Advanced Optical Microscopic Imaging Techniques for Imaging Amyloid Beta and Deciphering Alzheimer's Disease Pathogenesis
Funding: This work was supported by NIH (R01AG055413), (R01AG085562), (R21AG059134), (R21AG078749), and (S10OD028609) awards (C. R.). NIH Office of the Director, National Institute on Aging.
ABSTRACT
Alzheimer's disease (AD) is a neurodegenerative disease characterized by a progressive decline in cognitive functions. Given that AD undermines the quality of life for millions and has an extended asymptomatic period, exploring the full AD pathogenesis and seeking the optimal therapeutic solution have become critical and imperative. This allows researchers to intervene, delay, and potentially prevent AD progression. Several clinical imaging methods are utilized routinely to diagnose and monitor AD, such as magnetic resonance imaging (MRI), functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and single photon emission computed tomography (SPECT). Nevertheless, due to their intrinsic drawbacks and restrictions, such as radiation concerns, high cost, long acquisition time, and low spatial resolution, their applications in AD research are limited, especially at the cellular and molecular levels. In contrast, optical microscopic imaging methods overcome these limitations, offering researchers a variety of approaches with distinct advantages to explore AD pathology on diverse models. In this review, we provide a comprehensive overview of commonly utilized optical microscopic imaging techniques in AD research and introduce their contributions to image amyloid beta (Aβ) species. These techniques include fluorescence microscopy (FM), confocal microscopy (CM), two-photon fluorescence microscopy (TPFM), super-resolution microscopy (SRM), expansion microscopy (ExM), and light-sheet fluorescence microscopy (LSFM). In addition, we introduce some related topics, such as the development of near-infrared (NIR) Aβ probes, the Aβ plaque hypothesis, and Aβ oligomer hypothesis, and the roles of microglia and astrocytes in AD progression. We believe optical microscopic imaging methods continue to play an indispensable role in deciphering the full pathogenesis of AD and advancing therapeutic strategies.
Abbreviations
-
- AD
-
- Alzheimer's disease
-
- APOE
-
- apolipoproteins E
-
- APP
-
- amyloid precursor protein
-
- Aβ
-
- amyloid beta
-
- BBB
-
- blood brain barrier
-
- CAA
-
- cerebral amyloid angiopathy
-
- CM
-
- confocal microscopy
-
- dSTORM
-
- direct stochastic optical reconstruction microscopy
-
- ExM
-
- expansion microscopy
-
- ExR
-
- expansion revealing
-
- FDG
-
- fluorodeoxyglucose
-
- FM
-
- fluorescence microscopy
-
- fMRI
-
- functional magnetic resonance imaging
-
- IL
-
- interleukin
-
- LSFM
-
- light-sheet fluorescence microscopy
-
- MRI
-
- magnetic resonance imaging
-
- NIR
-
- near-infrared
-
- PET
-
- positron emission tomography
-
- RA
-
- reactive astrocytes
-
- ROS
-
- reactive oxygen species
-
- SIM
-
- structured illumination microscopy
-
- SPECT
-
- single photon emission computed tomography
-
- SRM
-
- super-resolution microscopy
-
- STAT3
-
- signal transducer and activator of transcription three
-
- STED
-
- stimulated emission depletion microscopy
-
- STORM
-
- stochastic optical reconstruction microscopy
-
- TPFM
-
- two-photon fluorescence microscopy
1 Introduction to Alzheimer's Disease and Imaging Approaches
1.1 Alzheimer's Disease: Incurable Disease Affecting Millions
Neurodegenerative diseases, caused by progressive neurodegeneration, significantly undermine the quality of daily life by affecting various fundamental activities, such as walking, talking, and learning. The most widely recognized and studied ND is Alzheimer's Disease (AD). As AD progresses, patients experience memory loss and other cognitive dysfunction. AD is an age-related disease, mostly diagnosed in the older population. It is estimated that over 50 million people are currently suffering from AD globally, and this number is expected to triple by 2050 [1]. Despite considerable ongoing efforts to elucidate the complete pathogenesis of AD and numerous clinical trials aimed at seeking effective therapeutic interventions, AD remains incurable. As AD advances, some pathological alterations gradually occur in the brain, including synaptic loss and neuronal death, leading to cognitive deficits and impaired information processing capability [2, 3]. The histopathological hallmarks of AD are the formation of amyloid beta (Aβ) senile plaques in extracellular space and neurofibrillary tangles by hyperphosphorylated microtubule-associated protein tau inside neurons [4]. Aβ exists in soluble forms (e.g., monomers, dimers, oligomers, and protofibrils) and insoluble forms (e.g., fibrils and plaques). Monitoring these biomarkers can offer valuable information about AD progression and the efficiency of therapeutic solutions.
1.2 Clinical Imaging Techniques: Foundations for AD Diagnosis and Monitoring
To date, various clinical imaging techniques have been routinely used in diagnosing and monitoring AD, including magnetic resonance imaging (MRI) [5-8], functional magnetic resonance imaging (fMRI) [9-11], positron emission tomography (PET) [12-17], and single photon emission computed tomography (SPECT) [18-21]. These imaging modalities provide researchers with noninvasive approaches to examine the functional state and anatomical details of the brain.
MRI, for instance, uses powerful magnets to generate a strong magnetic field, causing protons in the body to align with the field. A radiofrequency current pulse is then applied, which stimulates protons and causes protons to spin out of equilibrium. After turning off the pulse, the protons gradually return to realign with the magnetic field through various relaxation processes and release energy. These energy signals are detected and analyzed to reconstruct the images of organs and structures. One of the applications of MRI is to evaluate brain atrophy rates, which can sometimes serve as indicators of AD progression. In one study, it was shown that the entorhinal cortex in the medial temporal lobe of AD patients had a higher atrophy rate than that found in the hippocampus [5]. Further research has demonstrated a strong association between medial temporal lobe atrophy and cognitive deficits [6]. It was also suggested that atrophy in the temporal, parietal, and frontal neocortices correlates with cognitive decline, highlighting brain atrophy as a biomarker for AD [7, 8]. Several probes have been developed to detect Aβ plaques in MRI, including luminescent conjugated oligothiophenes [22], Gd-DO3A-Chal [23], and Gd (DOTA)-cyanine dyad [24].
While MRI can examine organs, tissue, or bones throughout the body, fMRI focuses on mapping the functional activity of the brain. Some fMRI results have revealed disrupted functional connectivity in several cognitive-related subregions, including the default-mode network, frontoparietal network, and visual network [9]. Notably, the connectivity between the hippocampus and posterior cingulate was lower in AD patients compared to healthy controls, corresponding to the hypometabolism of the posterior cingulate found in another early AD study [10]. fMRI scan has also been proven effective in distinguishing AD patients from individuals of healthy aging or mild cognitive impairment [11].
PET imaging utilizes radioactive tracers to assess metabolic brain activity. Before PET scanning, a tracer is usually injected into the vein. The tracer's unstable nuclei then emit positrons, which interact with electrons and generate gamma rays. In the scanner, a ring of detectors captures these gamma rays, enabling 3D reconstructions of tracer distribution [25, 26]. Multiple tracers have been developed for AD research and diagnosis. For instance, fluorodeoxyglucose (FDG), a glucose analog tracer, can detect hypometabolic areas in the brain, making it a powerful tool for early AD diagnosis [14]. PET scanning with FDG-PET has revealed focal hypometabolism in the parietal, temporal, and/or frontal cortices in AD patients [15]. More recent research reported that in older people with cognitive decline concerns, a normal brain F-18 FDG-PET scan can help rule out the short-term risk of dementia [16]. Another study suggested that combining F-18 FDG-PET and cerebrospinal fluid biomarkers can improve the accuracy of AD predictive diagnosis [27]. Some Aβ tracers have been developed to quantify pathological changes during disease progression, such as [18F] florbetapir, [18F] florbetaben, and [18F] flutemetamol [28]. In addition, some tau tracers, such as 18F-PI-2620 [29] and 18F-RO-948 [30], have also shown promising effects in AD diagnosis.
During SPECT imaging, a radioactive tracer (radionuclide) is injected into the bloodstream. This allows for the creation of a 3D map of radioactivity distribution within the patient, which helps assess the uptake of radiopharmaceuticals in the body. Although both PET and SPECT use radioactive tracers, they operate differently: PET measures photon emissions from positron annihilation, whereas SPECT detects gamma emissions from the tracers [18, 19, 31, 32]. SPECT is also actively used in the diagnosis and research of AD. For instance, research has shown that a positive SPECT result was associated with a 92% likelihood of AD, compared to an 84% likelihood from the clinical diagnosis of “probable” AD. This indicates that SPECT can provide valuable information in addition to clinical assessments [33]. Several SPECT tracers have been developed for imaging Aβ plaques. For instance, Dr. Cui's group designed the [CpRe/99mTc(CO)3] complex as Aβ probes for SPECT imaging. This tracer demonstrated strong binding to Aβ deposits in the blood vessels of brain sections from AD patients, as shown by in vitro autoradiography [34]. In another research, it was shown that radiolabeled (123) I-DRM106 detected Aβ deposits in transgenic mice with a higher sensitivity than the well-known probe (125I) I-IMPY. In addition, (125) I-DRM106 demonstrated a lower binding ratio of the diffuse plaque-rich lateral temporal cortex to the dense-cored/neuritic plaque-rich hippocampal CA1 area, compared with (11)C-PiB [35]. Another study highlighted the effectiveness of (123) I-ABC577, a radioiodinated imidazopyridine derivative, which showed significant retention in amyloid-accumulating regions, such as the frontal cortex, temporal cortex, and posterior cingulate, while exhibiting minimal retention in white matter [36].
While these clinical imaging methods provide avenues to study, diagnose, and monitor AD conveniently, their further application in AD research is limited due to several inherent drawbacks, including radiation concerns, high costs, long acquisition times, and limited spatial resolution. For example, even though Aβ PET scanning can precisely detect cerebral Aβ deposits, it cannot accurately detect aberrant Aβ or differentiate parenchymal Aβ from vascular Aβ. Due to spatial resolution limitations, PET imaging is incapable of providing structural characteristics of Aβ [37]. Furthermore, the abnormalities of the biomarkers, which signal the onset of AD, can appear over 20 years before the onset of clinical symptoms. This long asymptomatic period makes early-stage AD studies very challenging with current clinical imaging modalities [38, 39].
Therefore, in order to fully explore AD pathogenesis, adopting more accessible and reliable approaches that can overcome the limitations mentioned above becomes crucial. This would not only help us understand the causes of AD but also pave the way for early intervention, treatment, and potentially preventive therapies.
1.3 Optical Microscopy: Exploring AD at Cellular and Molecular Levels
Optical microscopic imaging methods offer several advantages over clinical imaging in AD research, including faster imaging speed, lower imaging costs, and higher spatial resolution. Through optical microscopic imaging, the exploration of AD pathogenesis is permitted at both molecular and cellular levels across a variety of models, ranging from cell culture in vitro to live animals in vivo.
In this review, we introduce several widely adopted optical microscopic imaging techniques and summarize their applications in understanding the causes of AD. Here, although neurofibrillary tangles are implicated in AD pathogenesis, we specifically focus on recent advancements and key findings in Aβ imaging.
In Section 2, we introduce the mechanisms of microscopic imaging techniques, including fluorescence microscopy (FM), confocal microscopy (CM), near-infrared (NIR) light, the developments in Aβ probes, two-photon fluorescence microscopy (TPFM), super-resolution microscopy (SRM), expansion microscopy (ExM), and light-sheet fluorescence microscopy (LSFM). A brief illustrative summary of these imaging methods is shown in Figure 1. In Section 3, we highlight the contributions of FM, CM, and TPFM to AD research by listing some major topics in AD research, including the Aβ plaque hypothesis and Aβ oligomer hypothesis and the dual effects from microglia and astrocytes. In Section 4, we focus on the applications and developments of SRM, ExM, and LSFM. Finally, we offer a perspective on areas that require further improvement.
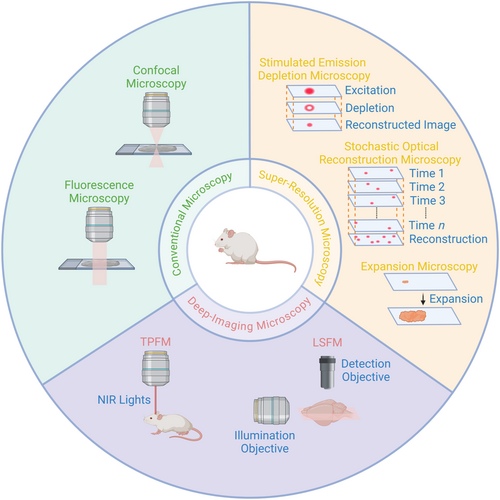
Optical microscopic imaging techniques in AD research. Created in BioRender.com. AD, Alzheimer's disease; NIR, near-infrared; TPFM, two-photon fluorescence microscopy.
2 Cutting-Edge Optical Microscopy Techniques in AD Research
Over the last few decades, microscopic imaging techniques have become increasingly important in AD studies. Conventional imaging techniques, such as FM and CM, are relatively simple to use. They enable visualization and tracking fluorescent labels on molecules with minimal sample preparation. However, they are limited to surface and shallow imaging due to light scattering and auto-fluorescence. Advances in NIR light permit researchers to perform in-depth imaging and analysis of thick samples, which drives the development of NIR probes for AD biomarkers, such as Aβ [40, 41]. TPFM, which utilizes NIR light, has been the major tool for in vivo imaging. Furthermore, the advent of SRM and ExM has significantly enhanced lateral imaging resolution to the scale of double-digit nanometers, allowing sub-diffraction imaging. Additionally, LSFM allows high-resolution volumetric imaging of intact AD mouse brains, providing another powerful approach for deep tissue imaging. In this section, we introduce these imaging techniques and briefly explain their operational mechanisms.
2.1 FM and CM: Accessible Solutions for Shallow Imaging
FM employs laser light to excite fluorophores, which label molecules of interest in specimens. Briefly, upon illuminating the specimen with light at a specific wavelength, the fluorophores are excited and enter an unstable and higher energy state. They then quickly return to the ground state and release energy in the form of light, which typically has a longer wavelength. This emitted fluorescence is captured by a photodetector or camera, revealing critical information about the specimens, such as molecule localizations, protein expression, and cell structures [42].
In contrast to traditional FM, which illuminates the entire specimen, CM adopts a spatial pinhole to block out-of-focus blurry signals. Despite the concern of photobleaching at the scanning points, CM effectively reduces background autofluorescence, improves signal-to-noise ratio, and boosts spatial resolution. As only a small sample area is illuminated at a time, the photobleaching of nonscanning fluorophores is thus minimized [43]. Both FM and CM serve as invaluable research tools for AD due to their ease of use and simple sample preparation. These imaging techniques are especially useful for imaging cultured cells or tissue slices in vitro.
However, when trying to image deeper proteins or structures, such as Aβ in an AD mouse brain in vivo, FM and CM are not applicable. This is because according to Rayleigh scattering [44], shorter wavelengths (e.g., blue and green) scatter more than longer wavelengths (e.g., orange and red). Therefore, the depth that a beam of light can penetrate in tissue is inversely correlated with its wavelength, making it challenging for deep-tissue imaging with shorter wavelengths. As a result, FM and CM are not the central tools for in vivo AD research.
2.2 NIR Light: Illuminating Deeper Brain Structures
NIR light has been employed for deep tissue imaging, owing to its exceptional tissue penetration ability and low background autofluorescence. Based on wavelength ranges, NIR light can be divided into three groups, including the first NIR window (NIR-I; 650–900 nm), the second NIR window (NIR-II; 900–1880 nm), and the third NIR window (NIR-III; 2080–2340 nm) [45, 46]. By combining NIR light with other advanced imaging modalities, deep tissue imaging is permitted [47]. For instance, by combining three-photon microscopy and NIR-III, an 1895 μm imaging depth was reported on brain vascular imaging after craniotomy [48]. In recent years, NIR light has been gradually used in AD imaging, and designing ideal chemical NIR probes for AD diagnosis has thus become a popular area of research [41, 47, 49, 50]. Here, we provide a brief overview of the development of some important NIR probes for AD.
2.3 NIR Aβ Probes: A Promising Path for Early AD Study
Over recent years, one of the imperative objectives of AD research has been synthesizing ideal fluorescent probes with superior blood-brain barrier (BBB) permeability, long emission wavelengths, enhanced specificity, and high quantum yield. Various NIR probes have been actively designed and synthesized, such as curcumin derivatives, cyanine derivatives, and BODIPY derivatives [51]. Here, we introduce some of the curcumin-derivative probes.
Curcumin has been used as a probe for imaging Aβ deposits. However, its short emission wavelength, poor BBB permeability, short blood lifetime, and low quantum yield restrict its application for in vivo imaging [52]. Consequently, designing and optimizing curcumin-derived probes has thus gained much popularity in recent years. In 2009, Ran et al. [53] designed a curcumin-derivatized NIR probe named CRANAD-2, which exhibits exceptional sensitivity and affinity (Kd = 38.67 nM) for Aβ aggregates. Upon binding to Aβ aggregates, the probe achieved a remarkable 70-fold fluorescence increase, with its quantum yields sharply increasing from 0.006 to 0.40, and an emission wavelength at 715 nm, making CRANAD-2 well-suited for NIR imaging. However, this probe is incapable of detecting soluble Aβ (monomer and oligomer), prompting further probe optimization.
In 2013, this group updated the design and synthesized a new probe, CRANAD-58. Apart from its long emission peak (750 nm), CRANAD-58 can detect both soluble and insoluble Aβ in vitro and in vivo. Moreover, CRANAD-58 can differentiate between AD and control mice at 4 months of age. It was suggested that CRANAD-58 has the potential to be adapted into PET probes [54].
To further improve the quantum yield in detecting soluble and insoluble Aβ, an updated design was brought up in 2014: CRANAD-28 (Figure 2). As expected, this curcumin analog probe displayed an outstanding quantum yield: 0.32 in Phosphate buffered saline and > 1.0 in ethanol (with rhodamine B as a reference). CRANAD-28 also inhibits the natural cross-linking of Aβ42 [55].
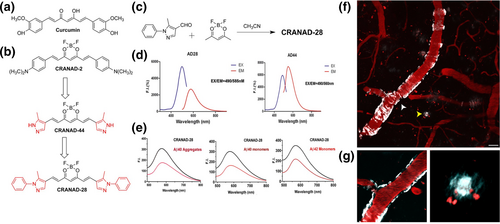
Design, excitation/emission spectra, and two-photon images of CRANAD-28. (a) Structure of Curcumin. (b) Design of CRANAD-28 through pyrazole replacement. (c) The synthetic route for CRANAD-28. (d) The excitation/emission spectra of CRANAD-28 and -44. (e) Fluorescence responses of CRANAD-28 with Aβ40 aggregates, Aβ40 monomers, and Aβ42 monomers. (f) and (g) Two-photon in vivo images of CRANAD-28 labeling in a 9-month-old APP/PS1 mouse. (f) Images were taken through a thinned-skull window 15 min after i. v. infusion of the dye. Both CAA and amyloid plaques were labeled with CRANAD-28. (g) Lower panels show zoomed-in single focal plane examples of CAA (white arrowhead, left panel) and amyloid plaque (yellow arrowhead, right panel). Blood vessels were labeled with Texas-red dextran (70,000 MW). Red punctate signals are auto-fluorescence intracellular structures. Scale bar: 25 μm. Reproduced/adapted with permission from Ref. [55].
In 2015, to design a probe to monitor Aβ levels during therapy, the group synthesized the curcumin analog CRANAD-3. In transgenic AD mice, CRANAD-3 successfully tracked Aβ reduction following drug treatment. This probe, with a maximum emission at 730 nm, exhibits a strong binding ability toward Aβ40/42 monomers, dimers, and oligomers [49]. Later, the group developed CRANAD-102, which specifically detects soluble Aβ [56]. The group also synthesized additional probes in the following years to image Aβ oligomers, such as PTO-41 [57], PTO-29 [58], and compound 37 [59]. Similar to the previously designed probes, these oligomer-specific probes exhibit similar exceptional traits, including large emission maxima, high affinity and specificity, and the capability to differentiate AD mice. Furthermore, the group also designed and synthesized some probes for Aβ fibrils, including ICTAD-1 [60], and PTAD-3 [61].
Ran research group also designed and developed the ADLumin-X series for Aβ imaging which used chemiluminescence [62]. For instance, two-photon imaging results showed that ADlumin-1 can quickly penetrate BBB and enter the brain, and it offers a significant contrast for both Aβ plaques and cerebral amyloid angiopathy (CAA), suggesting that ADlumin-1 is an excellent probe for two-photon imaging for Aβs in vivo. In addition, upon binding to Aβ species, including oligomers and fibrils, the chemiluminescence signals generated by ADLumin-1 were significantly amplified. Compared to the chemiluminescence in wild-type mice, ADLumin-1 in transgenic AD mouse brains produced approximately two-fold stronger signals [63].
These NIR Aβ probes offer tools to efficiently and accurately distinguish AD mice from controls, which can be beneficial for early-stage AD studies and monitoring.
2.4 TPFM: A Core Technique for Deep Brain Imaging
Since its invention in 1990 [64], TPFM has revolutionized brain imaging across various in vivo and in vitro models. By harnessing NIR light, TPFM enables deep-tissue visualization with an exceptional signal-to-noise ratio. In TPFM imaging, two longer wavelength photons are absorbed simultaneously to excite one fluorophore and thus achieve the same excited state as that excited by a single photon. As imaging depth is correlated with the wavelength of the lights, the long wavelength lights from TPFM can penetrate deeper tissue, enabling noninvasive visualization of inner structures. For instance, for in vivo mouse brain imaging, around 500 μm imaging depth can be commonly achieved [65]. In a more recent study, after combining superconducting nanowire single-photon detectors and TPFM, an over 1100 μm imaging depth was reported in mouse brain imaging [66].
In addition to the CRANAD-X series mentioned in the previous section, several other probes have also been designed and synthesized for the detection of Aβ aggregates and plaques in TPFM, such as QAD1 [67], IRI-1 [68], and PyrPeg [69].
In contrast to conventional FM which applies continuous laser lights, TPFM uses laser pulses to excite fluorophores on the focal plane, reducing the photobleaching of nonfocal plane fluorophores. Nevertheless, due to the low probability of near-simultaneous two-photon absorption, high-intensity laser pulses are required, raising photodamage concerns. Hence, various efforts have been reported to ameliorate photodamage during TPFM imaging (see [70]).
Even though the imaging depth of TPFM is not comparable to clinical imaging modalities, such as fMRI and PET, the short imaging duration and relatively high spatial resolution make TPFM an indispensable tool for in vivo AD research, especially in mouse models.
2.5 SRM, ExM, and LSFM: Revolutionary Advances in AD Research
Even though the imaging methods discussed above offer different advantages and have been routinely used in AD research, none can distinguish adjacent structures lower than the optical diffraction limit (∼200 nm). The advent of SRM breaks this spatial resolution barrier, significantly boosting the imaging resolution to a few nanometers. Various SRMs have been used in AD research, including stimulated emission depletion microscopy (STED), stochastic optical reconstruction microscopy (STORM), and structured illumination microscopy (SIM). STED was first brought up in 1994, and it typically uses two lasers: an excitation laser to illuminate the fluorophore and a donut-shaped depletion laser to quench fluorescence at the periphery, creating a sharp and clear central imaging point with lateral resolution down to 20–50 nm [71-73]. In contrast, STORM uses photo-switchable fluorophores, and only a fraction of fluorophores is switched on during each imaging cycle so that each active fluorophore is optically resolvable from the rest. The overall image is then reconstructed by processing a series of image scanning cycles, achieving an approximately 20 nm lateral resolution [74]. In SIM, a series of sinusoidal stripes of light is applied to the sample, which generates Moiré fringe patterns. With the Moiré fringe patterns and the low-frequency information from normal imaging, the high-frequency component, which cannot go through optical diffraction-limited microscopy, can be reconstructed to achieve sub-diffraction images [75]. Nonlinear SIM, which is a SIM variant [76, 77], has been reported to enable a 50-nm lateral imaging resolution.
However, these super-resolution techniques share some drawbacks, such as strict fluorophore selection, inevitable phototoxicity, and intense photobleaching. To address these challenges, Edward Boyden's lab introduced a new design in the SRM family, namely ExM in 2015 [78], which overcomes these constraints. During ExM sample preparation, the fixed samples are first embedded with hydrogel. Next, through gelation, homogenization, and expansion steps, the samples are physically enlarged [79, 80]. Different from SRM imaging modalities that focus on improving spatial resolution, ExM separates molecules isotropically, allowing standard CM to resolve previously closed structures. Even though incessant efforts have been made to increase the expansion factor, the fact that ExM can only be used on fixed samples restricted its applications for in vivo imaging.
In contrast to the sub-diffraction imaging techniques mentioned above, LSFM deploys a micrometer-thin plane of light to scan a thin layer of fluorophore-labeled transparent tissue or whole organism, achieving an optical sectioning of transparent tissues. Meanwhile, the photobleaching and phototoxicity during LSFM imaging are much weaker compared to other popular microscopic imaging methods, including CM and TPFM [81, 82]. Nevertheless, large tissue imaging with LSFM is restricted as light scattering precludes its application in imaging deeper tissues [83].
In Figure 3, we summarize the typical imaging resolution [43, 74, 84-89] and imaging depth [66, 82, 90-95] for each microscopic imaging method.
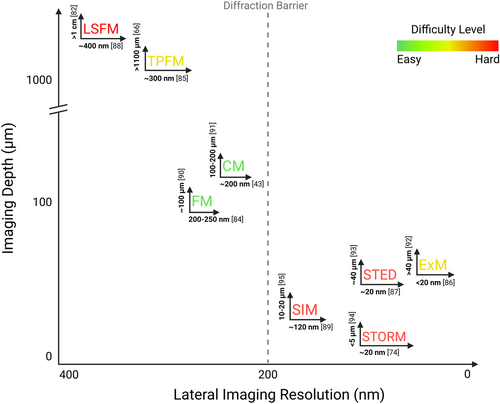
Summary of typical lateral imaging resolution and imaging depths for microscopic imaging methods. Each imaging method is labeled with its lateral resolution at the bottom and its imaging depth on the left side, followed by the reference. The difficulty of imaging is indicated by colors: red signifies the hardest and green denotes the easiest. Created in BioRender.com.
While these advanced techniques have intrinsic limitations and drawbacks, combining them or integrating them with other imaging modalities could significantly advance AD research and benefit us in investigating the full pathogenesis of AD.
3 Applications of FM, CM, and TPFM in Imaging AD Pathology
3.1 Aβ Plaques: Defining Markers of AD Pathology
Aβ is derived from a sequential proteolytic cleavage of amyloid precursor protein (APP) by β- and γ-secretases [96]. The length of Aβ ranges from 36 to 43 amino acids, with Aβ40 and Aβ42 being the most extensively studied species [97]. Approximately, 90% of the total Aβ is Aβ40, which primarily deposits in blood vessels, whereas Aβ42, the major component of plaques, accounts for less than 10% of total Aβ and accumulates in parenchymal deposits [98, 99]. One of the histological hallmarks of AD is the extracellular deposition of Aβ plaques. Notably, using TPFM and donor-acceptor-donor-type fluorescent probe (BTD-Aβ), researchers have observed Aβ42 aggregation within cells and in the brains of AD mice in vivo [100].
In 1992, Hardy and Higgins [101] proposed the amyloid cascade hypothesis, stating that the deposition of Aβ plaques leads to AD progression. Since then, evaluating the impacts of Aβ plaques in AD pathogenesis has become a significant area of research. Supporting evidence from dominantly inherited AD cases revealed that inherited gene mutations led to increased Aβ production or a higher Aβ42-to-Aβ40 ratio, with Aβ42 being more prone to aggregation [102].
Results from a series of studies have established a connection between plaque accumulation and AD progression. For example, individuals carrying the apolipoproteins E (APOE) ε4 allele face a higher risk of developing AD. Specifically, the APOE ε4 carriers have a slower Aβ clearance rate, higher Aβ aggregation level, and a concomitant enhanced cognitive decline compared to noncarriers [103]. Studies with CM and TPFM have demonstrated that while APOE ε4 expression exacerbates Aβ plaque buildup, plaque-related synapse loss, and dystrophic neurites, expression of APOE ε2 has opposite effects. These results highlighted the polarized effects of APOE isoforms on Aβ deposition and AD progression, suggesting a potential therapeutic strategy focused on increasing APOE ε2 expression in APOE ε4 carriers [104, 105]. In another study, in vivo TPFM imaging was performed through a thinned-skull cranial window on AD mice, and the results showed that Aβ plaques developed more rapidly in 6-month-old than in 10-month-old mice. A 20%–25% decrease in extracellular soluble Aβ reduced plaque formation in 6-month-old but not 10-month-old mice. Moreover, plaque formation rates were inversely correlated with their sizes (Figure 4) [106]. Long-term in vivo studies on AD mice using TPFM also suggested a relationship between the plaque size and the severity of neurite dystrophy [107, 108]. These results underscored the importance of early treatment in interfering with and delaying AD progression. Using spinning disc CM, it was shown that neuronal dendrites undergo spine loss near Aβ plaques. This leads to neuronal disconnection and degeneration, indicating a neurotoxic effect of Aβ plaques on surrounding neurons [109]. Additionally, other TPFM imaging results also supported this conclusion: not all neuronal activity is changed in the brains of AD mice, only neurons located close to Aβ plaques that exhibit hyperactivity are affected [110, 111]. Collectively, these findings underscore that Aβ plaque serves as a defining hallmark of AD, and it is associated with disease progression and cognitive decline.
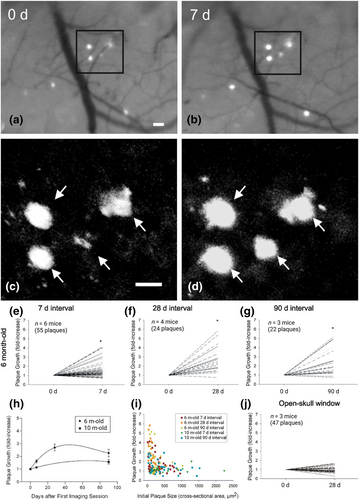
The growth of amyloid plaques is age- and size-related. (a–d) Serial in vivo multiphoton microscopy demonstrates growth of individual amyloid plaques. (a) and (b) Epifluorescence micrographs of 4 plaques labeled with methoxy-X04 and imaged over a 7 days interval in the brain of a 6-month-old APP/PS1 mouse. (c) and (d), Multiphoton micrographs of those same 4 plaques illustrate the growth of individual plaques over time. Scale bars, 20 μm. (e–j) Amyloid plaques exhibit age- and size-related growth under thinned-skull window preparations. Using serial intravital multiphoton microscopy, individual plaques were labeled with methoxy-X04 and imaged at multiple time points. Plaque growth was expressed as fold increases in a cross-sectional area relative to the initial plaque size. (e–g) In 6-month-old APP/PS1 mice, plaques exhibited significant growth over 7, 28 and 90 days intervals (paired t test, *p ≤ 0.05). (h) Time course of plaque growth in 6- and 10-month-old mice. In 6-month-old animals, the average fold increase in plaque size over a 28 days period was significantly greater than that observed over a 7 days period. There was no difference between the average fold increase over a 28 days period compared with that over a 90 days period (one-way ANOVA followed by Dunn's post hoc test). Plaques imaged in 10-month-old animals did not exhibit significant plaque growth at either the 7 or 90 days interval (paired t test). (i) Average plaque growth is plotted as a function of initial plaque size. Regardless of animal age, smaller plaques grew at a greater rate compared with larger plaques. (j) Plaques imaged in 6-month-old animals under open-skull window preparations did not exhibit significant growth over a 28 days interval. Reproduced/adapted with permission from Ref. [106]. Copyright © 2024 by the Society for Neuroscience.
3.2 Aβ Oligomers: The Real Culprits Driving AD Progression
In addition to the studies mentioned above, incessant studies have been performed to confirm that Aβ plaques drive AD progression. However, mounting evidence suggests that Aβ oligomers are implicated in AD pathology, and they are the main characters in triggering cognitive dysfunction instead of Aβ plaques [112-115].
For instance, using FM, MRI, and PET, several studies showed that the Osaka mutation (E693Δ) of APP, despite reducing overall Aβ production, induced severe cognitive impairment and elevated levels of Aβ oligomers in both patients and animal models [116-118]. In addition, in transfected cultured cell models, researchers found that Osaka mutation in APP leads to the intracellular formation of Aβ oligomers and cellular apoptosis [119]. One study demonstrated through FM imaging that reducing soluble Aβ significantly prevented the formation of Aβ plaques in transgenic mice. However, the levels of oligomers and the severity of cognitive deficits remained unchanged [120]. Similarly, results from CM and atomic force microscopy showed that promoting fibril formation decreased oligomer levels and mitigated cognitive impairments [121]. In vivo TPFM imaging showed that Aβ monomers elevate Ca2+ levels in cytosol and mitochondria, causing Ca2+ overload, synaptic loss, and neuronal death in AD [122-124]. Additionally, studies employing in vivo two-photon calcium imaging and CM suggested that preventing the aggregation of Aβ oligomer by monomer depletion could reverse early neuronal and synaptic dysfunction [125]. Together, these results indicate the strong neurotoxic effects of Aβ oligomers.
Several studies have also indicated that Aβ oligomers alone are sufficient for AD progression, and cognitive dysfunction is independent of Aβ plaques. For instance, in the APP Indiana mutation mouse model, CM imaging suggested that the neurotoxicity induced by Aβ is independent of Aβ plaque deposition, and the observed deficiency in synaptic transmission preceded Aβ plaque formation by several months [126]. In other AD mouse models, cognitive dysfunction also occurred before the formation of Aβ plaques and was correlated with the accumulation of intraneuronal Aβ [127-131]. Moreover, CM imaging results revealed that intraneuronal Aβ forms Aβ oligomers in an age-dependent manner in mouse models [132]. Intriguingly, under multiphoton microscopy, Aβ oligomers were observed forming a halo around plaques, interacting with a subset of postsynaptic densities in AD mice, which may contribute to synaptic losses [133]. In our previous study, TPFM results suggested that Aβ plaques have a reservoir function that sequesters and releases AkaLumine probes (Figure 5) [134]. Other studies also showed that Aβ plaques serve as reservoirs for toxic oligomers, which gradually aggregate into diffuse plaques. Once the plaque reservoir becomes saturated or loses capacity, toxic oligomers become free and disrupt adjacent neuron networks [135, 136].
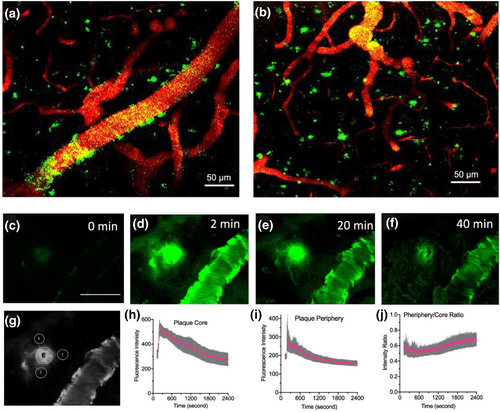
In vivo two-photon microscopic fluorescence imaging with AkaLumine. (a) CAA Aβ deposits (green) labeled by AkaLumine on blood vessels (red) in the brain of a 12 month-old 5xFAD mouse. (b) Aβ plaques (green) labeled by AkaLumine in a cortex area. (c)–(f) Representative images of a plaque at different time points after AkaLumine injection (scale bar: 50 μm). (g) Representative region of interest for quantification. (h) Time-course of plaques (n = 6). (i) Time-course of peripheral areas of plaques (n = 18). (j) Time-course of the intensity ratio of the periphery/core. Error bars: SEM. Reproduced/adapted with permission from Ref. [134]. Copyright © 2023 American Chemical Society.
Additionally, multiple studies on Aβ immunotherapy underscore the central role of oligomers in AD progression. For example, upon the administration of Aβ oligomer selective antibodies in AD mice, memory performance was rescued in various degrees [137-141]. Conversely, some studies reported that although immunization with Aβ42 led to the clearance of Aβ42 plaques in AD patients, neurodegeneration continued, along with severe dementia during the follow-up investigations [142, 143].
Together, these results and findings suggest that Aβ oligomers instigate neuronal degeneration and AD progression. Nevertheless, the complete mechanism of how Aβ oligomers are involved in AD development requires further investigation.
3.3 Microglia and Astrocytes: Double-Edged Swords in AD
Microglia and astrocytes are integral components of the glial cell family, which help maintain the homeostasis of the central nervous system. Microglia, as the primary immune cells of the central nervous system, destroy and remove pathogens and damaged cells, whereas astrocytes provide support for neurons, facilitate neuronal reparation, and maintain the BBB [144-146].
Following Aβ plaque formation, microglia become reactive in close vicinity of these plaques, and they can actively uptake Aβ plaques through microglial phagocytosis. A previous study using CM showed that lacking scavenger receptor class A induced a 50% decline in phagocytic activity, leading to increased Aβ plaque levels [147]. While Aβ accumulation can inhibit scavenger receptor class A expression in microglia through neuroinflammation, forming a vicious cycle that accelerates plaque growth and AD progression [148]. Results from TPFM and CM imaging indicated that activated microglia contribute to Aβ plaque formation and growth. This is because Aβ uptake leads to microglial cell death, causing the release of accumulated Aβ back into the extracellular space [149]. In addition, one recent study using FM, CM, and TPFM suggested that microglia function as carriers, spreading Aβ to previously unaffected tissues [150].
Microglia have two different polarization states: M1 and M2. The pro-inflammatory M1 phenotype, which acts as the first-line defender, is responsible for initiating inflammatory responses. In contrast, the function of the anti-inflammatory M2 phenotype is to repair the damaged tissues and suppress inflammatory responses [151]. Upon M1 activation, an immune cascade begins, and microglia release several pro-inflammatory cytokines, including tumor-necrosis factor-α, signal transducer and activator of transcription 3 (STAT3), interleukin (IL)-6, IL-12, and IL-23. Overexpression of these cytokines has been implicated in neuroinflammation and neurodegeneration. Aβ clearance from the brain is thus reduced, leading to an increased level of Aβ accumulation [152-156]. For instance, CM and TPFM imaging showed that the peripheral production of tumor-necrosis factor-α is implicated in synaptic instability and cognitive deficits [157]. The application of STAT3 inhibitors to AD mice was found to directly mitigate parenchymal Aβ plaques and improve cognitive functions [158]. Likewise, in another study, deleting STAT3 in astrocytes of AD mice decreased the level of Aβ. In addition to exhibiting a more complex morphology, the microglia, which were close to plaques, internalized more Aβ, promoting Aβ clearance of both overall Aβ and Aβ plaques. Meanwhile, in STAT3-deficient AD mice, astrocytes showed a decreased level of pro-inflammatory cytokine activation, and cognitive dysfunction was ameliorated. These results highlight the promising role of inhibiting STAT3 in treating AD, providing a potential therapeutic target [159]. On the other hand, the anti-inflammatory cytokines from M2 phenotype glial cells, including transforming growth factor beta, IL-4, IL-10, IL-11, and IL-13, prevent acute inflammation and inhibit the creation of pro-inflammatory cytokines [151, 160].
As the most abundant type of glial cells, astrocytes have been found to possess both neuroprotective and neurotoxic effects depending on the progression of the disease [161]. During the early stage of the disease, astrocytes could degrade and clear Aβ through phagocytosis, releasing Aβ-degrading proteases, such as neprilysin. It has been shown that applying neprilysin inhibitors to the hippocampus and cerebral ventricle of rats increased Aβ levels, together with cognitive dysfunction [162, 163]. The reactive astrocytes (RA) are classified into two phenotypes, A1 and A2. The A1 phenotype, activated by microglial cytokines, secretes pro-inflammatory substances and neurotoxins, leading to neuronal death. The A2 phenotype RA upregulates anti-inflammatory substances and promotes neuronal reparation [164-166]. As AD progresses, the presence and deposition of Aβ have been found to induce an increase in the pro-inflammatory phenotype A1 RA, which in turn causes A1 RA to release more inflammatory mediators, including reactive oxygen species (ROS), pro-inflammatory cytokines, and chemokines. These pro-inflammatory signals accelerate the A1 RA activation, leading to a vicious cycle that exacerbates neuronal damage and expedites AD progression [161, 167]. Targeting astrocytes to restore cognitive function, as demonstrated in studies combining FM, TPFM, and optogenetics, can potentially slow AD progression [168]. Using the NIR probe CRANAD-61, TPFM results suggest that the “active” Aβ plaques are surrounded by elevated levels of ROS, indicating greater neurotoxicity compared to “inactive” plaques with lower adjacent ROS levels [169]. This suggests a heterogeneous effect of neuronal toxicity among Aβ plaques.
These findings illustrate that microglia and astrocytes have both beneficial and detrimental roles during AD progression, depending on the stages of the disease. However, additional research is essential to better understand how each subtype influences AD progression.
4 Pushing the Boundaries of AD Imaging: SRM, ExM, and LSFM
4.1 SRM: Overcoming Diffraction Limits
The diffraction barrier once prevents researchers from visualizing structures smaller than approximately 200 nm with conventional microscopy, such as CM. Thanks to the design of SRM, including STED, STORM, and SIM, nanometer-scale details during imaging in AD studies have been routinely captured [170-172]. For instance, using STED, it has been shown that among AD patients, the parameters of Aβ aggregates, such as the aggregate number, size, and intensity, correlate with the severity of cognitive impairment [173]. In addition, this high-resolution imaging technique allows the visualization of Aβ aggregates in vitro and in the brain section of 17-month-old AD mice at a spatial resolution of 29 and 62 nm, respectively [174]. By combining array tomography and STED, it was shown that Aβ plaques consist of high-order Aβ species (∼0.22 μm3) at the dense core and low-order Aβ structures (∼0.003 μm3) around them, forming a peripheral halo [175].
In an early study, researchers utilized direct STORM (dSTORM) to not only probe the Aβ aggregation process but also image the morphology of the ensuing aggregates (< 20 nm resolution). Besides successfully distinguishing oligomeric assemblies from mature fibrils, the species formed in vitro and in vivo were found to have distinct morphologies. This study showed that monomeric Aβ40 peptides were rapidly internalized and sorted into endosomes of cultured Hela cells using SIM [176]. Later, it was shown in another study using dSTORM that the kinetic of Aβ42 is faster than Aβ40. Surprisingly, Aβ40 aggregates adopt a mostly spherical shape with an average diameter of around 160 nm. In contrast, Aβ42 aggregates consist of both spherical and elongated morphologies, and the average size is around 225 nm. This study also suggested that Aβ internalization into neurons promotes Aβ aggregation, probably due to the spatial confinement within cellular compartments [177]. In another study, SIM results showed that the aggregates formed by hereditary arctic mutation 42 (E22 G) were more compact and resistant to degradation than those formed by wild-type Aβ [178]. Notably, dSTORM imaging revealed that the growth of Aβ fibrils is highly polarized, with one end growing faster than the other [179].
Given that synaptic loss strongly correlates with cognitive decline and is a key feature of AD, various research studies have been conducted around synapses using SRM. For example, through STED and dSTORM, γ-secretase was found to localize at both presynapse and postsynapse, while its activity might correlate with synapse maturation [180]. In another study, Aβ42 was only observed in small vesicle presynaptic compartments, but not postsynapses in culture neurons [181]. Combining CM and STORM, researchers found that the synapses in the AD human brain exhibit decreased cluster areas and increased cluster counts compared to controls, suggesting some critical nanoscale alterations and reorganizations at presynapse during AD [182].
For imaging probes in STED, ATTO 488 has been reported as a secondary antibody [183]. Other studies utilized CRANAD-2 [184] and Thioflavin-T [185] for imaging Aβ fibers. In STORM imaging, HiLyte Fluor 488, HiLyte Fluor 647, and Alexa Fluor 647 all showed exceptional imaging quality [177, 186, 187].
In addition to the applications of SRM in the AD study mentioned above, considerable efforts have been made to integrate SRM into in vivo imaging [188]. Nevertheless, maximizing imaging depth while preserving lateral resolution and minimizing photodamage during SRM imaging will undoubtedly be a central focus for AD research in the coming decade.
4.2 ExM: Unlocking Sub-Diffraction Imaging via Physical Enlargement
In ExM, samples are physically enlarged by anchoring them to swellable hydrogels, enabling substantial improvements in imaging resolution based on the degree of expansion. When ExM was first introduced, it allowed a 4.5-fold linear expansion, achieving a lateral resolution of approximately 70 nm [78]. Over the last decade, multiple ExM variants have been designed that have surpassed this resolution. For instance, iterative ExM can expand samples over 20 times, resulting in an impressive 25 nm resolution [189]. Recently, Boyden's group designed a single-step ExM, which could also reach 20-fold expansion, achieving < 20 nm resolution [86]. ExM can also combine with other SRM techniques to maximize the final lateral imaging resolution, such as ExM + SIM (25–40 nm) [190], ExM + STED (∼10 nm) [191], and ExM + STORM (5–10 nm) [192, 193].
So far, studies that apply ExM in AD research are very limited. For example, in 2022, Boyden's group found that the previously crowded proteins, which could not be labeled by antibodies, were successfully separated and labeled using expansion revealing (ExR). By labeling Aβ after expansion, they observed that Aβ formed periodic nanoclusters, which could not be observed when labeling was conducted before expansion (Figure 6). Remarkably, these periodic Aβ nanostructures were found to co-localize with Nav1.6 and Kv7.2 channels. As Nav1.6 and Kv7.2 are involved in regulating neuronal excitability, this further provides evidence that Aβ aggregation is implicated in instigating neuronal hyperexcitability [194]. Another study showed that Aβ oligomers, rather than directly binding at the synaptic cleft, form nanoclusters encircling the postsynaptic membrane, with many attaching to presynaptic axonal terminals. It is thus believed that Aβ oligomers can disrupt the synaptic function through spatially restricted signaling mechanisms [187].
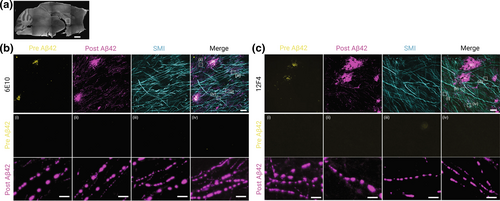
ExR reveals periodic nanoclusters of Aβ42 peptide in the fornix of Alzheimer's model 5xFAD mice. (a) Epifluorescence image showing a sagittal section of a 5xFAD mouse brain with the fornix highlighted (dotted box). Scale bar, 1000 μm. (b) and (c) Top row: ExR confocal images (maximum intensity projections) showing immunolabeling against Aβ42 peptide with two different monoclonal antibodies 6E10 (b) and 12F4 (c). From left to right: pre-expansion immunolabeling of Aβ42 (yellow), post-expansion labeling of Aβ42 (magenta), post-expansion SMI (neurofilament protein), and merged pre- and post-expansion staining of Aβ42 with post-expansion staining of SMI. Middle and bottom rows: insets i–iv showing the regions of interest highlighted (dotted boxes) in the merged images in the top rightmost panels of (b) and (c); middle row: pre-expansion Aβ42 labeling; bottom row: post-expansion Aβ42 labeling. Post-ExR staining reveals periodic nanostructures of β-amyloid, whereas pre-expansion staining can detect only large plaque centers. Scale bar, 10 μm (top); 1 μm (bottom, i–iv). Reproduced/adapted with permission from Ref. [194]. Copyright 2022, Springer Nature.
While ExM can breach diffraction limits by spatially separating closely packed structures, certain restrictions must be addressed before considering its applications in broader AD research. First, because ExM requires fixed samples, this technique is incompatible with in vivo studies. Second, if the labeling step is performed before expansion, the final fluorescence signals are much dimmer after expansion, which requires higher excitation laser power during imaging. Third, despite the efforts to maintain the best global isotropy during the expansion process, some double-digit nanometer distortions currently remain inevitable [194].
4.3 LSFM: 3D High-Volume Imaging
LSFM has emerged as a powerful tool for deep imaging. This technique illuminates transparent specimens from the side with a thin layer of beam. This beam excites the fluorophores within the narrow plane of light, achieving optical sectioning. LSFM enables cellular-level exploration of intact mouse brains at high acquisition rates [83], making it a valuable tool in AD research.
For instance, by combining LSFM with the iDISCO clearing method, researchers could visualize plaque formation in AD mice and patient brains. They found that Aβ plaques in human brains exhibit higher 3D complexity, along with large 3D amyloid patterns. Surprisingly, no comparable large amyloid pattern was observed in AD mice, underscoring a potential distinct difference in aggregation mechanisms between human and mouse species [195]. In another study, using macrolaser LSFM and hydrophilic tissue-clearing technique, it was shown that the average total surface volumes of Aβ accumulation in the brain of 44-week-old 5xFAD mice were significantly higher than the control group. They also suggested a higher number of astrocytes and microglia in aged AD mice, which indicates that second-wave and late-stage active neuroinflammation still occur in aging AD brains [196]. In a recent study, LSFM imaging results unveiled that oligodendrocytes, which are one type of glial cells, produce Aβ, contribute to plaque formation, and participate in establishing primary Aβ pathology [197]. Efforts are ongoing to improve the spatial resolution of LSFM. For example, combining SIM and LSFM achieved sub-100 nm lateral imaging resolution [198]. Later, the design of light-sheet localization microscopy further increased lateral resolution to approximately 75 nm [199].
These 3D, high-speed, volumetric super-resolution LSFM variants offer researchers diverse methods to visualize the Aβ plaques and oligomers at sub-diffraction resolution in the contact brain, which will play a major role in understanding the full pathogenesis of AD at the subcellular level.
5 Summary and Future Perspectives
5.1 Advantages and Limitations of Microscopic Optical Imaging Techniques: Versatile Tools With Inherent Drawbacks
Microscopic optical imaging techniques provide powerful tools for studying the pathology of AD. Here, we summarize the imaging techniques discussed in this paper and highlight their advantages and limitations.
As one of the most widely adopted imaging techniques, FM allows researchers to conveniently observe samples with simple preparation steps. Upon staining the samples with highly specific probes, researchers can quickly assess the target proteins or structures. With the advent of CM, the out-of-focus signals are efficiently minimized which significantly improved lateral imaging resolution with almost no changes in sample preparation protocols. These two imaging techniques have become the central tool for imaging cells and thin tissue slices. However, due to light scattering, thick sample imaging (> 100 μm) remains challenging with the current settings. Additionally, CM users often face trade-offs between lateral imaging resolution, scanning speed, field-of-view, and imaging bleaching of fluorescence probes.
TPFM utilizes NIR light, which has a longer wavelength that reduces light scattering and allows for deeper imaging. Since excitation occurs only at the focal point, this technique minimizes phototoxicity, making it particularly important for imaging live samples. TPFM has become indispensable for visualizing the Aβ species within live mouse brains, which serves as the critical model for AD research. However, due to the low probability of near-simultaneous two-photon absorption, the signal intensity is lower compared to one-photon excitation. Hence, to optimize imaging quality, laser power may need to increase, which raises the risk of photodamage. So far, imaging Aβ species within the AD mouse brain still requires the surgical preparation of a cranial window before TPFM imaging. In addition, compared to CM, TPFM has more complex laser settings, and some fluorophores lack optimal two-photon absorption properties, requiring careful selection of probes.
SRM breaches the diffraction barrier and enables visualization of the fine details of target proteins and structures at resolution from several tens to single-digit nanometers, allowing observation at single-molecule levels. These super-resolution techniques, including STED, STORM, and SIM, are powerful tools for studying the compositions and connections within each Aβ species, and they are likely to remain prominent topics in AD research. Nevertheless, several limitations hinder their use. For instance, STED requires robust probes that can withstand intense laser light during scanning. Despite using recommended fluorophores, such as abberior STAR RED or Alexa Fluor 594, even a slight increase in laser power can lead to significant bleaching of the fluorophores within the field of view after just one scan. Additionally, SRM scanning is generally time-consuming, and researchers must be cautious about potential drift, which can cause artifacts and lead to false interpretations and conclusions. This suggests that optimizing image quality necessitates extensive experience. Furthermore, as SRM techniques are incapable of deep imaging in live brains, their application in AD research is currently still limited to image single cells or thin tissue slices.
By physically enlarging the sample isotropically, ExM also offers high-resolution imaging down to a few nanometers. Different from SRM, ExM is generally applicable to most probes, and the expanded gel can be observed using conventional CM. The lower cost and less preparation complexity make ExM a promising approach for in vitro AD studies. However, some limitations of ExM are inevitable. First, the expanded gel, such as 20-fold expansion, would become so thick that it might exceed the working distance of high-index lens. Hence, users might need to opt for the low-index lens, which would sacrifice lateral resolution. This requires trade-offs between initial slice thickness and final imaging resolution. Second, some distortions during sample preparation and imaging are almost avoidable which can undermine the result accuracy. After all, despite the ongoing development for a higher expansion factor, ExM remains not applicable for in vivo studies, highlighting the need for further innovations.
LSFM, on the other hand, has been extensively used on living samples. It illuminates a thin slice of the sample with light perpendicular to the direction of observation. The photodamage and background are greatly reduced as only one plane is illuminated at one time, which enhances image contrast. Additionally, instead of scanning point by point in CM, LSFM scans the entire plane which drastically improves imaging speed, which makes it a perfect tool for scanning mouse brain. In the meantime, LSFM also has some inherent limitations. For instance, as LSFM requires two or more objectives, the implementation and alignment can be quite challenging. In addition, the preparation procedure for live samples is very complicated and there are various constraints. The sample must fit within the working distance of both the observation lens and the perpendicular excitation lens, which restricts the sample size. Lastly, LSFM's low lateral resolution poses a significant disadvantage compared to other microscopic imaging techniques.
5.2 Future Perspectives: AD Research in the Next Decades
Even though optical microscopic imaging methods have significantly advanced AD research, further advancements are needed to address certain limitations. For example, the current maximum imaging depth of TPFM is only slightly more than 1000 μm. Achieving greater depths without sacrificing the signal-to-noise ratio remains a challenge that will require extensive development and innovation. In addition, some SRM techniques, such as STED and STORM, have strict probe selection criteria and can cause considerable phototoxicity during imaging. Making SRM techniques compatible with a broader range of AD probes and reducing phototoxicity are crucial issues to address. Furthermore, integrating ExM with existing SRM techniques deserves much more attention as this can potentially achieve sub-nanometer imaging resolution with existing techniques. Finally, even though trials on combining LSFM with SRM are already in progress, achieving a better lateral and axial resolution while increasing scanning speed requires additional investigation and research.
Aβ plaques exhibit different morphologies, ranging from diffuse to dense-core structures [200-202]. Currently, it is believed that dense-core plaques consist of fibrillar amyloid aggregates, while diffuse plaques contain amorphous prefibrillar aggregates [203-206]. With the development of advanced microscopic imaging techniques, more pathological details regarding the heterogeneous nature of Aβ plaques are expected to be unveiled in the future.
In summary, optical microscopic imaging methods offer a powerful suite of noninvasive tools that enable researchers to visualize, monitor, and study AD progression across diverse models. They also offer insights into the complex mechanisms of AD pathogenesis, facilitating the seeking of optimal therapeutic solutions.
Author Contributions
Shiju Gu: conceptualization (lead), methodology (lead), validation (lead), writing–original draft (lead). Chongzhao Ran: funding acquisition (lead), investigation (lead), project administration (lead), resources (lead), supervision (lead), writing–review & editing (lead).
Acknowledgments
The authors have nothing to report.
Ethics Statement
The authors have nothing to report.
Consent
The authors have nothing to report.
Conflicts of Interest
Professor Chongzhao Ran is the member of the iRADIOLOGY Editorial Board. To minimize bias, he was excluded from all editorial decision-making related to the acceptance of this article for publication. The remaining author declare no conflict of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this article.




