting moieties such as peptides, antibodies, vitamins, carbohydrates and nucleic acids [117]. Liposomes have the ability to accommodate hydrophilic agents (nanoparticles) in their aqueous core and hydrophobic drugs (PSs) within their lipid bilayers allowing for dual drug delivery [73]. Yurttas and colleagues loaded phthalocyanine PS into liposomes and tested the therapeutic efficacy of this nanoformulation against breast cancer cells. In vitro studies showed that the PS-loaded liposomes considerably improved the cellular uptake load in cancer cells compared to free PS. As a result, the inhibitory effect of the liposomes was significantly greater than that of the free PS because of their higher intracellular ROS generation [118].
Lakshmi and colleagues successfully synthesised ruthenium and curcumin-based NPs and loaded them into liposomes to overcome off-target toxicity and poor water solubility. This nanoformulation showed significant anticancer effects on cancer cells, with prominent nuclear damage [119]. Aparicio-Blanco and colleagues incorporated CBD in lipid nanocapsules to overcome the dose dependence and poor bioavailability associated with cannabinoids [120]. The study revealed that the encapsulated CBD demonstrated a significant inhibitory effect, with a 3.4-fold increase in targeting in vitro the human glioblastoma cell line U373MG compared to free CBD. Fraguas-Sánchez et al. encapsulated CBD into poly-lactic-co-glycolic acid (PLGA) NPs [121]. CBD-NPs exhibited improved antiproliferative activity than free CBD and increased internalisation in SKOV-3 epithelial ovarian cancer cells. NPs cannot exclusively differentiate between malignant and healthy tissues and, to some extent, exhibit off-target cytotoxicity [39]. To circumvent limitation related to bare NPs such as high costs, poor safety and tolerability profiles, there are various functionalisation strategies adopted to improve nanocarrier systems [122].
Targeting entities, such as antibodies, is the most recommended and effective approach for active-targeted drug delivery. Functionalisation of NPs with targeting moieties that have high affinity for receptors overexpressed by cancer cells allows therapeutic components to selectively distribute to targeted tumour sites. Conceptually, immobilisation of antibodies onto the surface of the liposomes carrying NPs involves designing targeting moieties modified with linking molecules (e.g., peptide linkers) [117, 123]. These targeting moieties (monoclonal antibodies, protein fragments, carbohydrates, folic acid, etc.) have high affinity for extracellular receptors overexpressed by the surface of cancer cells [124]. In relation to melanoma, studies have stated that melanoma inhibitory activity (MIA) antigen is universally overexpressed by early stage and advance melanomas but not by normal melanocytes [125]. Therefore, immobilisation of targeting moieties such as Anti-MIA Ab onto the surface of the liposomes encapsulating NPs can selectively attach to MIA antigens expressed on the surface of MM cells to increase NPs accumulation in MM cells [126]. Alternatively, the physical and chemical properties of NPs embedded in liposomes could be enhanced by modification with photoactive compounds for improved PDT-mediated cytotoxicity outcomes [123, 127]. For example, encapsulation of green NPs and photoactive compounds in liposomes functionalized with cancer-specific antibodies can improve their accumulation in cancer cells and reduce their unwanted off-target toxicity (Figure 4).
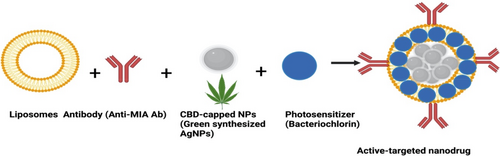
8 CBD NPS AND ACTIVE-TARGETED-MEDIATED PDT TO OVERCOME MM RESISTANCE
In recent years, various strategies have been adopted to circumvent melanoma resistance to PDT, these includes using photoactive compounds absorbing in the far-red and near-infrared ranges in order to evade melanin absorption. Melanin absorbs and scatters nearly across the entire spectral range used for PDT (400–700 nm) [128]. Virtually all the clinically approved PS absorb below 700 nm, and there is a dire need for novel PSs absorbing in the NIR, such as bacteriochlorins and Si(IV)-naphthalocyanine [44, 45]. Another strategy used to evade melanin absorbance is through the modification of PSs in Table 1 with NPs in Table 2, such as upconversion NPs and mesoporous silica nanoparticles (MSNs), since they can be excited with NIR light [129]. However, cancer selectivity and susceptibility still pose vital challenges that could hamper the therapeutic efficacy of anticancer agents and cause unwanted systemic toxicity. In order to improve increase cellular uptake and combat off-target cytotoxicity, PS nanocarrier platforms are further modified with active-targeting moieties that have a high affinity for receptor overexpressed by cancer cells [130]. However, due to the complexity of cancer cells, monotherapies rarely yield complete eradication of cancer cells. The heterogeneity of cancer cells allows them to rejuvenate and differentiate into different cells, leading to therapy resistance and tumour relapse [131].
Studies have successfully combined PDT with anticancer agents that enhance antitumour efficacy. Therefore, enhanced therapeutic efficacy can be achieved by the combined anticancer effects of CBD and PDT, since they induce cell death via the activation of apoptosis and necrosis pathways [27, 132]. CBD exerts its anticancer effects by binding to G-protein coupled receptors, referred to as type 1 and 2 cannabinoid receptors (CB1 and CB2), which are highly expressed in cancer [133]. These receptors can both induce pro-apoptotic, anti-inflammatory and antiproliferative effects, which may obliterative cancer cells [134]. The combination therapy of CBD and conventional cancer treatments may concurrently target multiple pathways that promote tumour growth and therapy resistance [135]. The modulation of pathways such as PI3K/AKT/mTOR and extracellular signal-related kinase (ERK) suggests that CBD can improve the susceptibility of certain cancers to other treatments. Indeed, studies have proved that the combination of CBD and conventional chemotherapeutic drugs such as cytarabine and vincristine can improve anticancer activity through alterations of these signalling pathways [136, 137]. Furthermore, combining CBD with conventional therapies may inhibit proliferation and metastases of residual melanoma cells, thus enhancing the patient's survival rate.
The combination therapy of CBD and photosensitizing agents, chemotherapeutics or radiotherapy drugs not only increases the susceptibility of cancer cells but also reduces the dose dependence of these agents, which could in turn limit their unwanted side effects on healthy tissues. CBD combination treatment with PDT may increase cytotoxic ROS yields, enhancing PDT treatment outcomes via activation of apoptotic cell death pathways. Moreover, the combination therapy of PDT and CBD should elicit a patient's immune system responses that alter the multiple signal pathways that accelerate tumorigenesis, drug resistance and metastasis, thereby augmenting the overall therapeutic synergistic efficacy of this suggested treatment for MM.
Melanin plays a vital role in melanoma resistance to PDT since it absorbs light aimed at tumour tissues and also acts as an ROS scavenger. A study conducted by Czech and colleagues demonstrated that CBD significantly inhibited cell viability, melanogenesis and tyrosinase activity of melanoma cells [138]. Czech et al went on to note that CBD not only had a significant cytotoxic effect on human melanoma cells, but also attenuated the melanin synthesis and release in αMSH-stimulated murine melanoma B16F10 cells, as evidenced by the decreased levels of extracellular and intracellular melanin. ABC proteins facilitate the movement of molecules across physiological membranes and contribute to drug resistance by limiting the bioavailability of an administered drug. ABCG2 has been found to predominantly affect the pharmacokinetics of PSs and other anticancer agents [139].
Holland et al. investigated the effect of CBD on ABCG2 and noted that CBD increased the intracellular content of mitoxantrone, a substrate of ABCG2, in the MEF3.8/Bcrp1A2 cell line overexpressing the ABCG2 protein [140]. Holland and colleagues concluded that CBD directly interacts with the ABCG2 transporter and counteracts the activation by substrates, which in turn inhibits the activity of ABCG2 transport. Undoubtedly, CBD can address the main challenges faced by PDT in MM treatment, suggesting that their combination could achieve complete tumour annihilation.
Despite the potential clinical use of CBD, its poor solubility hampers its bioavailability and ability to completely eradicate cancer cells [73, 141]. Therefore, green NPs may address this issue and induce cell death since they can be naturally capped with cytotoxic bioactive organic compounds such as cannabinoids [62, 105]. Recent studies by Chota et al. evaluated the phototoxic effect of green synthesised AgNPs coupled with pheophorbide-a mediated PDT on three-dimensional (3-D) doxorubin resistance breast cancer cells [142]. Chota and colleagues reported that this combination therapy resulted in high apoptotic rates compared to untreated cells only, suggesting that this combination therapy is very effective in cancer treatment.
There is no data to the authors' knowledge that has reported CBD-capped NPs cytotoxicity towards melanoma cells alone or in combination with PDT. To make up for the paucity of the literature, Table 3 summarises various studies that have tested the anticancer effects of green synthesised NPs on melanoma cells. This study merits further investigation into the anticancer effects of CBD-capped NPs on melanoma cells alone and in combination with PDT. As observed in Figure 5, combining active-targeted PDT with CBD-capped or CBD-loaded NPs could theoretically enhance its treatment outcomes by increasing cytotoxic ROS yields, which in turn prevents drug resistance and tumour recurrence in melanoma patients post-treatment.

9 CONCLUSION
As shown in Table 1, various second-generation PSs have demonstrated increased potency in the treatment of MM. Among them are phthalocyanines, MB, chlorins e6, and 5-aminolevulinic acid (5-ALA). In order to promote tumour specificity and enhance cellular absorption of second-generation PS for improved MM PDT primary treatment outcomes, researchers should be actively engaged in transforming them into third-generation PSs by combining them with NPs in Table 2 as well as functionalizing them with active targeting moieties such as anti-MIA antibodies or folic acid. Studies performed by Naidoo and colleagues conjugated zinc phthalocyanine to pegylated AuNPs and anti-MIA antibodies to actively target antigens exclusively overexpressed by melanoma cells. Naidoo and colleagues reported that the synthesised nanobioconjugate demonstrated increased cellular uptake and phototoxicity effects on melanoma compared to free PS [143]. Similar observations were reported by Montaseri and colleagues after modifying the same PS with pegylated AgNPs and folic acid to target folate receptors overexpressed by melanoma cells [144].
Melanoma is inherently metastatic and insidious, which makes it difficult to treat. Its heterogenicity and plasticity drive tumour progression, metastasis and drug resistance [145]. Thus, MM is a complex disease. Although the therapeutic landscape of cancer has broadened over the decades, single conventional or unconventional treatment modalities can effectively inhibit primary tumour growth but fail to control systemic spread. Additionally, current therapeutic approaches have impairing side effects and undesirable toxicity in patients, all of which weigh unfavourably on the efficiency of current treatments. Therefore, this calls for a multitargeted approach that can overcome the challenges of the present single-drug options. It is presumed that this approach could be derived from medicinal plants. This realisation has encouraged researchers to investigate combinatorial synergistic therapies that aim to inhibit primary tumour growth as well as evoke anticancer host immune reactions that can combat secondary disease [146]. Studies by Sham et al. (2015) pointed out that there is a dire need to develop PDT treatment modalities that can inhibit primary tumour growth as well as elicit host immune responses capable of promoting anticancer immune responses that prevent tumour relapse [146].
Accruing evidence suggests that CBD holds potential for inhibiting angiogenesis, metastasis, as well as prevents cellular proliferation by inducing cell death in cancer cells, thus counteracting metastasis [147, 148]. Lastly, studies have highlighted that CBD has the ability to depigment melanoma cells by inhibiting melanin synthesis, which suggests that its interaction with PDT can evade melanin absorption [138]. However, the hydrophobic nature of CBD drastically limits its bioavailability and overall efficacy. This issue can be addressed through the green synthesis of CBD NPs. Thus, this proposed combinatorial approach to MM treatment, utilising active-targeted third-generation PS and CBD NPs, warrants intensive investigation in order to enhance MM patients' treatment outcomes and quality of life.
AUTHOR CONTRIBUTIONS
Conceptualisation: Nkune Williams Nkune. and Heidi Abrahamse. Writing—original draft preparation; Nkune Williams Nkune. Writing—review and editing: Heidi Abrahamse. Supervision: Heidi Abrahamse. All authors have read and agreed to the published version of the manuscript.
FUNDING INFORMATION
This work is based on the research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation of South Africa (Grant no. 98337). The authors sincerely thank the Laser Research Centre, the University of Johannesburg, the National Laser Centre, and the National Research Foundation-South African Research Chairs Initiative (NRF-SARCHI) for their financial grant support.
CONFLICT OF INTEREST STATEMENT
No potential conflict of interest was reported by the author(s).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




