The Effect of Repeated Capillary Refill Tests on the Cutaneous Microcirculation
ABSTRACT
Objective
The capillary refill test (CR test) is often used in emergency care, and the capillary refill time (CR time) is used to assess a patient's circulatory condition. The objective of this study was to investigate if repeated CR tests affect CR time.
Methods
Thirteen healthy volunteers had repeated CR tests performed on the sternum, forehead, and fingers. The tests were filmed using polarized reflectance spectroscopy and dedicated software for objective quantification of the CR time.
Results
There were no statistical differences between the first CR test in a series and the following. However, there were statistically significant differences in CR time between the different anatomical sites.
Conclusion
Repeated CR tests, separated by a minimum of 2 min, do not affect CR time in healthy volunteers. The site where the test is performed is of importance for CR time.
1 Introduction
Since first described by Beecher et al. in 1947 [1] the capillary refill test (CR test) has become an integrated part in the assessment of critically ill patients worldwide, for instance in concepts such as Prehospital Trauma Life Support, and European Paediatric Advanced Life Support [2]. The test is quick and uncomplicated to perform, which probably has contributed to its wide use [3]. It measures the time it takes a distal capillary bed, such as those found in the fingers, to regain original color after a blanching pressure has been applied. This is called the capillary refill time—CR time. The initial rationale for the CR test was that the time to capillary refill could be considered as an indicator of macrocirculatory status. However, more recently, it has also been suggested as an indicator of microcirculatory function, and a guide to, for example, vasopressor treatment in sepsis patients [4]. Since organ perfusion ultimately relies on the function of both the macrocirculation and the microcirculation, and since direct measures of microcirculatory status will likely become more widespread as indicators of prognosis in critical illness, there is a need to physiologically characterize the capillary refill both in healthy subjects and in illness [5].
However, the performance of the test has been questioned [6] and there is little and conflicting data on the validity of the test [7, 8]. For instance, there is currently no consensus on how the capillary refill test should be performed. Specifically, there is no standardized protocol for the duration and amount of pressure to be applied. The most common application site is the finger, but other anatomical sites, such as the sternum and forehead have also been suggested [9]. Further, the accurate timing of CR time in a clinical setting is not always straightforward as the assessment is influenced by ambient light, the patient's skin pigmentation, and interpersonal differences among the clinical staff in estimation of time.
The CR test is currently described as clinically useful both as a single measurement or for repeated measures performed to follow the clinical course of a patient [4]. In situations where multiple capillary refill tests are performed in rapid succession, however, there may be a risk that the maneuver per se affects the appearance of the skin and consequently the interpretation of the test, as repeated stimulation of the skin may result in local erythema. Although repeated tests will be necessary to monitor the clinical course of a patient, there are—as far as we are aware—currently no studies on the effects of repeated CR tests on local erythema and the interpretation of the refill time.
Thus, the primary aim of the present study was to investigate whether repeated CR tests influence the measured refill time, objectively measured by polarized reflectance spectroscopy.
The secondary aim was to investigate any differences in capillary refill time between three different anatomical sites (forehead, sternum, and fingers), and to assess the intraindividual and interindividual variation in refill time among healthy volunteers.
2 Methods and Materials
This study was approved by the Linköping University Hospital Ethics Committee (approval no. 2015/99-31). All participants provided written informed consent prior to enrolment in the study.
Capillary refill time, here defined as the time in seconds for the blanched measurement area to regain its pre-pressure erythema intensity, was obtained by using polarized reflectance imaging. We have previously denoted this timepoint as “time to return to baseline 1” (tRtB1) [10]. The polarized reflectance imaging system utilizes a digital camera (Canon Eos 800D, Canon Inc.) fitted with an external LED broad spectrum light source and two orthogonal polarization filters. The camera was set to video mode, recording at 50 frames per second at high-definition resolution (1920 × 1080 pixels). The polarized reflectance imaging system has previously been described in detail [11]; however a brief description follows below. Light from the external LED light source becomes linearly polarized as it passes through the first polarization filter. A part of the light will be directly reflected from the surface of the skin, retaining its original polarization. This light is stopped by the second filter in front of the lens. Thus, surface glare is reduced and only a part of the light that has become diffusely scattered and randomly polarized by interacting with the tissue can reach the sensor of the camera to generate an image. By taking advantage of the wavelength-dependent differences in absorption between red blood cells and other tissue components of the skin within the 500–700 nm wavelength regions, an index is calculated for every pixel in the image. This index is linearly proportional to the concentration of RBCs within the measurement area and serves as an indicator of erythema (color change) of the skin during the refilling process. Pre-pressure erythema intensity was calculated as the mean of all index values within the measurement area (region of interest, ROI) during the baseline period of 5 s. Refill time was calculated as the time needed after the release of pressure for the ROI to regain this intensity value.
Video recordings and subsequent image analyses were made using software packages TiVi701 Camera and TiVi700 Analyzer, Versions 1.5.7, February 2018 and 1.2.20, November 2019, respectively, (WheelsBridge AB, Linköping, Sweden, wheelsbridge.se).
The capillary refill tests were performed with the volunteers in a supine position with one of their arms resting on an arm table at heart level (see Figure 1). After a 20-min acclimatization period, a blanching pressure was applied to the tissue using a 10 N dynamometer (TickIt 10 N, Hands-On-Science, Järfälla, Sweden) fitted with a 1.77 cm2 round plastic extension for an evenly distributed pressure (0.58 kg/cm2) over a standardized area. The dynamometer was calibrated using a digital force gauge model FH 50 (Sauter GmbH, Balingen, Germany). The camera of the polarized reflectance imaging system was positioned approximately 25 cm away from and directly above the measurement areas. Each measurement lasted 20 s with a 5 s pre-pressure period (baseline), a 5 s period of standardized pressure, and 10 s of refilling response (Figure 2). The imaging system was recording continuously at 50 frames per second for each measurement.
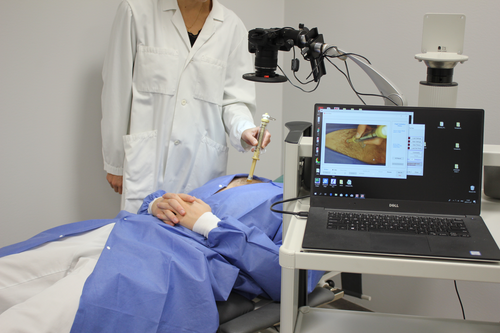
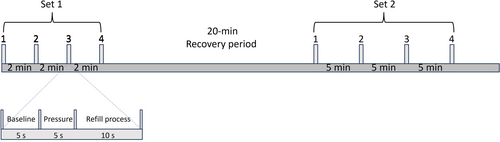
Capillary refill tests were performed on the sternum, forehead and on the finger pulp of one of the index fingers. The capillary refill tests were performed in two sets on each anatomical site, each set having either a 2- or 5 min recovery period between CR tests. Within sets, tests were repeated four times on the sternum, forehead, and the finger pulp.
The sets were separated by a 20-min period to allow the skin to recover from the previous set.
3 Statistical Analysis
Data were analyzed using Microsoft Excel and GraphPad Prism (GraphPad Prism version 9.1.2.226, 64-bit for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com) and intraclass correlation coefficient estimates and their 95% confident intervals were calculated using SPSS statistical package version 29.0.2.0 (20) (SPSS Inc, Chicago, IL).
Wilcoxon matched pairs test was used to find the difference in mean tRtB1 between sets (2- and 5-min separation time) within each subject. Kruskal–Wallis test, with Dunn's multiple comparisons test was used to compare the effect of repeated refill tests on tRtB1, and to investigate the effect of anatomical site on tRtB1.
Intraclass correlation coefficient estimates and their 95% confident intervals were based on a single-measurement, absolute-agreement, and two-way mixed-effects model.
An alpha level of 0.05 was used in all statistical tests.
4 Results
Thirteen healthy volunteers (6 males, 7 females) were enrolled in the study. Mean age and mean BMI values for the volunteers were 30.8 ± 6.2 years and 24.6 ± 3.8 kg/m2. All volunteers had fair skin (Fitzpatrick scale I or II).
The refill time (tRtB1) could be calculated for 71 out of 72 measurements performed on the forehead, 70 of 72 measurements on the sternum and for 72 out of 72 measurements on the finger.
The reason tRtB1 could not be calculated in three of the measurements was that the erythema intensity at removal of the pressure was higher than that of the mean baseline value, possibly due to insufficient or unevenly distributed pressure to the tissue.
No significant influence of separation in time (2 or 5 min) between repeated CR tests on tRtB1 within anatomical site and volunteer could be found (Wilcoxon matched pairs test). For the subsequent analyses, data from repeated tests within each anatomical site and volunteer was considered as one set. Figure 3 shows the individual scatter plots for tRtB1 values for each volunteer and site, with tRtB1 values with 2- and 5-min separation times as filled gray circles and black triangles, respectively.
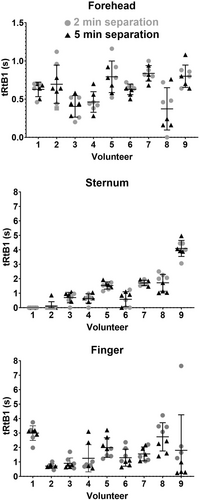
Figure 4 shows the tRtB1 values for the respective anatomical sites. Kruskal–Wallis test, with Dunn's multiple comparisons test was performed to compare the effect of anatomical site on tRtB1. The analysis revealed that there was a statistically significant difference in tRtB1 between all sites (p = <0.0083).
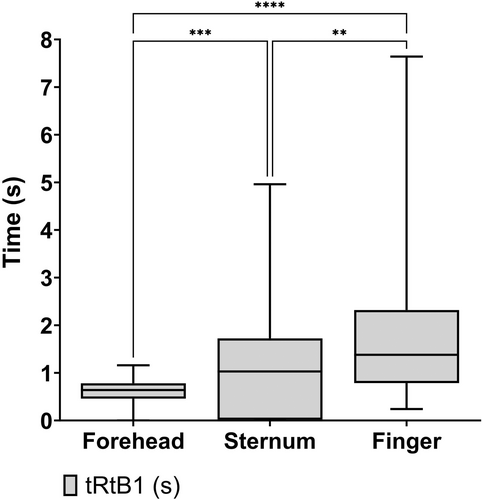
Table 2A shows the distribution of tRtB1 in each subject. The coefficient of variation for the tRtB1 values was calculated as 30%, 31%, and 60%, for the forehead, sternum, and fingers, respectively.
Table 2B shows the distribution of TiVi values in each subject. The coefficient of variation for the tRtB1 values was calculated as 8%, 10%, and 7% for the forehead, sternum, and fingers, respectively.
Intraclass correlation coefficient calculations for measurements separated by 2 and 5 min, respectively, at the different anatomical sites showed a test–retest reliability that ranged from “poor” to “excellent” (Table 1).
| Intraclass correlation coefficient (95% CI) | |||
|---|---|---|---|
| Forehead | Sternum | Finger | |
| 2 min | 0.81 (0.46–0.96) | 0.98 (0.94–0.96) | 0.73 (0.22–0.93) |
| 5 min | 0.75 (0.29–0.94) | 0.98 (0.94–0.99) | 0.91 (0.76–0.98) |
| Foreheada | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Repeats | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Mean tRtB1-value (s) | 0.63 | 0.70 | 0.41 | 0.46 | 0.79 | 0.68 | 0.84 | 0.43 | 0.80 |
| SD | 0.09 | 0.25 | 0.14 | 0.13 | 0.21 | 0.03 | 0.10 | 0.25 | 0.15 |
| Relative SD | 15% | 36% | 35% | 29% | 26% | 5% | 12% | 59% | 18% |
| Pooled repeatability | |||||||||
| %RSD pooled (CV) | 30% | ||||||||
| Sternum | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Repeats | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Mean tRtB1-value (s) | 0.00 | 0.11 | 0.79 | 0.73 | 1.53 | 0.75 | 1.72 | 1.81 | 4.12 |
| SD | 0.00 | 0.3 | 0.25 | 0.21 | 0.25 | 0.49 | 0.20 | 0.56 | 0.59 |
| Relative SD | a | a | 31% | 29% | 16% | 65% | 12% | 31% | 14% |
| Pooled repeatability | |||||||||
| %RSD pooled (CV) | 31% | ||||||||
| Finger | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Repeats | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Mean tRtB1-value (s) | 2.99 | 0.71 | 0.90 | 1.25 | 1.99 | 1.29 | 1.55 | 2.73 | 2.45 |
| SD | 0.51 | 0.16 | 0.37 | 0.94 | 0.68 | 0.59 | 0.48 | 0.98 | 1.80 |
| Relative SD | 17% | 23% | 41% | 75% | 34% | 46% | 31% | 36% | 136% |
| Pooled repeatability | |||||||||
| %RSD pooled (CV) | 60% | ||||||||
- a Refill times for sternum for volunteers 1 and 2 were zero (except for one measurement on volunteer 2), as erythema intensity at pressure release was the same as for baseline. This resulted in mean values and standard deviations of zero, making it impossible to calculate a coefficient of variation. Data for volunteers 1 and 2 were thus excluded from calculating a pooled variation coefficient.
| Forehead | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Repeats | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Mean TiVi-value (a.u.) | 246.15 | 176.30 | 199.59 | 222.34 | 249.19 | 235.50 | 223.17 | 194.76 | 236.67 |
| SD | 5.46 | 16.48 | 13.89 | 7.78 | 19.25 | 4.78 | 13.05 | 36.5 | 9.91 |
| Relative SD | 2% | 9% | 7% | 3% | 8% | 2% | 6% | 19% | 4% |
| Pooled repeatability | |||||||||
| %RSD pooled (CV) | 8% | ||||||||
| Sternum | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Repeats | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Mean TiVi-value (a.u.) | 113.70 | 113.20 | 122.49 | 151.26 | 146.71 | 225.19 | 198.50 | 115.27 | 187.07 |
| SD | 7.64 | 3.29 | 22.36 | 8.20 | 17.40 | 21.9 | 11.10 | 15.18 | 13.77 |
| Relative SD | 7% | 3% | 18% | 5% | 12% | 10% | 6% | 13% | 7% |
| Pooled repeatability | |||||||||
| %RSD pooled (CV) | 10% | ||||||||
| Finger | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Repeats | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Mean TiVi-value (a.u.) | 315.46 | 289.11 | 271.75 | 277.10 | 398.12 | 123.70 | 140.94 | 155.40 | 222.69 |
| SD | 15.25 | 8.83 | 19.94 | 9.28 | 9.21 | 4.02 | 6.09 | 6.78 | 34.63 |
| Relative SD | 5% | 3% | 7% | 3% | 2% | 3% | 4% | 4% | 16% |
| Pooled repeatability | |||||||||
| %RSD pooled (CV) | 7% | ||||||||
5 Discussion
In this study, done in a standardized setting with healthy volunteers, there were no clear effects on CR time (tRtB1) when CR tests were performed repeatedly in commonly used anatomical sites. Thus, under constant conditions, the CR time does not seem to depend on where in the test series the CR test was performed, or if the interval between the tests was 2 or 5 min. This indicates that repeated testing per se should not influence the interpretation of the test.
The polarized reflectance imaging technique used in this study is valid for quantification of erythema as an indirect marker for microcirculatory changes in the skin by using a light intensity index that correlates to the concentration of red blood cells within the tissue [10]. In the current study, the pooled repeatability of the TiVi values at baseline was overall low, indicating that the technique is stable and reliable for testing erythema of the skin.
In the current study, there was a clear difference in tRtB1 between anatomical sites, indicating the importance of localization to the test's outcome. The most common site to perform the CR test in adults is on the pulp of the distal phalanx of the index finger. The fingers are easy to access and less pigmented, which facilitates naked-eye assessment of capillary refill time. At the same time, blood flow to the fingers is also strongly affected by neural stimulation through the sympathetic nervous system, and it is known that low ambient temperature prolongs CR time in fingers when repeated CR tests are performed [8, 12]. The reproducibility for the CR time in the fingertips was low (CV = 60%), and lowest of all tested anatomical sites in this study. The use of fingertips as anatomical site for CR testing could be discussed in a clinical context.
The anatomical site with narrowest distribution of tRtB1 values was found on the forehead, both within and between volunteers. Circulation in the forehead and the area around the eyes originate from the internal carotid artery and is thus affected by blood flow to the brain, which is strictly regulated. The circulation in the skin of the face is also affected by the parasympathetic nervous system [13]. Stable local circulation at the measurement site is beneficial for the measurement of CR time. However, since blood flow to the brain is prioritized in a deteriorating patient, CR time measured on the forehead might not change until the patient is in extremis.
Thus, the sternum, which showed slightly more variation in obtained tRtB1 values could be a more suitable site for assessment of CR time if the goal were to capture dynamic changes in blood flow related systemic disorders. However, blood flow to the thoracic anterior wall differs between sexes, as the amount of subcutaneous tissue over the sternum. How this can affect CR time is to our knowledge not described in literature.
The 95% CI of the intra class coefficient showed both wide and narrow ranges. For example, tests on the sternum had ICC values and 95% CI indicating “excellent” level of reliability. However, several measurements on the sternum had a tRtB1 of 0 s. There was a considerable variability for the tRtB1 values for all sites, both within and between volunteers, though the inter individual differences were larger. It is known that adult females have a slightly longer normal CR time than males [14]. However, this does not fully explain our findings. One explanation could be that microcirculatory reactivity has a large variability in healthy individuals. For two of the volunteers (5 and 9), calculated CR time showed low variability. For those volunteers, mean tRtB1 values also surpassed the recommended cutoff for adults of 2 s, often suggested in guidelines to separate between healthy and ill, at two occasions. Larger observational studies could give more information on the distribution of CR times in healthy individuals and on the expected range of intra individual differences. Such studies should also, preferably, include patients with common cardiovascular and metabolic diseases. There are clear indications that early disturbances in the microcirculation precede manifest metabolic disease such as hypertension and diabetes [15] and it is unknown to what extent these changes affect CR time and its variability or repeatability.
In summary, this study shows that CR tests performed repeatedly with a minimum of 2 min, do not affect CR time. The anatomical site for testing CR time is of importance.
Further research should include repeated CR tests in a shock model to investigate its repeatability in hemodynamic instability, and further investigation of CR time in different anatomical sites in shock.
6 Limitations
This study has several limitations. First, it is not known if the CR test is a reliable indicator of microcirculatory changes in shock.
The use of a dynamometer instead of a human finger to perform CR tests might affect how capillary refill takes place in the tested skin area. The dynamometer was used as it standardizes the pressure and the area in contact with the study subjects' skin, but it is unknown if it reflects the amount of pressure used by an examiner in the clinical setting. The plastic cylinder fitted to the dynamometer is not flexible, which the human finger pulp of the examiner in the clinical setting is, and this might affect how blood is pushed out of the tested skin area.
The current method for calculating tRtB1 was chosen as it mimics how refill time is assessed clinically using naked eye assessment and manually counting time in seconds needed for the blanched skin to regain its original color. This method, however, when applied in combination with the digital refill data obtained in the present study resulted in missing data. CR test results were lost due to either a tRtB1 of 0 s or because no CR time could be calculated (at release of pressure the skin had already the same or higher TiVi value as before the application of a blanching pressure). Even though no value for tRtB1 could be obtained, in most cases a typical refill curve was generated. These curves likely contain information about the refill process, currently not assessed due to the chosen analysis method. In future studies we will aim toward a more thorough analysis of the refill process, including for example steepness of the refill curve, as this could potentially increase the usefulness of the CR-test.
In the present study an image-based measurement technique was utilized generating an erythema index over a rather large tissue area. This is only one technique available among several others, for example laser Doppler techniques, that may be as, or more, suitable for use with repeated CR tests.
7 Conclusion
Repeated capillary refill tests separated by at least a 2-min waiting period at one anatomical site does not affect CR time in healthy volunteers.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




