Bioresponsive fluorescent probes active in the second near-infrared window
Yuqin Chen and Jie Chen contributed equally to this work.
Abstract
Emerging stimuli-responsive composite probes active in the second near-infrared window (NIR-II, 1000–1700 nm) hold vast potential for improving in vivo imaging performance with minimized noise interference. The interactions among external irradiation, shell species, and the emissive core are key factors in the design of smart structures. The external irradiation provides energy for shell species and the emissive core to generate intense NIR-II fluorescence signals, while the energy transfer process hinders NIR-II emission in the inner structure of smart composite probes. However, if pathophysiological stimuli interrupt the above processes, then NIR-II fluorescence signals are recovered. This review covers NIR-II imaging based on diverse smart composite NIR-II fluorescent probes responding to various biological stimuli, including ONOO−, overexpressed reactive sulfur species, abnormally expressed enzymes, and abnormal levels of physiological metabolites. Finally, to appreciate these advances, the challenges and perspectives of stimuli-responsive composite NIR-II probes are highlighted.
Abbreviations
-
- ACIE
-
- absorption competition-induced emission
-
- AKI
-
- acute kidney injury
-
- CKD
-
- chronic kidney disease
-
- CT
-
- computed tomography
-
- DCNPs
-
- down conversion nanoparticles
-
- FAP
-
- fibroblast activation protein
-
- FI
-
- fluorescence imaging
-
- FRET
-
- fluorescence resonance energy transfer
-
- GSH
-
- glutathione
-
- HCC
-
- hepatocellular carcinoma
-
- MMP
-
- matrix metalloproteinase
-
- MRI
-
- magnetic resonance imaging
-
- NIR-II
-
- second near-infrared window
-
- PAI
-
- photoacoustic imaging
-
- PET
-
- photoinduced electron transfer
-
- QDs
-
- quantum dots
-
- RNS
-
- reactive nitrogen species
-
- RSS
-
- reactive sulfur species
-
- TME
-
- tumor-microenvironment
-
- UCNPs
-
- upconversion nanoparticles
1 INTRODUCTION
In contrast to mature imaging modalities, for example, magnetic resonance imaging (MRI) [1], computed tomography (CT) [2], positron emission tomography [3], and ultrasound (US) imaging [4], optical imaging uses a nonionizing radiation to obtain images noninvasively with high spatiotemporal resolution and sensitivity [5]. However, in the optical imaging of biological targets, light is easily dispersed and absorbed by endogenous biological tissues, including blood, fat, and skin, making it challenging to image deep tissues [6]. Fluorescence imaging (FI) is used to visualize and quantify molecular information as well as to monitor pathological changes in organisms because of its excellent features, such as high sensitivity, high spatiotemporal resolution, real-time detection, and noninvasiveness [7], which is associated with the optical emission window of numerous luminescent materials. However, light in the visible (400–650 nm) and first near-infrared (NIR-I, 750–900 nm) bands passing through tissues experiences significant scattering, absorption, and subtle autofluorescence from diverse endogenous biomolecules [8], resulting in a relatively low signal-to-background ratio (SBR). These factors represent significant challenges for achieving deep tissue imaging and clinical transformation. Nevertheless, by using long wavelength NIR-II light (1000–1700 nm), light scattering, signal absorption, and background noise can be eliminated to provide detailed physiological and pathological information [9, 10]. In contrast to visible and NIR-I light, NIR-II light is less susceptible to absorption as well as scattering, allowing imaging with a micron-level resolution at tissue depth [11, 12]. In this context, NIR-II optical nanomaterials are gaining considerable attention in the field of biomedical applications [13].
Luminescent nanomaterials comprising rare earth metal ion complexes [14-17] or composites [18, 19], inorganic quantum dots (QDs) [20-22], inorganic single-walled carbon nanotubes [23], organic polymers [24], and small-molecule organic dyes [25, 26] are representative NIR-II optical probes [27]. These probes can be used in therapeutic applications ranging from noninvasive diagnosis to imaging-guided surgery and real-time monitoring of physiological processes in living organisms. Whether they exist in vivo, most optical probes are “always-on” or nonspecific in that they require excitation by an external light source with an optimized wavelength to emit optical signals. Notably, high-resolution and high-contrast optical imaging needs high SBRs, which are dependent on the signals arising from the target tissue and background. In this regard, probes that accumulate in healthy tissues severely affect the SBR [28] and can interfere with diagnostic processes, particularly in the liver, kidney, and spleen. To this end, smart optical probes that can switch from an “off” state to an “on” state have been developed. More importantly, “off-to-on” switching can be activated by certain pathophysiological stimuli, for instance, abnormal pH [29], viscosity [30], abnormal levels of reactive oxygen species [31], reactive nitrogen species (RNS) [32], reactive sulfur species (RSS) [33], as well as overexpressed enzymes [34, 35]. Compared with conventional “always-on” and “non-specific” optical probes, stimuli-responsive optical probes can boost the accuracy and sensitivity of in vivo NIR-II imaging under pathophysiological conditions [36]. Moreover, damage to normal tissues can be minimized by keeping the probe “inert” in noncancerous locations [37]. With this regard, smart on-demand probes will facilitate the clinical application of NIR-II imaging [38].
Although stimuli-responsive small-molecule fluorescent probes are responsive to pathophysiological microenvironments, they usually respond a single stimulus only, which represents a roadblock in the exploration of clinic applications. Moreover, small-molecule organic probes are difficult to design, synthesize, and purify, which also hinder their clinical applications [39]. This review summarizes the recent advances in NIR-II stimuli-responsive composite probes, focusing on the interactions among external irradiation, shell species, and the emissive core, which are key factors in the design of smart structures. As shown in Figure 1, external irradiation provides energy to both shell species and the emissive core, while energy transfer processes, for example, intramolecular charge transfer, Förster/fluorescence resonance energy transfer (FRET), photo-induced electron transfer (PET), inner filter effect (IFE) [40], and absorption competition-induced emission (ACIE), hinder NIR-II emission in the inner structure of smart composite probes. However, if pathophysiological stimuli interrupt the above processes, then NIR-II fluorescence signals are recovered. This review covers NIR-II imaging based on diverse smart composite NIR-II fluorescent probes responding to various stimuli, including ONOO−, overexpressed RSS, abnormally expressed enzymes, and abnormal levels of physiological metabolites. Finally, the challenges and perspectives of stimuli-responsive composite NIR-II probes are discussed.
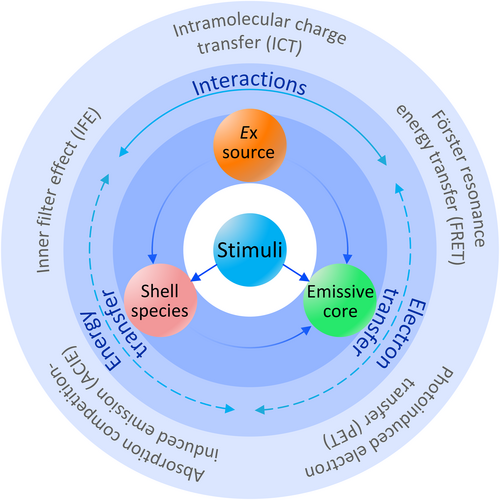
The nature of bioresponsive optical probes for in vivo imaging. By exploiting diverse energy transfer effects, second near-infrared stimuli-responsive optical probes can be used for biosensing and bioimaging applications, meeting multiple specific requirements and improving the precision and efficiency of disease diagnosis in the clinic.
2 ONOO−-RESPONSIVE NIR-II IMAGING
ONOO− is an RNS and a biomarker of the liver status because ONOO− overexpression induced by herbal medicines can cause hepatic damage [41, 42]. In ONOO−-responsive optical probes, strong oxidation by ONOO− can block the ACIE effect between organic species and downconversion nanoparticles (DCNPs). Peng et al. [43] assembled lanthanide-based NIR-II luminescent nanoparticles with a dye that absorbs energy in the NIR-II spectral window, thereby achieving highly accurate, efficient, and noninvasive detection of ONOO− in the liver. In detail, the downconversion emission of DCNPs at 1060 nm is effectively quenched on the surface of bioresponsive dyes via the ACIE effect (Figure 2a). As shown in Figure 2b, the surface of DCNPs was modified with small organic molecules through layer-by-layer modification. The bioresponsive dyes had a high absorption coefficient and absorption cross section and thus can compete with DCNPs and absorb excitation light (Figure 2c). When ONOO− in damaged hepatic tissues is encountered, the composite DCNPs can be turned “on” to enable NIR-II luminescence as the concentration of ONOO− changes (Figure 2d). Therefore, in vivo imaging based on this bioresponsive system can be used to screen hepatotoxic components.
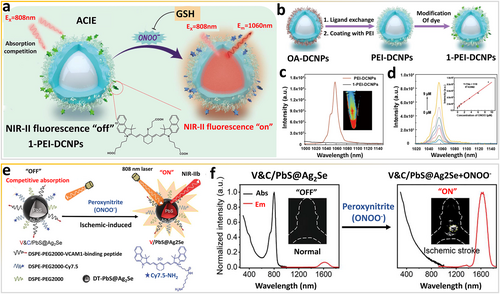
Exploiting the ACIE effect via ONOO− as an in vivo stimulus. (a) Working principles of 1-PEI-DCNPs responding to ONOO− in vivo. (b) Scheme of the synthesis of 1-PEI-DCNPs. (c) Luminescence spectra of PEI-DCNPs before and after coating with small organic molecules. (d) Luminescence spectra of 1-PEI-DCNPs responding to ONOO− (0–9 μM) in PBS. Reproduced with permission [43]. Copyright 2022, Elsevier Ltd. (e) Schematic illustration of the construction of activatable NIR-IIb probes and (f) detection of ONOO− in ischemic stroke mice. Reproduced with permission [44]. Copyright 2021, John Wiley and Sons. ACIE, absorption competition-induced emission; DCNPs, downconversion nanoparticles; NIR, near-infrared.
By exploiting the ACIE effect, Wang et al. [44] designed a smart ONOO−-targeting NIR-IIb nanoprobe (V&C/PbS@Ag2Se) for highly sensitive in vivo detection of early ischemic stroke in a photothrombotic stroke model (Figure 2e). ONOO−, involved in the development of ischemic stroke, is a prodromal biomarker of early ischemic stroke [45]. The bioresponsive V&C/PbS@Ag2Se is initially in the “off” state owing to the ACIE effect between Cy7.5 dye and PbS@Ag2Se QDs (Figure 2f). Subsequently, as observed in the ultraviolet (UV)–visible (vis)–NIR spectrum, the V&C/PbS@Ag2Se nanoprobe can be rapidly switched “on” via Cy7.5 bleached by ONOO− to produce NIR-II fluorescence signals, instantly illuminating the lesion tissues (Figure 2f).
More recently, Wang et al. [5] synthesized a smart fluorescent nanoprobe (V&A@Ag2S) for in vivo NIR-II bioimaging of traumatic brain injury (TBI) via responsiveness to ONOO− (Figure 3a). Instead of the ACIE effect, the FRET process from Ag2S to the A1094 fluorophore maintains V&A@Ag2S in the “off” state. Ag2S QD with an emission at 1050 nm is the energy donor and ONOO−-responsive A1094 dye serves as the energy acceptor owing to the substantial overlap between the absorption spectrum of A1094 and emission spectrum of Ag2S QDs (Figure 3b). As shown in Figure 3c, A1094 is oxidized in the presence of ONOO−, which reduces the FRET effect, and thus the absorption peak of A1094 at 1094 nm disappears. Therefore, Ag2S QDs are switched to the “on” state at 1050 nm (Figure 3d,e). Moreover, the probe is designed to target VCAM1 expressed by the inflamed endothelium in TBI tissues, allowing rapid diagnosis with high sensitivity.
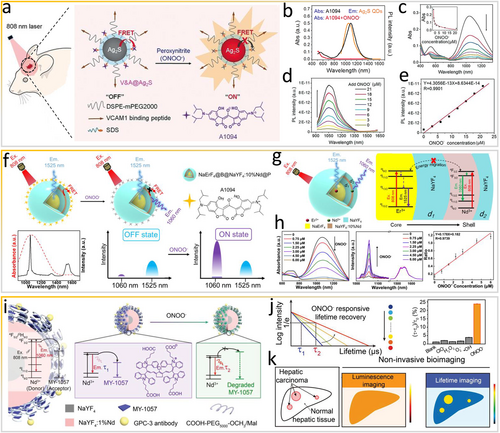
Exploiting the FRET effect via ONOO− as an in vivo stimulus. (a) Schematic of the synthesis of the V&A@Ag2S nanoprobe and sensing of ONOO− in vivo. (b) Absorption and emission spectra of A1094 and Ag2S. (c) Absorption spectra of A1094 (5 μM) upon the addition of ONOO−. (d) Photoluminescence recovery of V&A@Ag2S and (e) plot of fluorescence intensity versus ONOO− concentration. Reproduced with permission [5]. Copyright 2020, John Wiley and Sons. (f) Working principles of dye-coated downconversion nanoparticles responding to ONOO−. (g) Schematic diagram of core–shell nanoparticles and simplified energy flux between Er3+ and Nd3+. (h) Absorption and PL spectra of DCNP@A1094 upon gradual addition of ONOO− and the corresponding linear plot. Reproduced with permission [46]. Copyright 2021, American Chemical Society. (i) Scheme of lifetime-based detection of HCC in the NIR-II window. (j) Structure of energy acceptor MY-1057 degrading in the presence of ONOO−. (k) Scheme of noninvasive NIR-II intensity- and lifetime-based imaging of HCC mice after the administration of DCNP@MY-1057 nanosensor. Reproduced with permission [47]. Copyright 2020, John Wiley and Sons. FRET, Förster/fluorescence resonance energy transfer; HCC, hepatocellular carcinoma; NIR-II, second near-infrared.
Similarly, by exploiting the FRET effect among organic dyes, Zhang et al. [48] synthesized a series of organic NIR-II dyes (CX) with promising spectroscopic properties and constructed a smart fluorescent probe PN1100 by loading dyes CX-1 and CX-3 into a micelle for in vivo imaging of ONOO− species. CX-1 and CX-3 differ in reactivity with ONOO− because the HOMO energy level of CX-3 is higher than that of CX-1, resulting in different reaction rates. CX-1 dye can be regarded as an ONOO−-sensitive scaffold. CX dyes are chemo- and photo stable in aqueous environments. In particular, the optical properties of CX-3 are tunable with maximal absorbing/emitting wavelengths at 1089/1140 nm. The emission and absorption spectra of CX-1 and CX-3 overlap, and in micelles containing both CX-1 and CX-3 or only CX-1 or CX-3, the emission intensity of CX-1 under 808-nm excitation decreases, revealing efficient FRET between the CX dyes. When ONOO− is encountered, the emission peak of CX-1 at 920 nm steadily increases, while the peak of CX-3 at 1130 nm decreases. Both trends suggest that CX-3 is gradually oxidized by ONOO−, which disturbs FRET between CX-1 and CX-3.
Lanthanide-doped DCNPs possess outstanding optical properties, including sharp emission bands, large Stokes shifts, and relatively long fluorescence lifetimes, and furthermore, are resistant to photo-bleaching [49, 50]. Unfortunately, in the design of smart optical probes, energy loss is inevitable in multiple Ln3+-doped DCNPs with complex energy migration processes. To tackle this obstacle, Wang and colleagues constructed efficient NaErF4@NaYF4@NaYF4:10%Nd@NaYF4 nanoparticles (Figure 3f) [46]. These DCNPs are composed of two species emitting intensive NIR-II fluorescence signals under 808-nm excitation, specifically Er3+ emits a signal at 1525 nm and doped Nd3+ emits a signal at 1060 nm, both of which serve as activators. Furthermore, through efficient FRET from doped Nd3+ to the absorber, oxidation of ONOO−-responsive A1094 dye on the surface of DCNPs quenches the signal at 1060 nm only (Figure 3g) because A1094 has a high affinity for ONOO−. Owing to the dual emission property of the nanoprobe and the highly specific oxidation of A1094 by ONOO−, smart ratiometrically fluorescent nanoprobe DCNP@A1094 is in the “on” state in the presence of ONOO− (Figure 3h), and thus fluorescence signals with an intensity equivalent to that of the reference signal at 1525 nm are recovered. After the addition of ONOO−, the absorption of A1094 is inhibited, and the fluorescence at 1060 nm is gradually recovered. Interestingly, the ratiometric fluorescence intensity (I1060 nm/I1525 nm) of DCNP@A1094 has a good linearity toward the concentration of ONOO−.
In contrast to the smart “off-to-on” strategy, the lifetime strategy exploits the stable fluorescence lifetime in biological tissues for luminescence bioimaging via the time-domain technique [49, 51]. For example, Zhang's research group constructed a smart NIR-II ONOO−-responsive lanthanide-cyanine nanosensor [47] by integrating the lanthanide donor (Nd3+-doped nanoparticles) and dye MY-1057 acceptor undergoing FRET for lifetime-based in situ hepatocellular carcinoma (HCC) detection (Figure 3i). ONOO−-responsive NIR-II MY-1057 dye serves as the FRET acceptor. The luminescence lifetime can be changed by adjusting the number of the surface modified acceptor. When ONOO− in tumor tissues is encountered, the structure of energy acceptor MY-1057 is destroyed, which recovers the lifetime of the nanosensor (Figure 3j). Subsequently, HCC lesions are accurately distinguished from normal hepatic tissue in lifetime bioimaging owing to the recovery of lifetime signals (Figure 3k), while intensity-based luminescence bioimaging fails to distinguish tissues with low SBRs attributed to the high accumulation of nanomaterials in the liver. Owing to long-lived in vivo phosphorescence, pulsed excitation and delayed detection can be applied to lifetime bioimaging using the lanthanide-doped-nanoparticles donor@dye acceptor, thereby avoiding the autofluorescence noise to achieve high sensitivity and contrast. In contrast to conventional intensity bioimaging, the risk of thermal accumulation and damage to tissues can be minimized in lifetime bioimaging, allowing the use of high-power lasers as excitation sources.
3 RSS-RESPONSIVE NIR-II IMAGING
In contrast to traditional imaging methods for colorectal cancer, such as colonoscopy and X-ray CT, in vivo noninvasive optical bioimaging can significantly elevate the diagnostic accuracy and eliminate side effects [52]. By combining NIR-II fluorescence and photoacoustic imaging (PAI), which has high penetration depth/sensitivity, in the form of a complementary multimodal bioimaging probe, super spatial resolution and sensitivity can be achieved for colorectal cancer diagnosis [53]. For example, Zeng and colleagues synthesized a composite SiO2@Ag nanoprobe responsive to overexpressed endogenous H2S for in situ dual-mode NIR-II fluorescence/PAI bioimaging of colorectal cancer [54]. The concentration of H2S in colorectal tumors is higher (0.3–3.4 mM) than that in noncancerous tissues owing to the abnormal overexpression of enzyme cystathionine-β-synthase in colorectal tumors, and thus H2S molecules are considered important pathological analytes of colorectal cancer [55]. The composite SiO2@Ag nanoprobe can be transformed into SiO2@Ag2S after in situ biosynthesis via a sulfuration reaction with H2S in colorectal tumors, achieving high sensitivity and specificity for colorectal cancer in vivo via dual-mode NIR-II/PAI bioimaging (Figure 4a). The in vitro formation of H2S formed from S2– induces the dual-mode SiO2@Ag nanoprobe to produce NIR-II fluorescence signals at 1000–1400 nm and an outstanding PAI signal (Figure 4b,c).
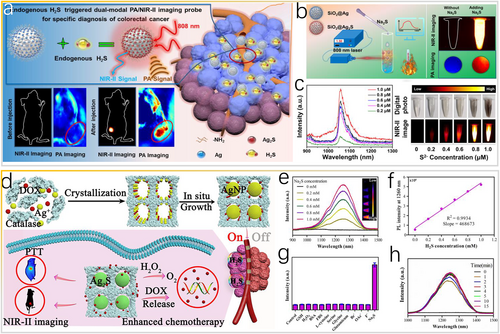
H2S as in vivo stimulus for the in situ generation of Ag2S. (a) Schematic of H2S-responsive dual-mode NIR-II/photoacoustic nanoprobe for highly specific imaging of colorectal cancer. (b) Schematic illustration of SiO2@Ag nanoprobes activated by Na2S solution. (c) Na2S concentration-dependent NIR-II emission spectra of SiO2@Ag under 808-nm excitation and corresponding in vitro NIR-II imaging of a phantom. Reproduced with permission [54]. Copyright 2021, American Chemical Society. (d) Scheme of the TME-activated NIR-II nanotheranostic platform for precise diagnosis and treatment of colon cancer. (e) NIR-II emission spectra of CatCry-AgNP-DOX in the Na2S solution under 808-nm excitation and (f) concentration dependence of the NIR-II PL intensity. (g) NIR-II PL of CatCry-AgNP-DOX activated by various bioavailable molecules and ions. (h) Time-dependent changes in the fluorescence of CatCry-AgNP-DOX after adding Na2S (5 mM). Reproduced with permission [56]. Copyright 2022, American Chemical Society. NIR-II, second near-infrared; TME, tumor-microenvironment.
Analogously, Qu et al. [56] used catalase nanocrystals (CatCry) as a “nanoreactor” for the in situ synthesis of controlled-size silver nanoparticles (AgNP) based on the biomineralization mechanism. Notably, CatCry can be loaded with anticancer drug doxorubicin (DOX) through a one-step cocrystallization method for the generation of O2 in vivo to relieve tumor hypoxia (Figure 4d, upper panel). By injecting CatCry-AgNP-DOX nanoprobes, intratumoral H2S can switch AgNP into Ag2S nanoparticles to display the “on” state with stable signals over time (Figure 4d, bottom panel). In normal tissues without an increase in the H2S concentration, the nanoprobe is in the “off” state without theranostic actions (Figure 4d, bottom panel). The addition of Na2S to the CatCry-AgNP-DOX suspension induces NIR-II emission at 1260 nm, and the signal increases as the Na2S concentration increases (Figure 4e,f). The high specificity of CatCry-AgNP-DOX toward sulfides (Figure 4g) is indicated by the highly efficient and complete conversion of AgNP in CatCry-AgNP-DOX to Ag2S within 5 min (Figure 4h).
By exploiting the in situ formation of NIR-II Ag2S nanoparticles and the optical properties of lanthanide-doped DCNPs, an orthogonal NIR-II window nanoprobe comprising NaYF4:Gd/Yb/Er@NaYF4:Yb@SiO2 coated with Ag nanodots was recently developed for the highly specific in vivo monitoring of hepatotoxicity via ratiometric fluorescence [57] (Figure 5a). The nanoprobe mainly accumulates in the liver, where Ag nanodots on the nanoprobe surface are subsequently converted into Ag2S via sulfuration by overexpressed endogenous H2S in the injured liver, finally turning “on” the orthogonal emission centered at 1053 nm (excited by 808-nm laser) and 1525 nm (excited by 980-nm laser) owing to the nonoverlapped spectral windows between lanthanide-doped nanoparticles (LnNPs) and Ag2S QDs (Figure 5b,c). In 2021, Wang et al. [58] constructed another smart H2S-responsive ratiometrically fluorescent nanoprobe in situ, which emits two NIR-II fluorescence signals to light up cancerous colon tissues intelligently (Figure 5d). Specifically, DCNPs are the main framework with NIR-II fluorescence at 1550 nm under 980-nm laser excitation (F1550Em, 980Ex). Human serum albumin (HSA) is an effective vehicle to load Ag+ because the carboxyl group of HSA can coordinate with Y3+ on the surface of DCNPs to form the DCNP@HSA-Ag+ nanoprobe. Similarly, as shown in Figure 5e, when H2S is encountered, Ag2S QDs are formed in the coated HSA, turning “on” the fluorescence at approximately 1050 nm under 808-nm laser excitation (F1050Em, 808Ex) via the in situ sulfuration reaction between H2S and Ag+. Moreover, the digital photographs confirm that the fluorescence signal of DCNPs at 1550 nm (F1550Em, 980Ex) is stable, which produces ratiometric fluorescence signals (F1050Em, 808Ex/F1550Em, 980Ex) with an outstanding linear relation with the H2S concentration (LOD = 0.22 mM, Figure 5f). Overall, the in situ formation of NIR-II Ag2S nanoparticles in colorectal tumor tissues is undoubtedly a highly efficient approach for activating bioresponsive probes.
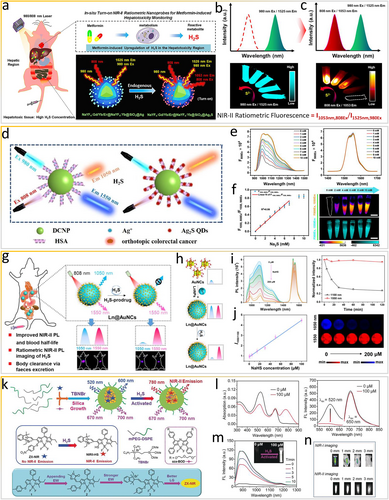
H2S as in vivo stimulus for the generation of ratiometric fluorescence signals. (a) Scheme of in situ ratiometric fluorescence imaging of metformin-induced liver injury based on (b, c) the endogenous H2S-activated orthogonal NIR-II nanoprobe under 808/980-nm laser excitation. Reproduced with permission [57]. Copyright 2021, American Chemical Society. (d) Scheme of the in situ bioresponsive ratiometrically fluorescent DCNP@HSA-Ag+ nanoprobe. (e) NIR-II FL spectra of the nanoprobe after treatment with Na2S under 808-nm (left panel) and 980-nm (right panel) excitation. (f) Linear relationship between NIR-II ratiometric fluorescence intensity and Na2S concentration. Reproduced with permission [58]. Copyright 2021, American Chemical Society. (g) Scheme of NIR-II Ln@AuNCs for in vivo ratiometric fluorescence imaging of H2S. (h) Synthesis of Ln@AuNCs and NIR-II PL changes. (i) Time-dependent PL spectra after incubation with NaHS at different concentrations. (j) I1550/1100 values as a function of NaHS concentration, corresponding with NIR-II PL images. Reproduced with permission [59]. Copyright 2022, American Chemical Society. (k) Scheme of the synthesis of multi-wavelength bioresponsive ZX-NIR nanoprobes. (l) Changes in the light absorption and fluorescence of NIR-II@Si upon the addition of NaHS in PBS. (m) Time-dependent NIR-II emission spectra of NIR-II@Si in the presence of NaHS. (n) Comparison of NIR-I and NIR-II fluorescence imaging of samples covered with pork tissue. Reproduced with permission [60]. Copyright 2018, John Wiley and Sons. NIR-II, second near-infrared.
Recently, Song and coworkers used ultrasmall gold nanoclusters (AuNCs) with fantastic luminescence and renal-clearable capacity as well as LnNPs to synthesize core-satellite Ln@AuNCs assemblies designed to anchor to ligands (Figure 5g) with a 2.5-times increase in the PL of AuNCs at 1100 nm and a prolonged blood circulation time compared with initial AuNCs [59]. Bioresponsive Ln@AuNCs assemblies with intense dual NIR-II PL based on AuNCs and Er3+ can effectively accumulate in the liver for ratiometric fluorescence NIR-II bioimaging of abnormally expressed H2S, which quenches the PL of AuNCs. Specifically, the NIR-II PL of Ln@AuNCs at 1100 nm is rapidly and effectively quenched within 30 min, while the NIR-II PL at 1550 nm is undisturbed (Figure 5h,i), allowing ratiometric PL imaging of H2S in the concentration range of 0–200 μM (Figure 5j). Increasing the PL intensity at 1550 relative to that at 1100 nm (I1550/1100) provides a good linear relationship with an LOD of 1.6 μM (Figure 5j). Additionally, increasing the luminescence efficiency of NIR-II AuNCs (e.g., through metal ion doping and ligand engineering) will further improve the detection sensitivity and serviceability of smart imaging platforms.
Undeniably, smart NIR-II optical probes based on rigid LnNPs have stable optical properties and thus deserve in-depth research. Nevertheless, organic luminescent dyes can be used to fabricate smart ratiometrically fluorescent NIR-II optical probes to further expand the field of theranostics. In 2018, Tian et al. [60] reported an H2S-responsive ratiometrically fluorescent organic probe in the hydrophobic interior of core–shell silica nanocomposites (NIR-II@Si, Figure 5k). In detail, the smart NIR-II nanoprobe comprises two organic luminescent dyes, a smart structural boron-dipyrromethene (ZX-NIR) dye to generate NIR-II emission in the presence of H2S and an aza-BODIPY (aza-BOD) dye to generate a reference signal that is independent of H2S. First, ZX-NIR within the water-dispersible nanocomposite responds to H2S. While ZX-NIR reacts with NaHS (100 μM), the NIR absorption peak at 780 nm is generated and the band at 520 nm decreases in intensity (Figure 5l). The robust fluorescence within the NIR-II window increases in intensity under 780-nm irradiation and reaches a plateau within 5 min, allowing real-time monitoring of H2S-related pathophysiological processes in vivo (Figure 5m). As shown in Figure 5n, NIR-I fluorescence is attenuated to the background level for samples covered by just 3 mm of pork tissue, suggesting that NIR-II fluorescence bioimaging has a better spatial resolution and deeper tissue penetration than NIR-I fluorescence bioimaging. By applying an analogous NIR-II window smart design strategy, Tian et al. [61] further fabricated a H2S-responsive theranostic nanoplatform (NPs@BOD/CPT) in situ, which produces NIR photothermal agents for in vivo bioimaging of colorectal cancer and photo-controlled on-demand drug release via the substitution reaction of 4-chlorin substituted boron-dipyrromethene.
In addition to H2S, in vivo glutathione (GSH) can influence the pathophysiological processes of the body, serving as one of the crucial reactive sulfide species underpinning tumor-microenvironment (TME)-responsive tumor therapy. MnO2 nanosheets are the basis of effective GSH-responsive materials. Released Mn2+ ions can be adopted as an MRI agent and a catalyst for chemodynamic therapy. Specifically, metals, such as Mn2+, Cu+, and Fe2+, can catalyze Fenton/Fenton-like reactions involving H2O2 to generate •OH, which can kill tumor cells [62, 63]. Accordingly, for self-enhanced photothermal/chemodynamic synergistic therapy, Jiang and colleagues recently developed a smart TME-activated biomimetic nanocatalyst with quad-mode bioimaging capabilities, namely NIR-II “off-to-on” fluorescence bioimaging, MRI, PAI, and photothermal imaging (Figure 6a) [64]. GSH can induce the degradation of MnO2 nanosheets in vivo, thus recovering the NIR-II fluorescence of Ag2S QDs at 1130 nm to enable tumor visualization. The FRET effect between MnO2 nanosheets and Ag2S QDs is evident from the spectrum (Figure 6b). Moreover, as the GSH concentration increases, the fluorescence at 1130 nm is gradually enhanced (Figure 6c).
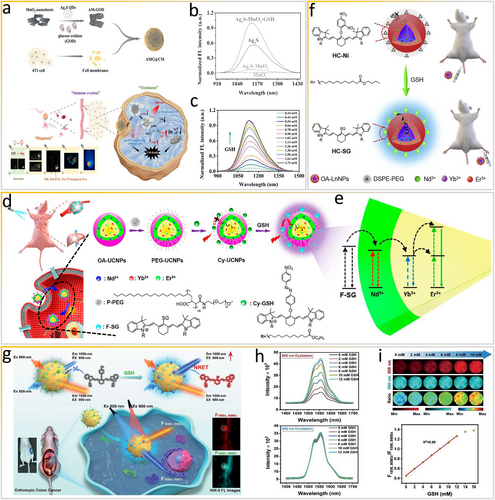
Exploiting the resonance energy transfer effect via GSH as an in vivo stimulus. (a) Scheme of the preparation of AMG@CM for visualized “off-on” detection of GSH and multimodal imaging-guided PTT/CDT. (b) FL spectra of different components. (c) FL spectra of AM-GOD at various concentrations of GSH. Reproduced with permission [64]. Copyright 2022, American Chemical Society. (d) Scheme of synthesis of Cy-UCNPs probe and sensing of GSH through target-modulated sensitization. (e) Scheme of the energy transfer process from F-SG to UCNPs. Reproduced with permission [65]. Copyright 2018, American Chemical Society. (f) Scheme of the NIR-II-bioresponsive LnNPs-based HC-Ni probe for bioimaging. Reproduced with permission [66], Copyright 2020, Elsevier Ltd. (g) Scheme of dye-sensitized DCNPs with emission beyond 1500 nm for ratiometric fluorescence visualization of cancer redox state. (h) NIR-II FL spectra of the nanoprobe after reaction with GSH under 808- and 980-nm excitation. (i) NIR-II FL imaging of the nanoprobe after treatment with GSH with the linear relationship between F1550, 808Ex/F1550, 980Ex and GSH concentrations. Reproduced with permission [67]. Copyright 2021, John Wiley and Sons. CDT, chemodynamic therapy; GSH, glutathione; LnNPs, lanthanide-doped nanoparticles; UCNPs, upconversion nanoparticles.
Similar to FRET, the luminescence resonance energy transfer (LRET) in organic–inorganic nanoparticles requires rigid upconversion nanoparticles (UCNPs) as the energy donor and various energy acceptors. Generally, organic dyes with a responsive moiety and strong NIR absorption can quench the upconversion luminescence (UCL). When the LRET process is inhibited or blocked by responsive dyes in the presence of biomarkers, the UCL is recovered immediately. The SBR and thus quality of in vivo bioimaging using smart optical probes depend on the extent of luminescence quenching, which undoubtedly rely on the LRET efficiency. Liu's group fabricated a bioresponsive optical probe sensitized in the NIR-I window [65] (Figure 6d) through an inverse LRET process. Specifically, the responsive dye serving as the energy donor absorbs NIR photons and then transfers excited-state energy to the lanthanide ions serving as the energy acceptor via nonradiative energy transfer, which is possible owing to the overlap between the emission spectrum of the dye and the absorption spectrum of the lanthanide ion. Before reacting with GSH, Cy-GSH dye is nonemissive and no sensitization occurs. After the reaction with abnormally overexpressed GSH, F-SG dye becomes strongly emissive, enabling the nonradiative energy transfer process and inducing UCL of the UCNP core. A thicker shell can resist the surface quenching effect and enhance the UCL intensity. Notably, the energy transfer process is distance-dependent. An increase in the shell thickness reduces the energy transfer efficiency both from the dye to Nd3+ and from Nd3+ to Yb3+ (Figure 6e). Subsequently, Liu and co-workers constructed similar smart NIR-II window dye-sensitization LnNPs [66]. As shown in Figure 6f, nonemissive dye HC-Ni, serving as the GSH recognition motif and a potential antenna, cannot sensitize NIR-II LnNPs. After reacting with GSH, HC-Ni transfers into emissive dye HC-SG, sensitizing and turning “on” NIR-II luminescence via the LRET process. Smart NIR-II luminescence based on the resonance energy transfer process is extremely effective for high-contrast in vivo bioimaging.
Similarly, bioresponsive ratiometrically fluorescent 4-nitrophenol-Cy7 (NPh)-conjugated lanthanide-based downconversion nanoparticles (DCNP@NPh-PEG) can be used to detect GSH in vivo for accurate assessment of the redox state and real-time visualization of pathophysiological processes via nonradiative resonance energy transfer (NRET) (Figure 6g) [67]. NPh molecules on the surface of DCNPs have no fluorescence signals owing to the intramolecular PET process. When NPh dye reacts with GSH to form Cy7-SG, the intramolecular PET process is eliminated. Excitation energy is then transferred to Nd3+ to sensitize DCNPs through the NRET effect, finally producing the enhanced fluorescence at 808 nm (F1550, 808Ex). Notably, the fluorescence of DCNPs at 1550 nm under 980-nm laser irradiation (F1550, 980Ex) is stable (Figure 6h). The ratiometric fluorescence, measured as F1550, 980Ex/F1550, 808Ex, has a linear relationship with the GSH concentration with an LOD of 0.3 mM (Figure 6i). As mentioned above, biorepsonsive ratiometrically fluorescent NIR-II optical nanoprobes developed by exploiting the FERT effect are reliable tools for accurate biosensing in vivo.
4 ENZYME-RESPONSIVE NIR-II IMAGING
Aminopeptidase N (APN) is a membrane-bound zinc metallopeptidase with a vital role in the growth of local tumors as well as the invasion and metastasis of tumors [68]. Because it is overexpressed on tumor vasculature and cells, APN is regarded as one of the crucial biomarkers of cancer, including lymphoma. Recently, a smart NIR-II nanoprobe, TX-APN, was constructed (Figure 7a) for the precise tracking of metastatic lymphoma and imaging-guided resection of the primary tumor and metastatic lymphoma [69]. The smart molecular probe consists of bovine serum albumin (BSA) as the matrix, tricyanofuran as the electron-accepting scaffold (electron acceptor), xanthene as the electron-donating scaffold (electron donor), and alanine as the APN-responsive scaffold (recognition moiety). Notably, after TX-APN@BSA reacts with APN in PBS, the intensity of the absorption band at 600–900 nm with a peak at 795 nm increases markedly (Figure 7b). The smart TX-APN@BSA nanoprobe emits a strong NIR-II fluorescence signal (900–1150 nm) with a peak at 922 nm under 808-nm irradiation, whereas TX-APN and TX-APN@BSA are weakly emissive in the absence of APN. As shown in Figure 7c, the intensity of the NIR-II fluorescence increases as the APN concentration increases.
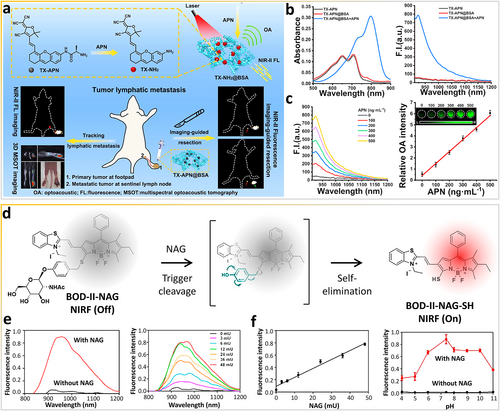
Enzymes as in vivo stimuli for molecular structure changes of composite probes. (a) Aminopeptidase N bioresponsive nanoprobe for tracking metastatic lymphoma and guiding tumor resection surgery via dual-mode imaging. (b) Optical spectra of different samples after incubation with aminopeptidase N. (c) NIR-II FL spectra of TX-APN@BSA after incubation with aminopeptidase N at varying doses. Reproduced with permission [69]. Copyright 2022, American Chemical Society. (d) Real-time monitoring of renal impairment due to drug-induced AKI and diabetes-caused CKD using NAG-bioresponsive NIR-II nanoprobes. (e) Fluorescence spectra of BOD-II-NAG in the absence and presence of NAG at increasing concentrations with 30 min incubation. (f) The linear relationship between the fluorescence intensity of BOD-II-NAG and enzyme concentration. Influence of pH on the fluorescence of BOD-II-NAG after incubation with and without NAG. Reproduced with permission [70]. Copyright 2021, American Chemical Society. AKI, acute kidney injury; CKD, chronic kidney disease; NAG, N-acetyl-β-d-glucosaminidase; NIR-II, second near-infrared.
Enzyme N-acetyl-β-d-glucosaminidase (NAG) serves as a sensitive and specific biomarker of acute kidney injury (AKI) and chronic kidney disease (CKD) [71, 72]. More recently, Gu et al. [70] fabricated a NAG-responsive NIR-II fluorescent nanoprobe, BOD-II-NAG-NP, for the monitoring of drug-induced AKI and in vivo bioimaging of diabetes-caused CKD (Figure 7d). NAG residues are incorporated into BODIPY-based NIR fluorophores via the self-elimination linker. The π-conjugation at the 3-position of the BODIPY core is extended to benzothiazole via a vinylene unit to achieve NIR-II fluorescence responsiveness. NAG can specifically react with BOD-II-NAG-NP to produce NIR-II fluorescence signals (Figure 7e). Furthermore, NAG can catalyze the hydrolyzation of N-acetyl-β-d-glucosamine residues and undergo 1,6-elimination to release the BODIPY-based fluorophore. The intensity of the NIR-II fluorescence at 1000 nm is remarkably enhanced as the NAG concentration increases with a calculated LOD of 0.72 mU/mL (Figure 7f). Moreover, the fluorescence intensity from pH 6.0 to 10.0 indicates that the probe has a stable response to NAG in complex pathophysiological environments. Finally, BOD-II-NAG-SH can deeply penetrate the relatively thick layers of fat in mice with diabetic nephropathy to provide NIR-II fluorescence for high-resolution in vivo bioimaging and potentially precise diagnosis of CKD.
Enzyme-responsive NIR-II nanoprobes are not limited to simple designs involving mono-molecular reactions. In particular, the specificity and sensitivity of these probes can be improved with certain electron/energy effects. By exploiting the PET effect, Kim and colleagues constructed a smart matrix metalloproteinase (MMP)-responsive NIR-II nanoprobe, PA-NIRQD (Figure 8a), which fluoresces in the microenvironments of colon tumors [73]. The bright and stable PbS/CdS/ZnS core/shell/shell QDs are a model NIR-II fluorophore. AcM is a compact surface ligand containing methylene blue (MB), a quencher operating via the PET mechanism. Additionally, AcM is accessible to enzymes and has a high activation signal, which can modulate the fluorescence of QDs. When the QDs are near MB, NIR-II QDs with quasi-type-II electronic characteristics are quenched via the PET process from the QDs to MB because electrons can facilely delocalize in the CdS shell and holes are confined in the PbS core [76]. Specifically, this configuration benefits the transfer of photoexcited electrons to the quenching molecules on the surface of QDs. In contrast, NIR-II fluorescence signals are recovered when the peptide sequence in the ligand is cleaved by MMP, separating the emitter from the quencher. As shown in Figure 8b, in vitro bioimaging of a small mouse model with colon cancer indicates that the intensity of the selective fluorescence of the smart NIR-II PA-NIRQD nanoprobe at the tumor tissue is enhanced by up to three-fold within 10 min.
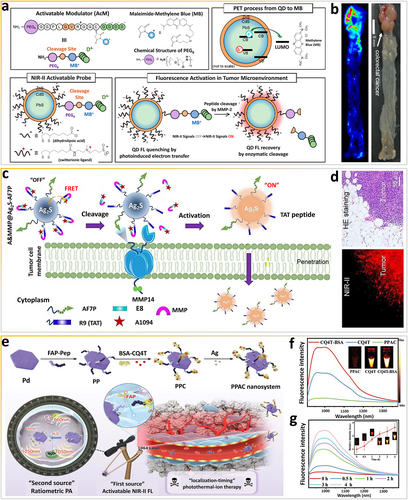
Exploiting the energy transfer effect via enzymes in vivo. (a) Scheme of TME-sensitive bioresponsive NIR-II quantum dots. (b) Ex vivo FL imaging of colon cancer by spraying with the activatable probe. Reproduced with permission [73]. Copyright 2017, American Chemical Society. (c) Scheme of unperturbed-tissue analysis for intraoperative cancer diagnosis using an enzyme-bioresponsive NIR-II nanoprobe. (d) HE staining and fluorescence microscopic images of cancer tissues. Reproduced with permission [74]. Copyright 2021, John Wiley and Sons. (e) Scheme of “dual-source, dual-activation” strategy for an NIR-II window theranostic nanosystem enabling optimal photothermal-ion combination therapy. (f) NIR-II FL spectra of different samples. (g) Time-dependent NIR-II emission spectra of PPAC at 1064 nm. Reproduced with permission [75]. Copyright 2022, John Wiley and Sons. NIR-II, second near-infrared; TME, tumor-microenvironment.
By exploiting the FRET mechanism, Wang's group fabricated another smart MMP14-responsive NIR-II nanoprobe (A&MMP@Ag2S-AF7P) for rapid, in vitro analysis of unperturbed tissues and in vivo diagnosis of neuroblastomas (Figure 8c) [74]. MMP14-responsive peptide (MMP) can be loaded on the surface of the nanoprobe via electrostatic interaction-directed assembly of polycationic cell-penetrating peptide R9 (TAT-peptide) and poly-anionic fragments (E8), in which E8 is modified with NIR-II A1094 dyes. Furthermore, AF7P can recognize and target MMP14, anchoring on the extracellular membrane of cancer cells. The membrane-penetrating peptide R9 is exposed via specific enzymatic reactions between MMP14 and MMP to achieve cellular uptake. Ultimately, the FRET effect between Ag2S QDs and A1094 can be disturbed to produce NIR-II optical signals originating in neuroblastoma (NB) tissues. As shown in Figure 8d, lesions can be instantly illuminated, providing well-defined tumor margins to guide cancer surgery or tissue biopsy.
Because fibroblast activation protein (FAP) is overexpressed on cancer-associated fibroblasts (CAFs), it is widely regarded as a promising species for theranostic applications [77]. More recently, Zhang and colleagues constructed a “dual-source, dual-activation” multifunctional nanotheranostic platform, PPAC (Pd-Pep-BSA-CQ4T-Ag), for bioresponsive NIR-II ratiometric fluorescence photoacoustic bioimaging-guided “localization-timing” photothermal-ion therapy [75]. The FAP-responsive peptide on the surface of Pd nanosheets has excellent NIR-II absorption ability and can efficiently cross-link BSA-CQ4T to realize efficient quenching of NIR-II fluorescence via energy transfer (Figure 8e). Specific cleavage by FAP via perivascular CAFs (the first source) releases BSA-CQ4T for rapid fluorescence recovery. Of note, after conjugation with BSA, CQ4T produces a fluorescence signal with a 6-fold increase in intensity (Figure 8f), which enhances the NIR-II fluorescence of PPAC. The fluorescence intensity of PPAC at 1064 nm increases over time (Figure 8g). More importantly, the fluorescence intensity at 4 h is compared to that of BSA-CQ4T, suggesting that the fluorescence of PPAC is completely turned “on” by FAP with extraordinary sensitivity. The “dual-source, dual-activation” strategy can be further developed to visualize tumors with a clear boundary for precise spatial positioning, thus precisely guiding NIR-II PTT-based “localization-timing” therapy.
Most biomarkers often distribute across multiple types of cells, and these overexpressed biomarkers in diseased tissues also express in normal tissues and plasma. Thus, current smart mono-responsive probes in complex and dynamic pathophysiologic environments can provide ambiguous results [78]. To avoid ambiguity and provide reliable information, Fan et al. [79] synthesized smart dual-source cooperatively responsive NIR-II fluorescent nanoprobes (HISSNPs) via self-assembly and crosslinking, by which hyaluronic acid chains and disulfide bonds act as “double locks” to lock quenched NIR II signals in dye aggregates for ultra-specific in vivo bioimaging of tumors (Figure 9a). The fluorescence of IR-1061 can be recovered by disassembly of the self-quenched aggregates only when the “double locks” are simultaneously opened in the tumor via reactions with the “dual smart keys,” namely overexpressed hyaluronidase (Hyal) and thiols. Undoubtedly, only one “smart key” (Hyal or thiols) cannot guarantee the accuracy of the obtained information about tumor tissues (Figure 9b,c). Moreover, the NIR-II fluorescence of “dual lock-and-key”-controlled HISSNPs can be dramatically amplified (13.3-fold) within 30 min in the presence of both Hyal and GSH (Figure 9d). Irrespective of the existence of the GSH “key,” the fluorescence of HINPs can be easily turned on by the Hyal “key” only. Therefore, the dual-key “off-to-on” strategy can be used to eliminate the interference from complex pathophysiological environments and to guarantee high-quality information.
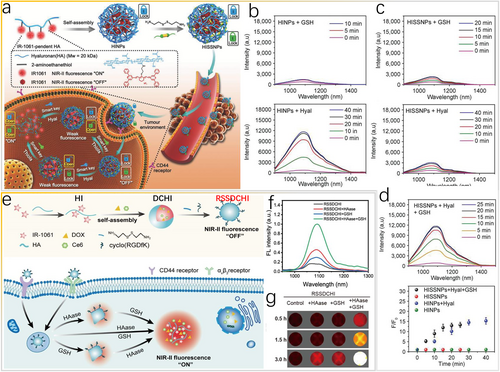
Dual stimuli in vivo. (a) Scheme of “lock-and-key”-controlled tumor-specific nanoparticles (HISSNPs) for bioimaging. (b–d) FL spectra of HINPs and HISSNPs incubated with Hyal or GSH. Reproduced with permission [79]. Copyright 2018, John Wiley and Sons. (e) Scheme of the NIR-II-bioresponsive drug delivery nanoplatform for combined photodynamic and chemotherapy of cancer. (f) FL spectrum and (g) NIR-II FL imaging of RSSDCHI incubated with HAase, GSH, or both. Reproduced with permission [80]. Copyright 2022, American Chemical Society. GSH, glutathione; NIR-II, second near-infrared.
Analogously, a smart nanoparticulate drug delivery system (nano-DDS), hyaluronidase (HAase)/GSH dual-responsive RGD-SS-DOX/Ce6@HA-IR-1061 (RSSDCHI), was designed for NIR-II fluorescence bioimaging to monitor the smart release of drugs, specifically cancer-targeting photodynamic and chemotherapeutic agents (Figure 9e) [80]. The nano-DDS remains silent with weak fluorescence signals (“off” state) because of aggregation-caused quenching (ACQ) of IR-1061 dye via hyaluronic acid chains and disulfide in the normal tissue microenvironment, preventing the release of doxorubicin (DOX) and chlorin E6 (Ce6). Specific reactions between hyaluronidases (HAase) and GSH in the tumor microenvironment can induce the disassembly of the smart NIR-II RSSDCHI, thus switching “on” the NIR-II fluorescence signals (“on” state). The fluorescence spectrum (Figure 9f) and NIR-II fluorescence image (Figure 9g) confirm that the HAase/GSH dual-responsive platform can effectively activate the smart “off-to-on” process. To obtain high-quality and high-resolution in vivo information about cancer tissues, the “dual-key” design strategy deserves further in-depth study to eliminate the background interference as much as possible.
5 PHYSIOLOGICAL METABOLITES
Methylglyoxal (MGO) is a byproduct of many metabolic pathways, for example, glycolysis and the polyol pathway, which produces advanced glycation end products that are more reactive than glucose [81]. Recent studies indicate that abnormally expressed endogenous MGO can induce certain pathologies, including diabetes, cardiovascular diseases, and cancer [82, 83]. To develop rapid and convenient methods for disease diagnosis and the characterization of disease pathogenesis at the molecular level, Xu et al. [84] fabricated a smart NIR-II fluorescent probe, TDTCD, and discussed the reaction of the probe with MGO. A novel smart MGO-responsive nanoprobe, MG-SLNP, was then constructed on the basis of rational composite engineering method, which can improve the stability, water solubility, and response rate of the nanoprobe (Figure 10a). In contrast to the conventional D-A-D structure of NIR-II probes, TDTCD contains carbazole as an innovative electron donor, instead of N-C single bonds, which have limited rotation (Figure 10b). Consequently, in the presence of MGO, the intermediate is easily trapped owing to intermolecular hydrogen bonding in polar media and the subsequent dehydration is decelerated to emit NIR-II fluorescence signals. The spectral curves reveal that MG-SLNP emits dose-dependent fluorescence signals in response to MGO. In particular, the NIR-II fluorescence signals in the range of 900–1400 nm is gradually enhanced, increasing the SBR by 18 times at 6 μM MGO. The linear range of the MG-SLNP response, from 0.3 to 5 μM, has an LOD of 57 nM (Figure 10c). By using biocompatible MB-SLNP, rapid response, a high-resolution detection range, and a relatively satisfactory SBR can be achieved, further enabling the real-time detection and in vivo bioimaging of MGO.
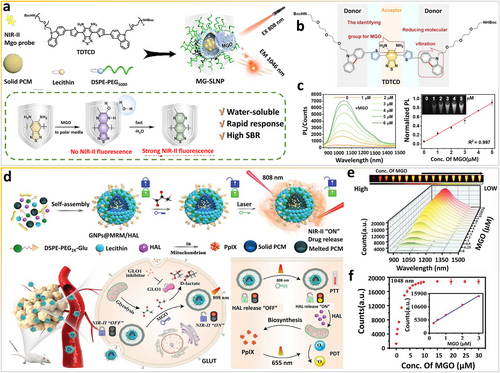
Physiological metabolites as in vivo stimuli. (a) Scheme of the bioresponsive NIR-II fluorescent nanoprobe for rapid detection and imaging of methylglyoxal facilitated by the local nonpolar microenvironment. (b) The molecular structure of MGO-bioresponsive nanoprobe. (c) FL spectra of MG-SLNP treated with MGO and corresponding fitted linear relationship. Reproduced with permission [84]. Copyright 2022, American Chemical Society. (d) Scheme of the reactive glycolysis metabolite-bioresponsive nanotheranostic platform for imaging-guided phototherapy of cancer. (e) FL spectra of probes treated with MGO at different concentrations and (f) the corresponding trend of emission intensity at 1048 nm. Reproduced with permission [85]. Copyright 2022, John Wiley and Sons. MGO, methylglyoxal; NIR-II, second near-infrared.
Likewise, Xu and co-workers synthesized another smart “dual-lock-and-key” MGO-based NIR-II fluorescent probe, MRM, and then incorporated a photodynamic prodrug (i.e., hexyl 5-aminolevulinic acid hydrochloride [HAL]) into the lipid nanoparticles for nanotheranostics (Figure 10d) [85]. The surfaces of MRM and HAL co-loaded nanoparticles are modified with glucose to construct GNPs@MRM/HAL nanoparticles, which target tumors by recognizing overexpressed glucose transporters (GLUT) on cancer cell membranes. The benzo[c][1,2,5]thiadiazole-5,6-diamine group in MRM is a weak electron acceptor and reacts with MGO (the “first key”) to form thiadiazole-fused methylquinoxaline, which turns “on” the NIR-II fluorescence of MRM. Next, under 808-nm laser irradiation (the “second key”), the NIR-II fluorophore induces the photothermal effect, which is tunable, to release HAL inside the tumor. The “dual-lock and dual-key” design concept is a convenient and effective strategy for achieving spatiotemporal tunable phototheranostics. As shown in Figure 10e,f, the NIR-II fluorescence signals of NPs@MRM nanoparticles are linearly correlated with the concentration of MGO with an LOD of 41 nM, demonstrating the high sensitivity of MRM for MGO. Overall, in vivo NIR-II fluorescence bioimaging using smart nanoprobes that respond to pathophysiological metabolites can provide novel insights for the development of precise cancer nanotheranostics.
6 CONCLUSIONS AND PERSPECTIVES
In this review, we surveyed recently developed bioresponsive NIR-II fluorescent probes for accurate bioimaging, in which ONOO−, RSS (H2S, GSH), enzymes, and physiological metabolites are critical analytes. These smart probes were introduced with an analysis of structural designs and stimuli-responsive strategies, focusing on the interactions among irradiation sources, shell species, and the luminescent core. More importantly, energy transfer and electron transfer are the essential mechanisms underpinning stimuli-responsive processes. Among smart NIR-II fluorescent probes, shell species can be divided into two categories: organic dyes with outstanding optical properties [86] and inorganic functional materials [56]. The small Stokes shifts (<50 nm) of organic cyanine-based smart fluorophores can result in severe crosstalk between excitation and emission spectra, which significantly compromise the imaging sensitivity and accuracy. Moreover, smart probes are designed to integrate various merits, for example, responsiveness of shell organic species and stable optical properties of fluorescent cores, and thus they exhibit excellent sensitivity and specificity as well as biological properties, which enables high-quality imaging in biomedicine. Compared with conventional “always on” and “nonspecific” fluorescence, we believe that recently advanced stimuli-activated fluorescence, with multi-channel and ratiometric signals, is more effective in achieving highly accurate and sensitive bioimaging in complex pathophysiological microenvironments. Despite substantial achievements, there are still urgent challenges that need to be further studied and addressed.
6.1 Increasing the quantum yield
In optical applications, the excitation/emission wavelengths, signal intensity, lifetime, and quantum yields are critical measurement parameters. An increase in the excitation/emission wavelengths can decrease the damage to tissues and guarantee signal acquisition, while the signal intensity is associated with the irradiation power and accumulated probe concentration. With this regard, the signal intensity is an unsuitable parameter for accurately measuring the performance of lesion imaging [87]. Generally, optical materials with higher quantum yields can easily produce stronger emission signals (Figure 11a). A high-fluorescence quantum yield is beneficial for enhancing the SBR and resolution of bioimaging. Emerging NIR-II stimuli-responsive probes are listed in Table 1. Increasing the quantum yield of smart optical probes can lead to reduced interfering signals from nondiseased tissues and background noise, which improves the quality of biosensing and bioimaging. So far, there is no effective strategy to synthesize one universal structural design with a high quantum yield, long-wavelength emission, and strong light absorption.
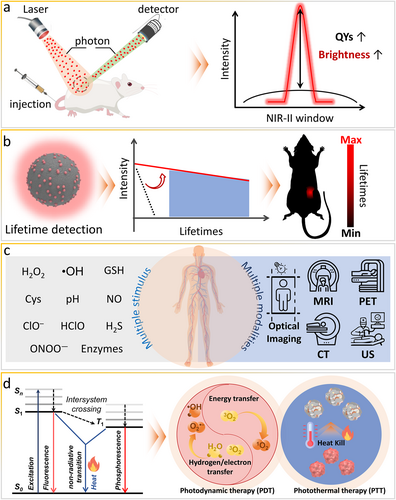
Prospects of bioresponsive NIR-II fluorescent probes, ranging from design, synthesis, and characterization to applications. (a) Increasing the quantum yield. (b) Development of lifetime imaging. (c) Multimodal imaging integration. (d) Therapeutics integration. NIR-II, second near-infrared.
| Smart NIR-II probes | Type of probes | Pathological analytes | Design principles | QYs (%) | Ref. |
|---|---|---|---|---|---|
| SIMM1000 | Self-assembly cyclopeptide dye | pH | Aggregation-induced emission (AIE)/enhancement | 0.51F | [29] |
| LET-1052 | Polymethine dye | pH | Molecular structure changing | 0.087F | [88] |
| NP-V | Polymethine dye | Viscosity | Rotation restriction | 3.7F | [30] |
| WD-X | BODIPY dye | Viscosity | Rotation restriction | 1.6F | [89] |
| PAT-BNT, TPAT-BSeNT | D-π-A-π-D thia/selena-diazolobenzotriazole | Polarity | Intramolecular charge transfer (ICT) | 49.2F, 17.3F | [90] |
| BHC-Lut | Benzoindolium heptamethine cyanine dye | H2O2 | Molecular structure changing | — | [91] |
| HP-H2O2 | Heptamethine-cyanine dyes | H2O2 | Molecular structure changing | ∼3.61F | [92] |
| IR-990 | D-π-A polymethine dye with phenyl borate moiety | H2O2 | Molecular structure changing | — | [93] |
| Hydro-1080 | Cyanine dye | •OH | Rigid planarization | 0.45F | [94] |
| QY-N | Bismethoxyphenyl-amine-containing dihydroxanthene, quinolinium | NO | Molecular structure changing | 0.48F | [95] |
| BOD-NH-SC | BODIPY dye | NO/H2S | Molecular structure changing | 4.56F (NO) | [96] |
| IRBTP-B | Benzothiopyrylium cyanine dye | ONOO− | Molecular structure changing | 0.1F | [97] |
| LET-7 | Anionic polymethylcyanide dye | GSH | Molecular structure changing | — | [98] |
| Nano-PT | BODIPY dye | H2S | Molecular structure changing | 0.0034F | [99] |
| WH-X | BODIPY dye | H2S | ICT | 0.206F | [33] |
| TPE-BODIPY-Cl | BODIPY dye | H2S | Fluorescence resonance energy transfer (FRET) | — | [100] |
| RHC-NO2 | Rhodamine hybrid polymethine dye | Nitroreductase | Molecular structure changing | 0.1F | [101] |
| BOD-M-βGal | BODIPY dye | β-galactosidase | Molecular structure changing | — | [102] |
| AuNNPs–Ag2S vesicle | Organic-inorganic nanocomposite | pH | Self-assembly Ag2S disassembly/losing quenchers | — | [20] |
| Ag2S vesicle | Organic-inorganic nanocomposite | pH | Self-assembly Ag2S disassembly (limited radiation pathways) | — | [21] |
| FEAD1 | Organic-inorganic nanocomposite | pH | FRET | — | [103] |
| HvMnO2@QDs-IR1061 | Organic-inorganic nanocomposite | pH | Absorption competition-induced emission (ACIE) | 62.61F | [104] |
| GSH-CISe nanotubes | Inorganic nanocomposites | pH | pH-induced fluorescence-to-long-lived phosphorescence switching (triplet exciton stabilization) | 12.4P (pH5.5), 39.8P (solid) | [36] |
| Ag/Ag2S JNPs | Inorganic nanocomposites | H2O2 | Plasmonic electron transfer in the JNPs | 4.91F | [105] |
| CCM@AT | Organic-inorganic nanocomposites | H2O2 | FRET | — | [106] |
| Er3+ sensitized UCNPs | Inorganic core-shell nanocomposites | H2O2 | ACIE | — | [18] |
| Er3+-doped DCNPs withCy7.5 | Organic-inorganic nanocomposites | ClO− | Inner filter effect | — | [19] |
| DCNP@SeTT@PEG | Organic-inorganic nanocomposites | HClO | Molecular structure changing | — | [107] |
| DCNP@MPS@IR-NO | Organic-inorganic nanocomposites | NO | Photo-induced electron transfer | — | [108] |
- Abbreviations: —, not available; BODIPY dye, difluoroboron dipyrromethene dye; DCNPs, downconversion nanoparticles; D-π-A, donor-π-acceptor; D–π–A–π–D, donor-π-acceptor-π-donor; F, fluorescent quantum yield; p, phosphorescent quantum yield; QYs, quantum yields of smart NIR-II probes after encountering specific stimuli; UCNPs, upconversion nanoparticles.
The development of stimuli-responsive optical probes with a high quantum yield and long-wavelength emission is still a significant challenge. According to the empirical energy-gap law [109], decreasing the energy gap (ΔE) increases the nonradiative rate constant (knr) and decreases the radiative rate constant (kr). The quantum yield is determined by kr and knr. A longer wavelength emission needs a smaller ΔE, and a faster non-radiative transition results in a lower quantum yield and shorter lifetime. Currently, the ΔE of small molecular probes can be increased by enhancing the rigidity of long π-conjugated organic skeletons, introducing bulky steric substituents to ligands, and avoiding heavy metal elements. Moreover, the quantum yield can be increased by populating the emission level through element doping and by decreasing lattice imperfection through a composite shell structure. Dye-sensitized functional NIR-II probes with high extinction coefficients have large absorption cross-sections to realize bright fluorescence.
6.2 Smart lifetime imaging
As mentioned above, in vivo bioimaging and biosensing based on the fluorescence is hindered by the signal intensity relying on the irradiation power and accumulated probe concentration. Generally, long-lived signals are selectively collected in intensity imaging, and thus, the accuracy of quantitative intensity imaging is difficult to improve owing to various interferences in complex biological microenvironments, photobleaching of fluorescent probes, and light scattering and absorption by biological tissues. In addition to the intensity, the lifetime is another critical optical parameter of probes, which can be exploited for imaging to eliminate the interference of autofluorescence as well as light scattering and absorption by tissues because of the long decay time of emission. Therefore, lifetime imaging can be as competitive as intensity imaging in the long-wavelength spectral window (Figure 11b). Photoluminescence lifetime imaging reports the decay information in each pixel. Long-lived signals are differentiated from background noise in the “time domain,” and the imaging resolution and sensing sensitivity can be significantly improved without background noise. Because phosphorescence has a relatively longer lifetime than fluorescence, it has been exploited for lifetime imaging [36] using NIR-II probes responsive to stimuli, such as pH and hypoxia [36, 110]. The long afterglow, a radiative emission lasting for a long time after the cessation of excitation, also needs further research to improve in vivo bioimaging using NIR-II stimuli-responsive optical probes because they can eliminate tissue autofluorescence [111, 112]. According to the energy gap law mentioned above, long-wavelength emission probes have decreased lifetimes. However, in intensity imaging, long-lived NIR-II stimuli-responsive optical probes can selectively collect long-lived signals, leading to accurate pathophysiological information. The rational design of smart optical probes needs to comprehensively balance various targets, including long lifetimes, high quantum yields, and long wavelength, to promote the translation of NIR-II probes into clinical applications.
6.3 Multiple stimuli-responsive design
Generally, most of the stimuli-responsive probes summarized in this review only sense and image one single stimulus in the pathophysiological milieu. The development of diseases induces various changes in biological tissues, such as abnormal pH levels, unbalanced RNS/RSS levels, and overexpressed enzymes. Multiple biosubstances are generally involved in a single/diverse pathophysiological or metabolic process, which can directly affect a variety of pathophysiological events to trigger the development of related diseases, such as Alzheimer's disease and Parkinson's disease. However, some specific bio-substances exist in both normal and diseased locations, and imaging of slight differences using stimuli-responsive NIR-II probes can be inaccurate. Stimuli-responsive probes active in a single smart site may show imaging specificity. Owing to the dynamic variation of the pathophysiological milieu, probes at a certain concentration in nontarget tissues may be activated nonspecifically during delivery, giving false or inaccurate results. Thus, NIR-II probes designed to respond to a single stimulus cannot provide enough disease-related information, lacking the requirements for accurate bioimaging and biosensing of a specific disease. Multiple stimuli-responsive probes can be easily realized in composite probes that integrate multiple functional units, for example, organic dyes and rigid inorganic nanocomposites. Note that multiple stimuli-responsive probes need to engage with the targeted stimulus for effective activation of fluorescence [96]. Multiple stimuli-responsive designs can improve imaging specificity and are promising for future medical treatments.
6.4 Multimodal imaging integration
Currently, single-mode imaging has certain advantages and drawbacks in terms of sensitivity, spatiotemporal resolution, and tissue penetration depth. For example, stimuli-responsive FL has high sensitivity but shallow tissue penetration. US can be used to realize deep tissue penetration, high spatial resolution, and real-time imaging at the cost of poor bone and gas penetration and non-whole-body imaging [113]. PAI combining optical excitation and acoustic detection can provide a sub-millimeter spatial resolution with centimeter-level penetration depth [114-116]. Moreover, CT has a high resolution but cannot display functional information. PET has deep tissue penetration but poor spatial resolution. MRI can obtain structural and functional information but has low sensitivity and poor responsiveness to small changes. These limitations restrict the type of information obtainable with single-mode imaging. In this regard, to obtain comprehensive and accurate pathophysiological information in a complex and dynamic biological milieu, multimodal imaging can provide highly sensitive and superior spatial resolution to monitor biomarkers and processes in vivo. Thus, by integrating the merits of different imaging modalities, specificity can be boosted for disease diagnosis (Figure 11c). Moreover, intraoperative assessments and resection of tumors are possibly achievable via multimodal imaging.
The use of hybrid imaging systems integrating MRI and PET can provide morphological, functional, metabolic, and even whole-body information in a single examination, revealing pertinent diagnostic, prognostic, and predictive information. For example, PET/MRI is superior to MRI alone in some respects, including enhanced soft tissue visibility and extra functional imaging capabilities. As expected, dual PET/NIR FI based on anti-tumor antibodies can offer impressively synergistic features, including excellent resolution compared with conventional PET imaging. Although integrating several imaging modalities can provide complementary information, carefully designed probes are required because the mechanisms underpinning the above-mentioned imaging modalities are different. For example, dual NIR-II FI/PAI requires rational allocation and conversion of irradiation energy, while dual NIR-II FI/MRI requires integration of functional structures. All new advanced techniques need to be tested with research. We believe that emerging modalities integrating NIR-II stimuli-responsive optical probes may address current unmet obstacles of disease diagnosis and treatment in the foreseeable future.
6.5 Therapeutics integration
Current NIR-II stimuli-responsive optical probes are mainly used for in vivo bioimaging and biosensing of biomarkers. More interestingly, optical probes integrating the therapeutic potential of organic and inorganic species can be used to realize disease diagnosis and treatment simultaneously. NIR-II imaging is an attractive imaging modality for integration because of attractive features, such as the use of nonionizing radiation, portability, and low cost. Therefore, NIR-II imaging has been integrated with PDT and photothermal therapy (PTT) to provide multiparametric anatomical and biologic information about therapeutic processes (Figure 11d). The absorption/emission wavelengths, brightness, photothermal conversion efficiency, and reactive species production efficiency are critical measurement parameters of NIR-II stimuli-responsive optical probes in therapeutic applications. Notably, during energy dissipation, three pathways leading to the generation of optical signals, heat, and reactive species are always in competition. To tackle this dilemma, smart NIR-II probes need to be carefully designed to achieve balance and maximize the merits of each mode for both diagnosis and treatment. Each part of the NIR-II stimuli-responsive optical probe plays a different role and cannot interfere each other in the complex milieu. Generally, composite smart optical probes with long-wavelength emissions have great potential for theranostic applications.
In summary, with increasing efforts devoted to the field of optical imaging, NIR-II stimuli-responsive optical probes have demonstrated potential for biosensing and bioimaging applications, thereby meeting multiple specific requirements. Eventually, diverse biological events will be revealed and detailed molecular information and mechanisms of basic pathophysiologic processes will be delivered, which will significantly improve the precision and efficiency for disease diagnosis and treatment in the clinic.
AUTHOR CONTRIBUTIONS
Baisong Chang: Conceptualization (lead); Funding acquisition (lead). Yuqin Chen: Formal analysis (equal); Writing – review & editing (equal). Jie Chen: Data curation (equal); Writing – original draft (equal).
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The author declares no conflict of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




