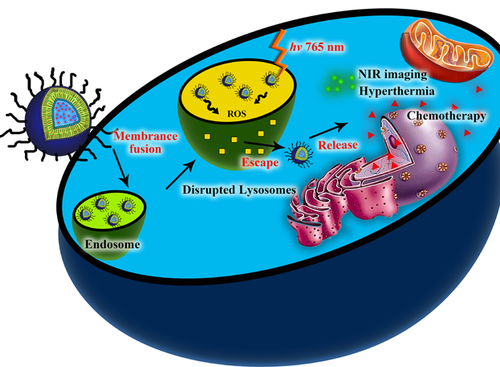
Cypate-mediated thermosensitive nanoliposome for tumor imaging and photothermal triggered drug release
Abstract
It is an emerging focus to explore controlled release drug delivery systems for simultaneous cancer imaging and therapy. Herein, we synthesized a photothermal sensitive multifunctional nano-liposome drug delivery system, with doxorubicin wrapped in the hydropholic layer as the therapeutical agent and cypate doped in the hydrophobic layer as the diagnostic agent. A series of in vitro and in vivo characterization demonstrated the stability of synthesized liposome, as the DL% was 9 ± 1.5 and the EE% was 82.7 ± 2.1. And the liposome achieved the functions of target-delivery, enhanced photochemical internalized drug release, and simultaneous chemotherapy and thermal therapy, indicating that this multifunctional nano-liposome is a promising drug delivery system for tumor diagnosis and targeting therapy.
Introduction
Nowadays, more and more tumor patients died of excessive treatment 1. The real killer is always not tumor itself but the side effects of the chemotherapy or radiotherapy. Up to the present, the chemotherapy drugs in clinical are still limited by low aqueous solubility, insufficient stability, rapid metabolism and non-selective drug distribution, which lead to high organ toxicity and dose-limiting side effects. Nanoscale drug delivery systems are attracting considerable attention as an alternative strategy to overcome above limitations. Because of the well biocompatibility, biodegradable property, low toxicity, and easy loading capability for both hydrophilic and lipophilic drugs, the nanoscale drug delivery exhibits great potential in modern pharmacy. Liposomes 2, micelles 3 and mesoporous silicas 4 are three kinds of commonly used nanoscale drug delivery systems.
The functional liposomes are outstanding among the three mentioned nanoscale drug deliveries because of their good biocompatibility and biodegradability. The biocompatible phospholipids can be well digested and weakly immunological rejected by our human body 5. What's more, the improved solubility, long-circulation time, tumor targeting and controlled release, also make functional liposomes a promising drug delivery system. Meanwhile, controlled released liposome drug delivery system are wildly attractive, such as pH-sensitive liposomes 6, temperature-sensitive liposomes 7, reduction-sensitive liposomes 8, and light-triggered liposomes 9.
As a carboxylic acid derivative of indocyanine green (ICG) 10-12, cypate is a typical near infrared (NIR) dye for in vivo imaging. Compared with ICG, the structure of cypate shows low cytotoxicity in living systems, high quantum yield, and high photothermal conversion efficiency 13, 14. At present, studies also focused on cypate's application in photothermal therapy because of its photothermal conversion ability, and photodynamic therapy because of the laser induced-generation of reactive oxygen species (ROS), primarily singlet oxygen 15.
Herein, we designed and synthesized a novel multifunctional nano liposome drug delivery system, the photothermal sensitive liposomes (PTSL). The doxorubicin (DOX) was wrapped in the hydropholic layer and cypate was encapsulated in the hydrophobic layer of the thermal sensitive liposomes (TSL) (Figure 1A). The PTSLs with a diameter of 100–200 nm were tended to gather at the tumor site by the EPR (enhanced permeability and retention) effect. Under the NIR light of 780 nm, the cypate could transform the light energy into heat and singlet oxygen. The raised temperature could not only ablate the solid tumor, but also crack the thermal-sensitive liposome and release the wrapped chemotherapy drugs. At the same time, the generated singlet oxygen could crack the organelle membrane of lysosomes or endosomes, releasing the Doxs into the cytoplasm and then the nuclear for pharmacological effect.
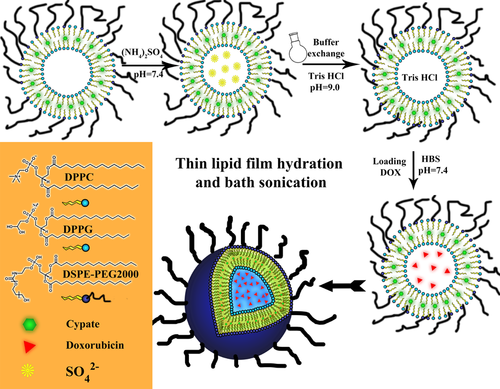
The structure and synthetic routine of DOX@PTSL.
Results and discussion
Preparation and characterization of the multifunctional liposomes
The TSL, PTSL and DOX@PTSL were successful synthesized and the diameters of the three nano structures gradually increased from 90 nm to 160 nm (Figure S1A-C), which was suitable for EPR effect. The TEM images suggested that the synthesized liposomes are spherical and have a narrow size distribution (insert pictures of Figure S1A–C). The size observed by TEM is smaller than the hydrodynamic diameter, which was attributed to the swollen state of TSL during hydrodynamic diameter detection.
The efficacy loading of DOX and cypate into the liposomes was confirmed by UV-Vis spectrometry (Figure S1D). The composites of DOX@PTSL clearly exhibited the two absorption peaks of DOX and cypate at 493 nm and 788 nm respectively. More importantly, after encapsulated into the hydrophobic layer of TSL, cypate's maximum absorption and fluorescence wavelength didn't change and still retained the strong spectral characteristics. And the thermosensitive property was confirmed by DSC analysis, with the Tm of 43.1 °C.
The stability of multifunctional liposomes for morphologic distribution and drug loading
The size, polymer dispersity index (PDI) and zeta potentials (ζ) of the TSL containing different ratios of DSPE-PEG2000 and their stability over two weeks at storage temperature (4 °C) are summed up in Figure 2. Incorporating DSPE-PEG2000 into the bilayer of the liposome resulted in the minimization of the size and best dispersion as the lowest PDI values in three weeks. The TSLs with different ratios of DSPE-PEG2000 all showed ζ potentials higher than 30 mV, which is the proper range for being stably preserved. We also studied the properties of liposomes based on DPPC alone without DPPG or DSPE, the liposome size changed from 96.5 ± 0.1 nm to 187.3 ± 10.2 nm after just one week, which may due to the neutral zeta potential that leading to aggregation of the particle and thus the increased size.
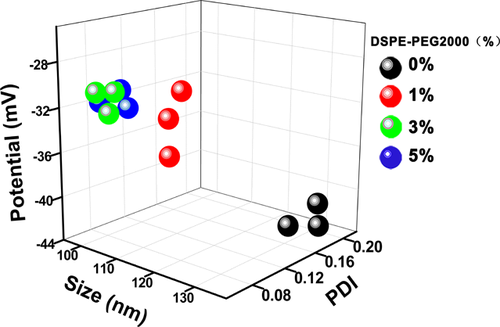
The dynamic light scattering (DLS) parameters of TSL over a period of 2 weeks.
For the DOX@PTSL and the DOX@TSL, the DL% and EE% against different dialysis buffers are shown in the Table 1 below. Obviously, the Tris HCl is a better choice of dialysis for easy dropping the DOX into the hydrophilic core of the liposomes. The stability for drug loading is shown in Supporting information which verified by the DOX leakage (Figure S2).
|
DL % |
EE % |
|||
|---|---|---|---|---|
|
Tris HCl |
HBS |
Tris HCl |
HBS |
|
|
DOX@PTSL(Dox) |
9 ± 1.5 |
6.4 ± 1.9 |
82.7 ± 2.1 |
66.3 ± 0.5 |
|
DOX@TSL(Dox) |
8.2 ± 2.4 |
5.1 ± 2.2 |
75.1 ± 3 |
66.4 ± 2 |
|
DOX@PTSL(Cypate) |
4.5 ± 0.5 |
62.5 ± 1.6 |
||
In vitro drug release
The result of TSL's temperature-dependent DOX release is represented in Figure 3A. TSL with different DSPE-PEG2000 densities were all stable up to 39 °C, with DOX leakage less than 5%. TSL started to release DOX at 40 °C with an abrupt release at 41 °C and reached its maximum level of 65% at 43 °C. Meanwhile DSPE-PEG2000 free TSL were less stable with already over 10% release at 37 °C. Their highest DOX release level was at 43 °C or so, which is coherent with the results of DSC (Figure 3B) and infrared thermal images of DOX@PTSL+laser treated mouse. The laser group's infrared image as insert of Figure 3B showed that the right leg's temperature was 40.5 °C, a little lower than the result of in vitro experiment (Figure 3A), which was caused by the infrared thermal imager can only capture the surface temperature. For all TSLs, DOX release levels gradually decreased from 43 °C to 45 °C. In general, DOX's release increased with the increasing DSPE-density and TSL with 5% molar ratio of DSPE-PEG2000 showed the best release quality.
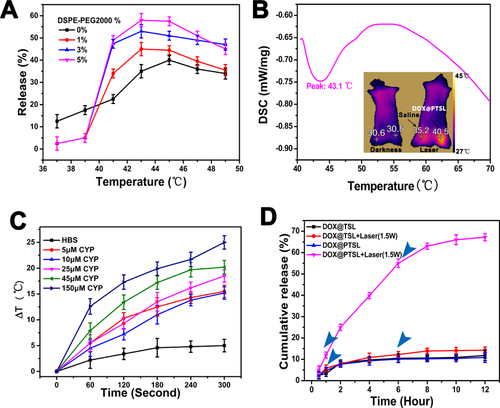
(A) The thermosensitivity of TSL evaluated by their ability to release Flu-Na after incubation at different temperature for 15 minutes. (B) The differential scanning calorimetry (DSC) results for phase transition temperature of PTSL. The infrared thermal image comparison of the mouse treatd with PTSL before and after exposed to 780 nm NIR laser (1.5 W/cm2) for 5 minutes; (C) the results of photothermal conversion ability for cypate of different concentrations in vitro; (D) DOX release profile under NIR irradiation (DOX@PTSL and DOX@TSL were put under the same experimental conditions. After being incubated at 37 °C, samples were irradiated with 780 nm NIR laser at 1 and 6 hours and the DOX release was compared with non-irradiated samples).
The dissolution curve of DOX release at 43 °C in 50% serum was monitored for 1 hour (Figure S3). TSL with 3% and 5% of DSPE-PEG2000 released DOX faster than TSL with 1% DSPE. A robust DOX release of 60% and 88% was noted from TSL with 5% molar ratio DSPE-PEG2000 in the first 10 minutes and 1 hour respectively, compared with 35% and 48% released from DSPE-PEG2000 free TSL at the same respective time points.
Exposing different concentrations of cypate to 780 nm NIR laser resulted in an elevation of temperature as shown in Figure 3C. With a starting temperature of ∼25 °C, the solution of cypate (25 mM) all rise to a temperature of over 43.5 °C (ΔT ≥ 18.5 °C) in five minutes. Under NIR irradiation, DOX@PTSL released almost 70% of their cargo in the 12 hours totally. On the other hand, DOX@TSL showed slight release after irradiation, because the NIR laser can make the sample's temperature a little warm, thus accelerating the DOX's movement. While DOX@PTSL or DOX@TSL with no irradiation show less DOX release. (Figure 3D)
Locating property of the functional liposomes in vitro
The laser scanning confocal microscopy (LSCM) pictures for the locating properties study are shown in Figure S4. For the Lyso Green stained cells, after 40 minutes, the red light began to scatter to the whole cell. The lysosome started to collapse and the wrapped drugs started to leakage into the cytoplasm, as shown in Figure 4A–D. As a carboxylic acid derivatives of indocyanine green (ICG), cypate's photochemical internalization as well as the lysosomal disruption mediated drug release can give great help to the drug release in cytoplasm, which mainly results from the formation of reactive oxygen species, primarily singlet oxygen 15. Light activation of photosensitizers located in the membranes of endosomes and lysosomes may therefore destroy the membranes and thus change the internal cellular environment.
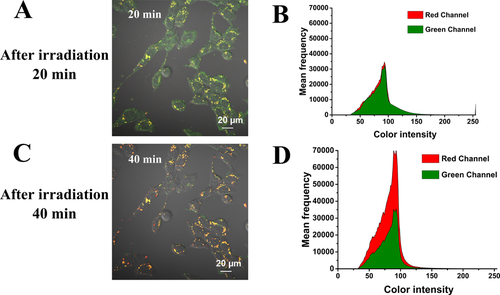
The LSCM pictures of dynamics of DOX@PTSL after incubated with U87 cell lines and irradiated with NIR laser (1.5 W/cm2) for 5 minutes, at different incubation time points, 20 minutes (A) and 40 minutes (C). The semi-quantitative results of 20 minutes (B) and 40 minutes (D) for flurescence intensity of two different channels. The green fluorescence of Lyso Green and the red fluorescence of DOX were collected under lasers (488 nm and 568 nm) excitation.
In vivo tumor-targeting evaluation of the functional liposomes
At 2 hours post-injection, the liposomes predominantly accumulated in the excretory organs such as liver, gastrointestinal system, (Figure 5A) because of its lipophilic property. At 12th hour, the liposomes were almost cleared from the body. The results of in vivo dynamic biodistribution of DOX@PTSL in H22 tumor-bearing mice were shown in Figure 5B. 1.5 hours later, the liposomes started to accumulate in the tumor site. And the tumor accumulation fluorescence was not disappeared until 24 hours. The synthesized liposomes was around 100–150 nm, which showed visible EPR effect and tended to gather at the tumor site. 36 hours later, the liposomes began to excrete out of the body. On the other hand, with PEG modified on the surface, the functional liposomes can avoid the capture of the reticuloendothelial system (RES), improve the passive target ability to the non-RES system and get prolonged cycle time as well as enhanced hydrophilic property 23. The NIR images of all dissected organs show that 4 hours after injected with DOX@PTSL, the nano-composites mainly gathered at the tumor, liver and intestine tissues, which consists with that of the in vivo imaging study. All the results above showed that the functional liposomes we synthesized can realize controlled and prolonged release to some degree. The fluorescence's semiquantitative results of can also prove the tumor-targeted and prolonged cycle time properties of the functional liposomes (Figure 5C).
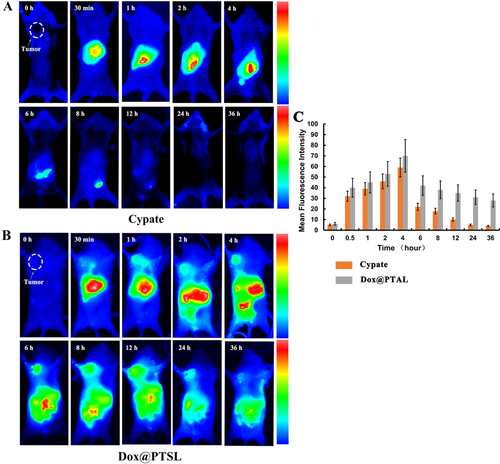
The near infrared images of H22 tumor-bearing mice under 765 nm laser excited after injected with (A) free cypate and (B) DOX@PTSL via the tail vein. (C) The semi-quantitative results of tumor-accumulation were calculated in Image J software, p < 0.05.
In vitro cytotoxicity study and in vivo acute toxicity test of DOX@PTSL
Upon the results of two cancer celllines, DOX@PTSL+laser showed the greatest cytotoxicity (Figure S5). The reasons could be summarized as 3 items, photochemical internalization, chemotherapy and thermotherapy. The acute toxicity was further assessed by evaluate the blood levels of ALT, AST, CK and BUN. Mice in saline and blank liposomes(PTSL) groups had almost the same serum levels of ALT, AST, CK and BUN. All of them fell in the normal range. The levels of ALT, AST and CK of DOX@PTSL treated mice were normal while those of BUN slightly high. As for DOX treated mice, ALT levels were normal, but the other three indexes, including AST, CK and BUN were higher than those of the other groups (Figure S6A). Figure S6B shows the histopathological study of the organs after sacrificing the mice at the third day of treatment. After the administration of DOX, a clear structure of the myocardial tissue and a minor myocardial cell swelling was observed. Results also showed a light cytoplasm dye loss. The left ventricular endocardial shallow was more obvious. For DOX@PTSL group, no myocardial cell necrosis, vasodilatation or interstitial inflammatory cells infiltration was noticed. In short, DOX@PTSL+laser combines a variety of treatment factors and stands out as an effective multifunctional drug model with lower toxicity.
Therapeutic efficacy of drug loaded functional liposomes in H22 tumor-bearing mic
The tumor sizes were recorded as Figure S7 Whether laser irradiated or not, the mice in the saline group all died at the first eight days. The PTSL +laser group started to die at the tenth day, and for the DOX@PTSL group without laser, one mouse died at the tenth day. The mice in DOX@PTSL+laser, DOX+laser or DOX group didn't die until the treatment ended. For the mice in the DOX@PTSL+laser group, tumor sizes increased slightly with time, the tumor growth was controlled to some degree compared with that of saline or PTSL group. The photothermal conversion ability can also be read on the infrared thermal images of Figure 6A and semiquantitative results of temperature are shown in Figure 6C. The temperature of the tumor site could reach 43.3 °C. Last but not least, the DOX-contained groups show better anti-tumor effects than the no DOX group, indicating that the DOX can leakage out of the liposomes more or less, and the chemotherapy is the most important mechanism for anti-tumor effect.
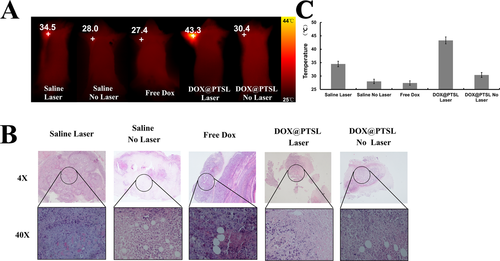
(A) The infrared images of tumor-bearing mice after injected with different agents with laser irradiated or not. The laser dose were 1.5 W/cm2 in five minutes for one day. (B) The microscopic pictures of tumors when the treatment reached 7 days or when the tumor volumes exceeded 200 mm3. (C) The semi-quantitative results of temperature were measured by Smart View software, p < 0.05.
Figure 6B showed the histopathological study of the dissected tumors after sacrificing the mice at the 12th treatment day, followed by H&E staining of tumor sections. In DOX@PTSL group, we observed more severe cancer necrosis with more cancer cells and extensive hemorrhagic inflammation under no laser treatment as compared to those under laser treatment. For those mice under DOX treatment, only hemorrhagic inflammation and some sporadic necrotic regions surrounded by malignant cells with nuclear atypia were observed. For tumors in DOX@PTSL group, hollow cross-section shapes were seen with pronounced cell damages, loss of tissue architecture and less intense tissue staining. Tumor tissues in free DOX group showed clear normal tissue boundaries and decrease in distribution of tumor cells. Obviously, the distribution of tumor cells decreased and no vacuole-like structures were observed.
In contrast, saline group as the control group showed no obvious tumor necrosis with or without laser irradiated. The results suggest that the treatment of DOX@PTSL+laser exhibit superior synergistic effect on tumor suppression compared with other treatments.
Conclusion
In summary, a multifunctional drug loading system was developed based on thermal sensitive liposomes that carried cypate and DOX for tumor diagnosis and therapy. The morphological characteristics, stability and loading ability of this system was investigated in the first step. The synthesized nano composites featured a delicate design with favorable tumor targeting property, good stability and superior drug loading efficiency. Under 760 nm laser irradiation, the photothermal effect of cypate could disrupt the membrane of liposomes, which facilitated liposomal escape of DOX for abundant accumulation in cytoplasm. As a result, DOX@PTSL exerted obvious cytotoxicity in tumor cells due to the combination of photothermal therapy and the tumor inhibition efficacy of DOX. DOX@PTSL showed excellent biocompatibility and tumor targeting ability in vivo. Finally, potent therapeutic efficacy of DOX@PTSL was demonstrated in tumor-bearing mice. Therefore, the attractive features of DOX@PTSL promise great potential of this drug loading system for combined tumor targeting therapy.
Experimental Section
Reagents: The phospholipid 1,2-dihexadecanoyl-sn-glycero-3-phosphocholine(DPPC), 1,2-dipaimitoyl-sn-glycerol-3-phospho-(1′-rac-glcerol)(DPPG), 1,2-distearoyl-an-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000](DSPE-PEG2000) were purchased from A.V.T (Shang Hai, China). Cypate was synthesized in our laboratory. Triton X-100, 1,3-diphenylisobenzofuran (DPBF) 3-(4,5-Dimethylthialzol-2-yl)-2,5-diphenylte-trazolium bromide (MTT), RPMI1640 medium, fetal bovine serum (FBS), penicillin, streptomycin and trypsin-EDTA were purchased from Wanqing (Nanjing, China). TX-100 and 1,3-diphenylisobenzofuran (DPBF) were purchased from Aladdin (Shanghai, China). All other solvents and reagents used in the study were certified analytical reagent grade.
Equipment
Morphological characterization of gold nanoshells was accomplished by transmission electron microscopic (TEM) imaging on a Philips FEI Tecnai G2 20s-TWIN (Netherlands) with an accelerating voltage of 200 kV. Particle size measurement was obtained on a Mastersizer 2000 Laser Particle Size Analyzer (Malvern, British). Zetasizer Nano instrument (Malvern Instruments, Malvern,British) was used to examine the Zeta potential. The UV-vis and fluorescence spectra were obtained by a 754-PC UV-Vis spectrophotometer (JingHua technological instrument corporation, China) and a spectrofluorophotometer (RF-5301PC, Shimadzu, Japan) respectively. Cell imaging was performed using the laser confocal fluorescence microscope (LCFM, FV1000, Olympus, Japan).
Preparation of thermosensitive liposomes (TSL)
The liposomes in this study were synthesized through the thin lipid film hydration and bath sonication method according to a previously reported method 18, 19. TSL was composed of DPPC/DPPG: DSPE-PEG2000 in a molar ratio of 4/5 (100 – x): 1/5(100 – x): x (x = 0, 1, 3, 5). The lipids were weighed out in a glass vial and dissolved in CHCl3 : MeOH (2 : 1, v/v)(every 5 mL solvent for 50 mg substrates). The solution was transferred into a round bottom flask and rotated for 10 minutes at 55 °C before starting the rotary evaporation under 1 bar pressure for 30 minutes to evaporate the solvents and get lipid film. The synthesized lipid film was put under nitrogen for 10 minutes before being put in vacuum desiccator for at least 4 hours to remove the solvents residues thoroughly. 10 mL HBS was used to hydrate the film at 50 °C. After short vortexing, the Multilayer vesicles (MLV) was formed and then downsized using bath sonication at 50 °C for 10 minutes to form single layer vesicles (SLV). The SLV of thermosensitive liposome solution was kept at 4 °C until further use.
Preparation of photothermal sensitive liposomes (PTSL)
The PTSL was prepared similarly to TSL. 1.5 mM cypate was first dissolved in 200 mL CHCl3 prior to its addition to the phospholipids and 5 mL of CHCl3/MeOH (2 : 1, v/v) mixture. The mixture was incubated at 40 °C for 1 hour to create hydrophobic interactions and then transferred into a round bottom flask. The subsequent steps were the same with that of the synthesis of TSL. Finally, the samples were dialyzed against HBS and gel size extruded through sephadex G-50 column and eluted with HBS to remove the unencapsulated cypate.
Encapsulation of DOX into the TSL or PTSL
The DOX was encapsulated into the inner hydrophilic core of the PTSL or TSL using ammonium sulfate gradient 20. TSL and PTSL thin lipid films, prepared as mentioned above was hydrated with 250 mM/L (NH4)2SO4 for 40 minutes at 50 °C. After formation of SLV, the samples were heavily dialyzed against HBS pH 7.4 or Tris HCl buffer pH 8.0 for 24 hours to exchange the exterior (NH4)2SO4 with the buffer and create the gradient. DOX (0.2 mg/mL) was added to the liposome solution and incubated together with TSL or PTSL at room temperature for 12 hours. Free DOX was removed by gel size extrusion through sephadex G-50 column and eluted with HBS.
Stability for morphologic distribution and drug loading
Firstly, the size, PDI and ζ of the TSL containing different ratios of DSPE-PEG2000 and their stability over two weeks at storage temperature (4 °C) was studied every one week. Similarly, the TSL's and PTSL's drug loading stability in 50% FBS at 4 °C for 3 weeks and at 37 °C for 1 hour were also studied by monitoring DOX leakage from liposomes using UV-Vis spectrometer. Absorbance (y) of different concentrations (x, from 10 m to 500 mM) of DOX at 493 nm using UV-Vis spectrometer was used to establish a standard curve. In detail, 0.2 mL of TSL or PTSL was added into 1.8 mL of 50% FBS, the mixture was exposed to 1% TX-100 at 50 °C for 15 minutes at 180 rpm to disrupt the liposomal membrane thus allowing the reception of maximal DOX release, that's IDT. Then the same amount of liposome-FBS mixture was transferred into a dialysis tubing (14000MWCO), and then submerged into 100 mL HBS (pH 7.4) under stirring (180 rpm) at 4 °C or 37 °C. The absorbance at 493 nm was measured at the designed time point, that's IDt. Samples without incubation were measured as negative controls (IDo). DOX retention was calculated as retention (%) = 1 – (IDt − IDo)/(IDt − IDo))
Drug and Cypate loading content and entrapment efficiency
The drug loading content (DL) and entrapment efficiency (EE) were determined by UV-Vis spectroscopy. EE and DL were calculated based on the following equations:
DL(%) = We/(Wl + We); where We is the weight of encapsulated DOX/Cypate, which can be got from the UV absorbance after being completely released by exposing PTSL or TSL to TX-100, Wl is the weight of the liposome.
EE(%) = We/Wt; where We is the weight of encapsulated DOX/Cypate and Wt is the total weight of DOX/Cypate.
In vitro drug release dynamics
The scheme of the in vitro drug release studies was similar to that of the drug loading stability study as mentioned above.
Temperature-dependent DOX release
Temperature's influence on TSL's content release rate in 50% FBS was determined. Samples were first mixed with 50% FBS and then measured after twenty minutes incubation at desired temperatures from 37 °C to 50 °C and DOX release (%) was calculated as release (%) = (IDt − ID0)/(IDT − ID0).
Time-dependent DOX release
The rate of TSL's content release was monitored by incubating the TSL suspensions at the optimal temperature achieved from temperature-dependent release experiments for 1 hour period of time. DOX release (%) was calculated as the above equation.
Photothermal release of DOX
Firstly, The PTSL's photothermal conversion ability was characterized through the free cypate diluted in DMSO. Different concentrations of cypate (0.5 mL) from 5 mM to 150 mM were exposed to 780 nm laser (1.5 W/cm2) for 5 minutes. The temperature change was monitored using a thermocouple. On the other hand, 0.2 mL of saline was intramuscularly injected into the left leg of the mice and 100 mg/ml DOX@PTSL was injected into the same mice from the right leg. Then the mice were irradiated both on the right and left legs by 780 nm laser and taken infrared pictures using the Fluke Ti300 infrared thermal imager and the temperature was read through Smart View software. The amount of DOX released under 780 nm laser's irradiation was determined as it follows: 1 mL of DOX@PTSL was exposed to 1% TX-100 at 50 °C for 15 minutes at 180 rpm to disrupt the liposomal membrane thus allowing the reception of maximal DOX release, that's IDT. 1 ml of DOX@PTSL or DOX@TSL was transferred into a dialysis tubing (14000MWCO), and then submerged into 100 mL HBS (pH 7.4) under stirring (180 rpm) at 37 °C. The samples were taken out from the dialysis tubing for twice at 1 hour and 6 hours after dialysis, and put into a transparent glass tube to be irradiated with 780 nm NIR laser at an output power of 1.5 W/cm2 over a period of 5 minutes and then the samples were put back into the dialysis tubing to continue the release study. The absorbance of solution outside the dialysis tubing at 493 nm was measured at the designed time point, that's IDt. The release of DOX under no NIR laser irradiation was used as control. A493 of the samples without incubation were measured as IDo. DOX release (%) was calculated as (IDt − IDo)/(IDt − IDo).
Cell culture
Brain glioma cell line (U87) and liver hepatocellular cell line (HepG2) were all purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) for in vitro studies. Cells were cultured in RPMI-1640 medium supplemented with 100 mg/mL streptomycin, 100 U/mL penicillin and 10% (v/v) fetal bovine serum. They were maintained at 37 °C in a humidified atmosphere containing 5% CO2.
Locating properties of the functional liposomes in vitro
For the fourth dish of cells, after treated with DOX@PTSL, irradiation and incubation, the cells, mainly the lysosomes structures, were stained with Lyso Green (200 mL, 2.5 mM). After another 20 minutes' incubation, the samples were observed again. The green fluorescence of Lyso Green and the red fluorescence of DOX were collected under laser (488 nm and 568 nm) excitation.
In vivo acute toxicity
Animals received care in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals. Six-to-seven week old female nude mice were maintained under aseptic conditions in a small animal isolator and were housed in standard cages with free access to food and water. All animals acclimated to the animal facility for at least 7 days before experimentation. 12 Kunming mice (aged 3–4 weeks, weighed 18–20 g) were divided into 4 groups with 3 in every group. Group 1–4 were intraperitoneal injected with 0.2 mL saline, PTSL, DOX@PTSL(1 mg/kg Cypate), and 2 mg/kg free DOX, respectively.
The biodistribution of the functional liposomes in tumor-bearing mice
The H22 tumor bearing mice (with the tumor size of 0.4–0.7 cm3 in the upper left axillary fossa) were used to determine the dynamics and biodistribution of free cypate as well as DOX@PTSL in vivo. Firstly, 100 mg cypate was weighed and dissolved with 1 mL HBS (pH 7.4), and 0.2% DMSO was added to help the dissolution. Secondly, 0.2 mL of cypate and 0.2 mL of DOX@PTSL solution (1 mg/kg Cypate) was respectively intravenously injected into the tumor-bearing mice via the tail vein. Before that, the fluorescence images of the mouse before treatment was acquired from the NIR fluorescence imaging system and set as background imaging, the later fluorescence images were acquired at different time points (30 min, 1 h, 2 h, 4 h, 6 h, 8 h, 12 h, 24 h and 36 h) post injection. Thirdly, 4 hours after injected with cypate or DOX@PTSL, the mouse was sacrificed and dissected for different organs' NIR imaging studies. The fluorescence of overall distribution in mice and different organs dissected were all semi-quantitated through off-line programming software Image J, with the background as the fluorescence baseline.
Therapeutic efficacy of drug loaded functional liposomes in H22 tumor-bearing mice
The mice in DOX@PTSL, DOX and saline groups with laser-irradiated or not were taken infrared thermal pictures using the Fluke Ti300 infrared thermal imager and the temperatures were read through Smart View software.
Histopathological studies
The tissue mass (usually less than 0.5 cm thickness) were fixated in Bouin's solution (made of 1.22% saturated picric acid, 10% formalin and 2% glacial acetic acid). All the slides were stained with H&E staining.
Statistical analysis
Significant differences were determined using the Student's t-test where differences were considered significant at p < 0.05. All dates were expressed as mean ± standard error.
Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (NSFC 81220108012, 61335007, 81371684, 81000666, 81171395 and 81328012); the 973 Key Project (2015CB755504); and the Priority Academic Program Development of Jiangsu Higher Education.