Threatened species are disproportionately important interactors in a seed dispersal network in Southeast Asia
东南亚地区濒危物种在种子散布网络中扮演着关键性角色
Abstract
enSoutheast Asia is a conservation priority region due to its high biodiversity—including megafauna—and high rates of defaunation, which has negative impacts on key ecological processes such as seed dispersal. Yet, seed dispersal interactions at the community level have rarely been described in this region. This is a major knowledge gap because medium-size and large animals are disproportionately affected by defaunation and they also have critical roles as seed dispersers. Hence, community-wide studies that encompass a full range of animal body sizes across diverse regions are required, to enable an improved understanding of defaunation impacts. Here, we (a) describe a highly diverse Southeast Asian seed dispersal network (Khao Yai National Park, Thailand), (b) assess the role of body size in identifying important animals and (c) determine if threatened species are disproportionately important in the network. The network is highly nested and modular, with species phylogeny, body size and seed size having a major influence on modularity; mammals and birds occupied different modules. Generalist species playing important roles in the network were mainly medium or large-sized. However, the largest disperser (elephants) played a relatively minor role in seed dispersal in this community, and bulbuls were important despite their small size. Many threatened animal species were important within the network as connector species and through their interactions with a larger number of plant species. Consequently, the resilience of this biodiversity hotspot is at threat by the potential nonrandom loss of the most important seed dispersers.
Abstract
zh因具有极高的生物多样性(包括大型兽类),且面临能对种子传播等关键生态过程产生重要影响的动物群落的快速丧失,东南亚地区成为生物多样性保护的重要优先地区。然而,该地区在群落水平上进行种子散布相互作用的研究仍然较少。大中体型动物是关键的种子传播者,且受到更大的动物群落丧失的影响。因此,为深入了解动物群落丧失的影响,在不同地区开展涵盖各类体型动物的群落水平研究就显得十分必要。本文以泰国考艾国家公园为研究对象,旨在(a)描述这一高度多样化的东南亚种子散布网络,(b)研究体型对评估动物的种子散布功能的作用,进而(c)判断受威胁动物在种子传播网络中的作用是否更为重要。结果表明:该网络高度嵌套和模块化,且系统发育、体型和种子大小对网络模块化均有重要影响;哺乳动物和鸟类占据不同的模块。作为泛性物种的大中型动物在该网络中发挥重要作用。然而,体型最大的大象所起的种子散布作用相对较小;而体型很小的鹎类,却起到重要的作用。作为该网络中的连接物种,许多受威胁动物通过与大量植物物种的关联在该网络中起到重要作用。因此,重要种子散布动物类群的潜在非随机丧失正在对该生物多样性热点地区的群落恢复力造成威胁。
1 INTRODUCTION
Seed dispersal is fundamentally important for determining the spatial and genetic structure of plant populations (Dick et al., 2008; Voigt et al., 2009) and in ensuring their long-term ability to adapt to change (Corlett & Westcott, 2013; McConkey et al., 2012). In tropical forests, most seeds are dispersed by animals (Howe & Smallwood, 1982) which vary widely in their fruit selection and their seed dispersal capabilities (Bueno et al., 2013; Martínez et al., 2008). Determining how these communities of diverse seed-dispersing animals are structured is essential for understanding the relative roles and potential importance of different animal species in seed dispersal, which is helpful to predict the resilience of ecosystems to the various disturbances that impact them (Fricke et al., 2017; Garcia et al., 2013; Markl et al., 2012).
Defaunation is a major problem in tropical forest communities and the animals most at risk tend to have medium-to-large body sizes (Bogoni et al., 2020; Wen et al., 2020). Because these animals often play especially vital roles in seed dispersal (Donatti et al., 2011; Naniwadekar et al., 2019; Ong et al., 2022; Timoteo et al., 2018), documenting their broad contributions within the dwindling intact communities is essential to understand the potential impact of their declines (Dugger et al., 2019; Vidal et al., 2013). However, we lack community-wide assessments of the roles of larger animals across a broad range of habitats. For example, elephants are especially important seed dispersers (Campos-Arceiz & Blake, 2011); yet only two community-wide studies have been conducted that encompass elephants (Ong et al., 2022; Timoteo et al., 2018). Defaunation also impacts smaller animals such as deer and primates, for example, all of which are important frugivores and seed dispersers (Bogoni et al., 2020; Osuri et al., 2020; Wen et al., 2020).
The animal and plant communities that maintain tropical forest ecosystems are diverse, both within single ecosystems and across tropical forests around the world (e.g., de Almeida & Mikich, 2018; Donatti et al., 2011; Fricke et al., 2017; Heleno et al., 2013). This diversity is apparent in the range of plant taxa available, and also the combinations of animal species that are present. For example, Southeast Asian rainforests, have unique frugivore taxa such as Asian barbets and gibbons, and the forests also have characteristic supra-annual fruiting schedules (Corlett, 2007b; van Schaik et al., 1993). These fundamental differences mean that we require community studies from regions across the globe so that patterns, processes and their vulnerabilities singular to particular regions can be identified. It is also important that community-wide studies encompass a large proportion of the animal community, rather than be focused on a single taxonomic group. This can be difficult to achieve, which is why many studies from highly diverse regions are focused on selected frugivore groups. Without capturing the diversity of tropical forests both within and across regions, our capability to identify the strongest interactors at a community level will remain limited, as well as our understanding of the vulnerability of tropical forests.
Southeast Asia is characterized by the dipterocarp forests that dominate the Sundaic region and have extreme supra-annual fruiting patterns, which make the forests difficult environments for frugivores (van Schaik et al., 1993). A seed dispersal network constructed in this region showed a forest dominated by mammal seed dispersers with the most important dispersers being able to handle large seeds—either because they were very large (elephants), could carry large seeds (rats), or had the capacity to swallow large seeds (gibbons) (Ong et al., 2022). Other regions of Southeast Asia have more reliable fruiting patterns, even though the animal and plant communities are somewhat similar. One of these regions is the semideciduous forests of Khao Yai in Thailand, where seed dispersal and frugivory in different sectors of the community have been studied over decades (e.g., Kitamura et al., 2002). Here, plants still exhibit supra-annual fruiting but there are reliable sources throughout the year and the forests lack the periods of extreme scarcity shown in the dipterocarp forests to the south (Suwanvecho et al., 2017). Throughout the region, deforestation is being exacerbated by strong hunting pressure, and this is already having major impacts on seed dispersal and tree communities in the region (Brodie et al., 2009; Chanthorn et al., 2019; Corlett, 2007a; Harrison et al., 2013, 2016; Laurance, 2007). It is imperative that we improve our understanding of community-level interactions in more forests so that the consequences of frugivore loss can be assessed.
Here, we describe a highly diverse Southeast Asian seed dispersal network based on a literature review; we took advantage of the numerous studies performed within the study site—Khao Yai National Park. The community of fruit-eating animals that we studied includes the full-size range of vertebrate seed dispersers, from small birds to elephants. The main animal groups missing from the data set (but not the forest) are nocturnal rodents such as rats and porcupines, and bats. We had three main objectives: (a) to describe the structure of a binary seed dispersal network that includes the full range of body sizes; (b) to identify whether larger frugivores tend to be the most critical species for seed dispersal as described by key network metrics (connectors, module hubs); (c) to determine if the important animals identified (via network metrics) were disproportionately threatened.
2 MATERIALS AND METHODS
2.1 Study site
Khao Yai National Park (2168 km²; 14°05′–15′ N, 101°05′–50′ E) was established in 1962. It is mainly covered by seasonally wet evergreen forest (Forest Restoration Research Unit, 2000), with some grasslands, and an elevational range of 250–1326 m asl. The climate is monsoonal, receiving 1200–3000 mm of rainfall per year. Maximum fruit availability occurs from April to June and sometimes in August. The Park supports a diverse bird (320 species) and mammal fauna (more than 71 species) (Lynam et al., 2006).
2.2 Data collection
Frugivory and seed dispersal have been well-studied in Khao Yai over the last 20 years, beginning in 1998 (Kitamura, 2000). We collected information from 17 published and four unpublished data sets (Supporting Information: Table S1) from all research conducted at the study location on these topics (most of it by the current authors). The available data include information on all major fruit-eating vertebrates with the exception of bats, rats and porcupines. There were insufficient studies on the nocturnal rodents (rats and porcupines) to understand their broad diets and to distinguish between seed dispersal and predation. Some bat species are present in Khao Yai but we found no evidence of feeding roosts, and no papers have been published on their role. All trees and lianas for which data were available and which have fleshy fruits are included in the network. Because the network is based on multiple studies conducted at different times and with different methods (Supporting Information: Table S1) the recorded interactions are binary and do not include the frequency of the interactions. This is a major limitation in the study as frequency is an important determinant of species' importance (Carlo & Yang, 2011).
Of the 60 animal species included in the final network, the majority of their interactions were identified in plant-focused studies (representing around 1 year of sampling effort in total, but spread over multiple years). These included direct observations of plants, camera-trapping (terrestrial and arboreal), and often supplemented with animal feeding trials (informally with habituated, wild animals—not captivity studies; McConkey et al., 2018), and indirectly through dung checks and identification of feeding signs (see references in Supporting Information: Table S1). Nine animal species had their interactions identified from animal-focused studies (representing at least 1 year of study for seven of the species) (Supporting Information: Table S1). These studies included animal follows, and systematic investigation of dungs. Studies were often focused on these animals because they were assumed to play important seed dispersal roles but this could potentially have led to them being over-represented in the network. If this focused effort for nine species biased the results, we expected that these species would be disproportionately represented in the nine most generalist species (i.e., those with the most interacting partners, which is termed as having the highest degree); this was not observed, with data collated through plant-focused studies for six species, and from animal-focused studies for three species. Hence, we assume that there is no major bias in combining interactions from both study methods.
We present the results from a seed dispersal network. We define seed dispersal as the confirmed or expected deposition of some undamaged seeds away from the parent crown (and thereby reaching sites where germination is more likely; Terborgh, 2020). ‘Confirmed’ interactions include those in which intact seeds were found in faeces, or were spat out, regurgitated or dropped away from fruiting trees. ‘Expected’ interactions were assigned when only frugivory was observed, but the seed was small enough to be dispersed by the animal interactor, and the animal interactor is a known seed disperser. Explanations of these decisions are given in Supporting Information: Table S2. For most species without at least one confirmed seed dispersal interaction, dispersal was confirmed in co-generic taxa or at the family level within the Khao Yai data sets. For three families (Phasianidae, Trogonidae, Psittacidae) we used published studies from other regions to confirm the taxa could function as a seed disperser (Blanco et al., 2016; Case et al., 2022; Wenny, 2000). For animals that were also seed predators, we included (i) confirmed seed dispersal interactions, and (ii) frugivory interactions in which the seed was very hard, and where the animal did not consume depulped seeds. We assigned these interactions with seed predators conservatively and excluded interactions for which we had no personal knowledge of the seed hardness. Overall, 49% of the 1148 interactions were confirmed and 51% were expected; four animal species had fully confirmed interactions (13–133 interactions per animal species; 306 interactions in total), 23 animal species had confirmed and expected interactions (mixed) (2–119 interactions; 731 interactions in total) and 32 animal species had only expected interactions (1–9 interactions per species; 111 interactions in total) (Supporting Information: Table S2).
Plant and family names follow Plants of the World Online (POWO, 2022). We only considered seed dispersal interactions involving fully identified plant species (78% of recorded species). Some animal species (Paradoxurus hermaphroditus, Paguma larvata and Arctictis binturong; Callosciurus finlaysonii and Ratufa bicolor; Muntiacus muntjak and Rusa unicolor; Helarctos malayanus and Ursus thibetanus) have rarely been clearly differentiated in seed dispersal studies, so we pooled them into their respective taxonomic group (civet, squirrel, deer and bear).
Data on fruit and seed width (the second largest dimension which is the limiting factor for ingestion [Forget et al., 2007] [Supporting Information: Table S3] and on body mass [Supporting Information: Table S4]) were compiled from the literature (Supporting Information: Table S1), Plants of the World Online (POWO, 2022) and EltonTraits (Wilman et al., 2014). For animals, we collected data on body weight, IUCN conservation status (IUCN, 2021) and conservation status in Thailand (Office of Natural Resources and Environmental Policy and Planning, 2021) (Supporting Information: Table S4).
2.3 Determining the structure of the network
We organized our network as a binary matrix where a seed dispersal interaction between plants (in columns) and animals (in rows) was represented by 1, and no interaction was represented by 0 (Supporting Information: Table S5). This binary network lacks information on the frequency of interactions and therefore has limited application to understanding animal importance, beyond the richness of species dispersed. To study the network structure, we estimated the modularity and nestedness (Tylianakis et al., 2010). We used NODF as a measure for Nestedness, which is based on the overlap and decreasing fills (Almeida-Neto et al., 2008; Ulrich et al., 2009) through the ANINHADO programme version Bangu 3.0.3 (Guimarães & Guimarães, 2006). We generated 1000 random matrices using a null model in which the probability of interaction between a plant and an animal species is proportional to their total number of interactions (Bascompte et al., 2003) and compared the outcomes with results from the original matrix.
Modularity (Q) was measured using the Bipmod function from BipartiteModularityMaximization R Package (T. Chen et al., 2022), based on a simulated annealing algorithm (Guimerà & Amaral, 2005b). Modularity (Q) describes how well interactions are divided among modules, with higher values of modularity expected for larger data sets (Dormann & Strauss, 2014). To assess the statistical significance of the metric, we compared the Q value obtained for the original matrix to the ones obtained for 50 random matrices, using the same null model as for the nestedness analysis (‘null model 2’; Bascompte et al., 2003).
We pictured the position of species in the network based on its interactions within modules (z) and among modules (c). Connectors (c > 0.62) were important for linking modules together, while module hubs (z > 2.5) are highly connected within their own module. Supergeneralist species, acting as both connector and module hub, are considered network hubs (z > 2.5 and c > 0.62) and potentially play a highly generalized role within the community because they disperse seeds from broad types of fruits (Guimerà & Amaral, 2005a; Olesen et al., 2007). A species with a few links inside its own module and rarely linked to other modules is considered a peripheral species (with z < 2.5 and c < 0.62; Guimerà & Amaral, 2005a; Olesen et al., 2007).
To test whether animal and plant traits had a significant phylogenetic signal (Blomberg & Garland, 2002), we calculated Abouheif's Cmean (Abouheif, 1999). This index is known to have among the best performances under a Brownian motion model of trait evolution (Münkemüller et al., 2012). We used the function ‘phyloSignal’ in the ‘phylosignal’ package (Keck, 2015) in R. We used the phylogenetic tree reconstructed by Hinchliff et al. (2015) for animals and the Phylomatic tree R20120829 (Webb & Donoghue, 2005) for plants. We also tested whether modules had a significant phylogenetic signal, such that the composition of species within modules can be explained by the phylogeny. We used the function ‘phylo.signal.disc’ in R that corresponds to the ‘Fixed Tree, Character Randomly Reshuffled’ model proposed in Maddison and Slatkin (1991) (see Donatti et al., 2011 for details). All branches in animal and plant trees were set equal to 1, which is assumed to have only negligible impacts on the performances of Abouheif's Cmean (Münkemüller et al., 2012).
To compare body weight, seed width and fruit width among modules, we performed phylogenetic ANOVAs and post hoc tests for pairwise comparisons with a Holm–Bonferroni sequential correction (Holm, 1979) using the function ‘phylANOVA’ in the ‘phytools’ package. For this purpose, we log-transformed values of body weight, seed width and fruit width. We used Pearson product-moment correlations to highlight the relationship between the body weight of dispersers and (a) the number of plant species they interact with (i.e., their degree of interactions), (b) c-values and (c) z-values. T-tests were used to compare the c-value and z-values of threatened (Endangered or Vulnerable species on the IUCN Red List) and nonthreatened species (Least Concern, Near Threatened). These tests were done to determine if the most important animals in the network were more likely to be threatened.
3 RESULTS
3.1 Overview of network structure
The network of potential and confirmed seed dispersal interactions included 60 animal species (Supporting Information: Table S4) and 271 plant species (Supporting Information: Table S3) involved in 1148 unique dispersal interactions (Supporting Information: Table S5). The diversity of fruit-consuming animals and fleshy-fruited plants is not known in the study area, but species accumulation curves suggested a good proportion of the animal and plant diversity was sampled (Supporting Information: Figure S1), although it is likely that actual interactions were undersampled. Animals included 53 bird species (36 passerines, 17 nonpasserines) and 7 mammal taxa (two primate species, elephant, and deer, civet, bear, and squirrel categories that included multiple species). The main plant families in the network were Moraceae (33 species), Lauraceae (19), Rubiaceae (18) and Annonaceae (17).
The network was significantly nested and modular with six modules identified (Figure 1). Four modules contained only birds and two modules contained only mammals; no modules contained both birds and mammals. There were slightly more plant species contained within the bird modules (155 species), compared to the mammal modules (119 species). We detected a phylogenetic signal in the modularity of plants as well, with the species of some plant families being particularly associated within certain modules. For example, most species in the plant families Anacardiaceae, Moraceae and Clusiaceae were associated with mammal module M1, while most Rubiaceae species were associated with the small bird module B3 (Supporting Information: Table S1). The effect of phylogeny on the distribution of plant and animal species within modules was confirmed by a significant phylogenetic signal in modules (p < 0.0001 for animals and plants).
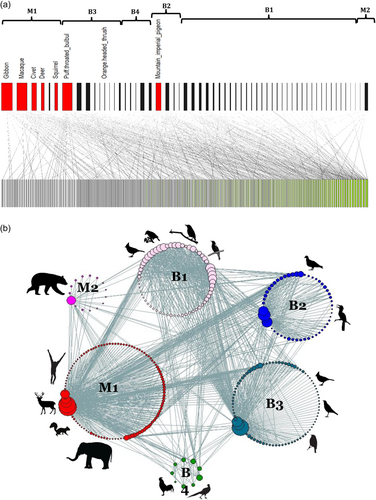
3.2 Important species in the network
Species considered to have especially important roles in the network were the connectors (c > 0.62) and within module hubs (z > 2.5). Eight animal connectors were identified and two module hubs (Table 1, Figure 2 and Supporting Information: Figure S2). There were no animal network hubs, and most species (90%) were peripheral species. There were 52 plant species found to be connectors between modules, with the families Moraceae (8 species) and Lauraceae (5 species) contributing most to this group. Eight plant species were module hubs, spread across the families (including two species of Rubiaceae). Three plant species (Desmos dumosus, Melicope pteleifolia and Macaranga denticulata) were network hubs (Figure 2 and Supporting Information: Figure S2). The remaining 78% were peripheral species.
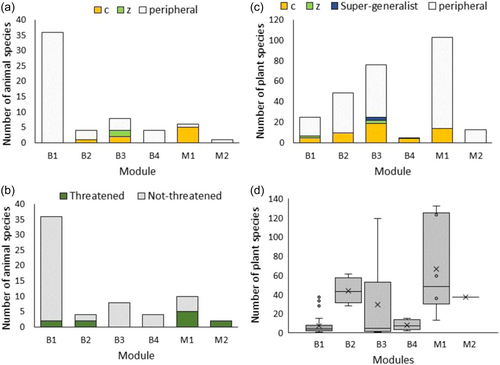
3.3 Description of modules
The modules with the highest proportion of threatened animal species were bird module B2 (containing large birds: hornbills and imperial pigeons), and the mammal modules M1 (most mammals) and M2 (the two bear species only) (Figure 2, Supporting Information: Tables S6 and S7). The mammal module M1 was also the module that contained the most plant species (102 species) and in which each taxon interacted with the highest number of plants (Figure 2). The majority of mammals in this module were connector species, with no module hubs. Species within module B2 also interacted with a high number of plant species, on average, but only one connector species (imperial pigeon) was within the module.
Bird module B1 contained a higher diversity of birds (a mixture of passerines and nonpasserines), but a low diversity of plants (Figure 2). All animal species were peripheral species interacting with few plant species on average. Bird module B3 had few animal species (all passerines), but the second highest number of plant species (76). This was the only module with super-generalist species (3), but none of these bird species were threatened. Bird module B4, contained ground-foraging birds, with few animal and plant species, few interactions and no threatened species.
3.4 Importance of animal size
The body weight of animals, seed width and fruit width showed a significant phylogenetic signal (body weight: Cmean = 0.711, p = 0.001; seed width: Cmean = 0.271, p = 0.001; fruit width: Cmean = 0.083, p = 0.019). There was a significant difference in body weight among modules (ANOVA: F(5,55) = 33.64, p = 0.001) (Figure 3). The post hoc test revealed that body weight was significantly higher for the bird modules B2 (pigeons and hornbills) than B3 and B1 (Figure 3), with no differences among other combinations. The mammal modules did not show a significant difference in body size, with M1 having a large spread of body sizes—from the largest (elephant) to the smallest (squirrels), and M2 containing only bears.
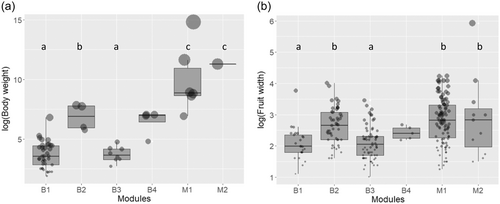
According to plant species, there was a statistically significant difference in seed width among modules (ANOVA: F(5,207) = 7.08, p = 0.001). However, the post hoc test revealed that only B1 differed from other modules according to seed width, with the smallest seeds (B3–B1: p = 0.982; M1–B1: p = 0.253; M2–B1: p = 0.022). There was also a statistically significant difference in fruit width among modules (ANOVA: F(5,207) = 12.05, p = 0.001) (Figure 3). The post hoc test revealed that the B1 and B3 contained significantly smaller fruit than the two mammal modules, and B2 had larger fruit than B1 (Figure 3).
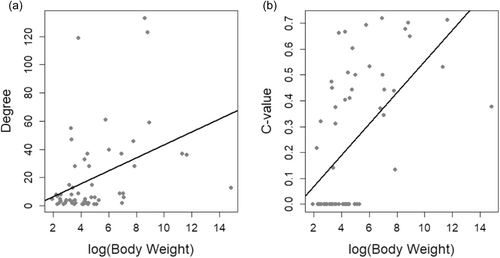
There was a positive correlation between the number of plant species the dispersers interact with and body weight (r = 0.395, d.f. = 59, p = 0.001752) (Figure 4), and also c-value (between modules connector) and body weight (r = 0.574, d.f. = 59, p = 0.00001), but not z-value (within module hubs) and body weight (r = 0.10, d.f. = 59, p = 0.4212). However, elephants—the largest disperser in the network and one of the better-studied species—had low c- and z-values. They interacted with few plants and were therefore an outlier to this pattern.
3.5 Threatened species
Ten animal species listed as threatened by the IUCN are included in the network (Table 1). Four of these species are connectors interacting with a large number of species, and are, therefore, likely to be important in the community and for network cohesion (or how connected the network is). The two bear species are peripheral species but are the only animal species within their own module. While the two threatened hornbill species are also peripheral species, they interacted with a large number of species and they are contained within a module of only four animal species and many plant species. One of the threatened species (the migrant, grey-sided thrush) was recorded in only one interaction.
| Family | Species | Common name | IUCN status | Status in Thailand | Network role | No. interacting plant species | Habitat | Module |
|---|---|---|---|---|---|---|---|---|
| Turdidae | Turdus feae | Grey-sided thrush | VU | VU | Peripheral | 1 | Forest | B1 |
| Bucerotidae | Buceros bicornis | Great hornbill | VU | VU | Peripheral | 28 | Forest | B2 |
| Bucerotidae | Rhyticeros undulatus | Wreathed hornbill | VU | NT | Peripheral | 46 | Forest | B2 |
| Ursidae | Ursus thibetanus | Asiatic black bear | VU | EN | Peripheral (in its own module) | 37 | Forest | B2 |
| Helarctos malayanus | Sun bear | VU | EN | |||||
| Columbidae | Ducula badia | Mountain imperial pigeon | LC | LC | Connector | 61 | Mixed | B2 |
| Pycnonotidae | Alophoixus pallidus | Puff-throated bulbul | LC | LC | Connector | 119 | Forest | B3 |
| Turdidae | Geokichla citrina | Orange-headed thrush | LC | LC | Connector | 3 | Forest | B3 |
| Pycnonotidae | Iole propinqua | Grey-eyed bulbul | LC | LC | Module hub | 55 | Mixed | B3 |
| Pycnonotidae | Pycnonotus flaviventris | Black-crested bulbul | LC | LC | Module hub | 47 | Forest | B3 |
| Cercopithecidae | Macaca leonina | Pig-tailed macaque | VU | LC | Connector | 123 | Mixed | M1 |
| Cervidae | Rusa unicolor | Sambar | VU | VU | Connector | 36 | Forest | M1 |
| Muntiacus muntjak | Muntjac | LC | NT | |||||
| Hylobatidae | Hylobates lar | White handed gibbon | EN | VU | Connector | 132 | Forest | M1 |
| Proboscidea | Elephas maximus | Asian elephant | EN | EN | Peripheral | 13 | Mixed | M1 |
| Viverridae | Arctictis binturong | Binturong | VU | NT | Connector | 59 | Forest | M1 |
| Paradoxurus hermaphroditus | Asian palm civet | LC | LC | |||||
| Sciuridae | Ratufa bicolor | Squirrels | LC | LC | Connector | 37 | Forest | M1 |
| Callosciurus finlaysonii | LC | LC |
- Note: Network roles are defined as connectors (c > 0.62), module hubs (z > 2.5). Habitat is broadly defined as whether they are confined to forest or can occupy forest and disturbed areas (mixed).
Overall, threatened animal species (EN, VU, n = 8) had higher c-values (2.6×) than nonthreatened species (NT, LC, n = 51) (mean ± SD c-value, threatened = 0.483 ± 0.246, nonthreatened = 0.0.188 ± 0.242; t = −3.637, p = 0.0003), while there was no difference in z-values (z-value, threatened = 0.172 ± 0.844, nonthreatened = −0.039 ± 0.984; t = −0.658, p = 0.256). Threatened animal species also interacted with 3.7× more plant species in the network (mean ± SD, 49.00 ± 44.88) compared to nonthreatened species (12.60 ± 21.50) (t = −3.807, p = 0.0002).
4 DISCUSSION
Threatened animal species that were represented within the Khao Yai seed dispersal network were disproportionately important based on their position in the network. Of the 10 animal species listed as threatened by the IUCN, four were connector species (lar gibbons, pig-tailed macaques, binturong, sambar) reflecting the large number of interactions they were involved in, and across modules that varied according to fruit and seed size, and probably other traits as well. Two threatened hornbill species (great hornbill, wreathed hornbill) and two threatened bear species (Asiatic black bear, sun bear; recorded as one taxa in the network) were ‘peripheral’ species, involved in fewer interactions and with lower values for connectedness, but they were contained within two specialized modules. The threatened hornbills shared a module with a third hornbill species and imperial pigeons, along with significantly larger-fruited species, suggesting these large-bodied birds are especially important for large-fruited species in the forest. The bear species were the only dispersers within its module, suggesting that some of the plants they disperse might be particularly dependent on them. The remaining threatened species were the grey-sided thrush, a peripheral species in module B1, and the elephant. The grey-sided thrush is a rare winter visitor to Thailand (Lekagul & Round, 1991) and, therefore, its low importance in the seed dispersal network might indicate its rarity rather than its capability as a disperser. Only two of the threatened species (pig-tailed macaques and elephants) are tolerant of some level of disturbance (Supporting Information: Table S4).
A further six animal taxa (five bird species and squirrels) were revealed as important to the network because they were connectors (four species) and module hubs (two species), but these were not threatened species. Four of these six species (two connectors, two module hubs; puff-throated bulbul, orange-headed thrush, grey-eyed bulbul, black-crested bulbul) were found in a single module that comprised smaller-bodied birds and smaller fruits (module B3). The three bulbuls (family Pycnonotidae) in this group represented half the bulbul species in the network. Bulbuls are recognized as important dispersers of small-seeded fruits in Asia (Corlett, 2017; Khamcha, 2009), and some species are tolerant of habitat disturbance (Corlett, 2017); however, only the grey-eyed bulbul at Khao Yai is considered to be able to occupy disturbed habitats (Supporting Information: Table S4). The six bulbul species were also distributed across two modules (B1 and B3), both comprising small birds and small fruits, which could indicate important differences among their feeding patterns. Imperial pigeons were another nonthreatened connector species, sharing the module with three hornbill species and several large-fruited species. The role of imperial pigeons in seed dispersal has received relatively few studies (Corlett, 2017; McConkey et al., 2004), but this network suggests they could play key roles as dispersers of an array of fruits. Finally, two squirrel species were nonthreatened connector species and are common frugivores at fruiting plants with a high capacity to consume many fruits (McConkey & Brockelman, 2011; McConkey et al., 2015, 2018; Ong et al., 2022). While they drop most seeds undamaged under the parent crowns where successful establishment is unlikely, small proportions of seeds can be carried short distances away (Becker et al., 1985; Brockleman et al., 2022; McConkey et al., 2015). This suggests that their seed dispersal roles should receive closer attention given their capacity for processing many seeds.
Bears (two species) were the only taxa in its own module potentially reflecting a unique role in seed dispersal at the community level. Their diet and seed dispersal role overlap with that of other mammals and, to a lesser extent, birds (Kitamura et al., 2002), but we have a poor understanding of their role at community levels. Of the 37 plant species recognized as bear-dispersed in this study, we failed to identify alternative dispersers for seven of them (19%). However, many other plant species contained within the module were fed on by several other mammal species, with the highest overlap shown with lar gibbons (57%) and pig-tailed macaques (46%); the birds with the highest overlap were puff-throated bulbul (27%) and imperial pigeons (24%). The plant taxa contained within the module were also scattered among several families (Supporting Information: Table S1). Therefore, the extent to which certain plant species might depend on bears for dispersal is not clear. Two bear species co-exist in Khao Yai and they might also vary in their seed dispersal roles, although they consume similar plant species (Steinmetz et al., 2013). Given the threatened status of these two bear species, their seed dispersal roles require urgent assessment.
Medium-sized and larger animals (but not the largest) tended to interact with more plants and be module connectors, suggesting they play dominant roles as seed dispersers in the forest. This pattern has also been observed in the Neotropics (Donatti et al., 2011; Mello et al., 2011a, 2011b) but the absence of very large dispersers in this region hindered a comprehensive assessment of body size. Because large animals are less constrained by seed and fruit size (S.-C. Chen & Moles, 2015), they are expected to be generalists consuming and dispersing a wider range of plant species than smaller dispersers. Indeed, in our study, the modules with the largest animals also contained the largest fruits and seeds, and we found a positive relationship between body size and the number of species dispersed. Overall, we identified especially important roles for medium-sized primates and the largest arboreal birds.
The largest seed disperser in the Khao Yai community is the elephant, but elephants dispersed few species and had low c- and z-values, even though elephants were among the better-studied species. This result contrasts with seed dispersal networks in Mozambique (Timoteo et al., 2018) and Peninsular Malaysia (Ong et al., 2021, 2022) where this megafaunal herbivore was among the most important dispersers. The contrast with Malaysia is particularly surprising given the close proximity of the study sites and the almost identical faunas, highlighting the need for community studies from a wider range of localities. However, the sampling protocol differed between the two studies, and the Malaysian network was a weighted one.
The herbivorous, rather than frugivorous, diet of megafauna (Campos-Arceiz & Blake, 2011) could mean their seed dispersal role at the community level could be site or habitat-dependent. It is possible that elephant access to grasslands is higher in Khao Yai, and the elephants preferentially consume this resource (Campos-Arceiz & Blake, 2011). The Sundaic dipterocarp forest of Malaysia might also contain more fruit species with traits attractive to large herbivores, perhaps a result of its very irregular fruit availability and low densities of frugivores (van Schaik et al., 1993). Indeed, megafaunal fruits are not common in Khao Yai's seasonally wet evergreen forests (eight species recorded in studies conducted over about 10 years; Kitamura et al., 2002), while many megafaunal-sized fruit species are described for Peninsular Malaysia (37 species identified in a 2-year study; Ong et al., 2022). This discrepancy is surprising considering Indochina, generally, has a diverse range of megafaunal fruits (McConkey et al., 2022), further reinforcing the need for frugivory and seed dispersal studies from a range of locations. The lack of a strong community role in frugivory for elephants in Khao Yai does not down play their high importance in seed dispersal for the plant species they do consume in Khao Yai, due to their capacity to consume many fruits (which was not measured in this study) and the high survival rates in elephant dungs (McConkey et al., 2015, 2018), nor the other pivotal ecological roles they might perform (Terborgh et al., 2017).
The importance of small-bodied bulbuls (as mentioned earlier) makes them also outliers to the general trend of large-bodied dispersers being important within the network. These findings highlight the importance of recognizing the roles of all key consumers within mutualistic networks, a recognition that can be overlooked when large body size is emphasized. Some of the most important small-bodied dispersers in seed dispersal networks are frequently not included. For example, rats (Muridae) were included in a Brazilian network (Carreira et al., 2020) and in the network from Peninsular Malaysia (Ong et al., 2021, 2022) and were found to be among the most important interactors, but few community studies include the synzoochoric and endozoochoric contributions of rats, and other rodents. Generating more networks from diverse communities could help identify key traits besides large body size, such as diet type and movement patterns (Mello et al., 2015).
None of the modules in the network contained both mammal and bird species, suggesting the two groups primarily disperse seeds from different subsets of fruits, despite an overlap in fruit diets. The differences in fruit traits that define this modularity were seed and fruit size and possibly other, untested, traits as well. This distinction in modules has also been found in other studies, highlighting consistent differences in the community-level roles of these taxa (Donatti et al., 2011; Mello et al., 2011b). Further, while the diverse bird community was split among four modules, almost all mammals were contained within a single module, suggesting a greater overlap in the dispersal role of these mammals, compared to the overlap among bird species. We also detected a phylogenetic signal in the modularity of plants, with the species of some families being particularly associated within certain modules. To understand these relationships and module composition would require more studies documenting more fruit traits other than fruit and seed size, such as dehiscence and pulp nutrients.
There are four significant limitations of our network for understanding community-wide seed dispersal interactions. First, we could not include the seed dispersal roles of bats and rats, which disperse seeds by stomatochory and synzoochory, as well as endozoochory (Aziz et al., 2021; Gomez et al., 2018). Rats have rarely been included in published networks but these few studies demonstrate they can play pivotal roles (Carreira, 2020; Ong, 2021). Bats are also known to have important seed dispersal roles in Neotropical communities and are recognized as important seed dispersers in the Paleotropics (Aziz et al., 2021; Ong et al., 2022). The second limitation is that the available data did not allow for a weighted, quantitative network and, therefore, we cannot include the important role of interaction frequency in determining network metrics. A quantitative network would have reflected how often animals visited each fruiting plant to give a more robust assessment of their importance. Third, although we sampled a good proportion of the available plants and animals present at the study site, the interactions will be undersampled, with many disperser-plant combinations not identified. In particular, we potentially underestimated the roles of nocturnal arboreal animals, such as civets. As further studies accumulate more interaction information, future studies could reassess species' roles and determine the robustness of the findings presented here. Finally, the interaction data set was built from heterogenous data sets, each of which had different goals and limitations, and potentially introduces bias and error into the final data set.
5 CONSERVATION IMPLICATIONS
All the 10 threatened animal species in the seed dispersal network have decreasing populations, facing major threats from habitat loss and degradation and/or hunting across their distributions (IUCN, 2021). However, three of these species were not considered threatened in Thailand (great hornbill, wreathed hornbill, pig-tailed macaque) (Office of Natural Resources and Environmental Policy and Planning, 2021), suggesting stable populations are being maintained within the country. Conversely, Austen's brown hornbill was listed as threatened in Thailand, but not by the IUCN; this species was not a strong interactor in the network, which might reflect a low density or its diet. Similarly, the two bear species in the network are listed as Endangered in Thailand, while being considered less threatened when their entire range is considered (Vulnerable on the IUCN Red List).
As mentioned previously, four of the threatened species (lar gibbons, pig-tailed macaques, binturong, sambar) likely play critical roles in maintaining seed dispersal interactions—and therefore contribute to the maintenance of plant populations—and it is essential that conservation attention includes maintaining or enhancing their current distribution and population sizes. However, it is important that attention is also directed to the nonthreatened species that hold especially vital roles in the seed dispersal network. Of the nine (six groups of species) nonthreatened animal species shown to be connectors or hubs in the network, only three have stable populations (grey-eyed bulbul, puff-throated bulbul, Finlayson's squirrel) (IUCN, 2021). The remaining six species (Mountain imperial pigeon, orange-headed thrush, black-crested bulbul, black giant squirrel, Indian muntjac, Asian palm civet) have decreasing populations with some species being hunted outside major protected areas. According to the IUCN no conservation action is noted to be occurring for these species. It is essential that these animals and their crucial seed dispersal roles do not ‘silently’ disappear and actions are required to stabilize their populations.
Khao Yai National Park is part of the Dong Phayayen-Khao Yai Forest Complex, but the forests of Khao Yai are disconnected from the other parks and sanctuaries within this complex (Lynam et al., 2006). Hence, the borders of Khao Yai's forest form a hard boundary with human-occupied regions and the forests and wildlife at the edges of the national park are threatened by habitat encroachment and hunting (Lynam et al., 2006). Indeed, despite having an extensive system of protected areas, forest loss in Thailand continues to be a major problem, with the protected area system being biased to habitats that usually show low economic value (Tantipisanuh & Gale, 2022). The seed dispersal network shown here represents the better-protected region of Khao Yai, close to the park headquarters. Hence, the network showed a diverse set of fruit-eating animals, with many strong interactors. Towards the edges of Khao Yai National Park, the seed disperser community could be missing many important interactors as populations are limited by hunting, collection of nontimber forest products and habitat loss (Lynam et al., 2006). The extent of the park that maintains a largely ‘intact’ seed disperser community is not known and it is possible that large areas are already missing key seed dispersers with potentially serious repercussions for plant recruitment and forest regeneration. Indeed, integrating a spatial component to the study of ecological networks would certainly improve our understanding of their functioning in relation to the spatial distribution of pressures (Fortin et al., 2021).
It is not only forest-dependent species that might be affected by habitat degradation, as more disturbance-tolerant animals (e.g., pig-tailed macaques) can exploit human-derived food sources, which can result in decreased fruit consumption and a reduced seed dispersal role (Sengupta et al., 2022). Sambar and macaques are common visitors to tourist-occupied areas within the park where they scavenge food, which drastically reduces their ranges and consequently their dispersal capacity (Domínguez et al., 2015). A major missing interactor in the network is the Sumatran rhino, which was likely to have occurred in Khao Yai's forests in historical times and was an important seed disperser (McConkey et al., 2022). In the mid-1970s, Sumatran rhinos were recorded close to Khao Yai's forests (McNeely & Laurie, 1977), but this rhino now has a highly restricted and critically low population on Sumatra, and possibly Borneo (IUCN, 2021).
What might the seed dispersal network look like if the important threatened species declined, or became locally extinct? We did not test network fragility (or how the network structure might change) by removing threatened species; this procedure does not allow for the formation of new interactions (network rewiring) as ‘gaps’ in the network develop and therefore, might not provide an accurate picture of the impacts of local extinctions (Moran-Lopez et al., 2020). However, we suggest that network rewiring could be limited for several reasons. Threatened species were among the most generalist in the community, with broad fruit diets that encompassed a range of fruit sizes (and potentially other traits), leaving far fewer generalist species to fulfil the seed dispersal roles. In particular, the mammal module M1 contained many threatened, generalist animal species and also a large number of plant species that are probably disproportionately dispersed by these animals. A community without these medium-sized and larger-bodied generalist consumers could have significant repercussions for plant communities in this tropical Asian rainforest.
6 CONCLUSION
In this Indo-Burma biodiversity hot spot, we show that threatened species were disproportionately important in the seed dispersal network. These species were among the most generalist and well-connected, suggesting a fragility to seed dispersal interactions at the community level if populations of these species decline. Nevertheless, our results highlight important roles for threatened primates, sambar and binturong because of their broad diets, and for hornbills and bears because the modules that contained them had few animal species and many plant species. We also identified an important role of medium-sized and larger animals in community-level seed dispersal interactions. However, important roles were also performed by small frugivores (bulbuls), while the largest disperser (elephants) played a minor role, emphasizing the importance of understanding community-level seed dispersal across a broad range of regions. Overall, the structure of the seed dispersal network in this Indo-Burma hotspot is dependent on the maintenance of populations of several currently endangered and vulnerable species.
AUTHOR CONTRIBUTIONS
Aurélie Albert-Daviaud: Conceptualization; data curation; formal analysis; investigation; methodology; visualization; writing – original draft; writing – review & editing. Kim R. McConkey: Conceptualization; data curation; investigation; methodology; visualization; writing – original draft; writing – review & editing. Nidhi Jha: Data curation; formal analysis; visualization. Colin Fontaine: Supervision; writing – review & editing. Shumpei Kitamura: Investigation; writing – review & editing. Anuttara Nathalang: Investigation; writing – review & editing. Chution Savini: Investigation; writing – review & editing. Tommaso Savini: Investigation; writing – review & editing. Pierre-Michel Forget: Resources; supervision; writing – review & editing.
ACKNOWLEDGEMENTS
We thank Dusit Ngoprasert, Daphawan Khamcha, Wangworn Sankamethawee and Umaporn Matmoon for providing part of the data, Enrico Rezende for providing the ‘phylo.signal.disc’ function and Elisa Thebault for providing a personalized version of ‘Bipmod’. Aurélie Albert-Daviaud, Colin Fontaine, and Pierre-Michel Forget were funded by LabEx BCDiv.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
No ethics approval was required for the research reported in this article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the supplementary files associated with the article.




