Identification of Priority Areas for Conservation of Threatened Agamid Lizards of Sri Lanka Using Species Distribution Modeling
斯里兰卡濒危蜥蜴 (鬣蜥科) 保护优先区域的识别:基于物种分布模型的研究
Editor-in-Chief & Handling Editor: Ahimsa Campos-Arceiz
ABSTRACT
enTo ensure the survival of threatened species, identifying biodiversity hotspots is essential for determining where and how conservation should be prioritized. Sri Lanka along with Western Ghats of India is a global biodiversity hotspot, with reptiles among its most threatened taxa. Agamid lizards in Sri Lanka are both threatened and evolutionarily distinct, with 19 of 22 species endemic to the island. We assessed the distribution of 14 threatened agamid lizard species using Species Distribution Modeling (SDM). The generated distribution maps were used to identify highly diverse target areas for agamid conservation, both within and outside protected areas. We assigned conservation priority ranks for species using different criteria such as IUCN status, the number of occurrence records, and the size of the estimated range. Our results indicated that agamid hotspots are primarily concentrated in the wet zone of Sri Lanka; specifically, Sinharaja Forest Reserve, the Peak Wilderness Sanctuary, and the Knuckles Mountain Range emerged as high priority conservation targets. Although all species targeted in our SDM had some portion of their range within protected areas, over 40% of high-priority agamid habitats remain unprotected. We advocate for the expanded use of SDM for hotspot identification and conservation planning, particularly for threatened and endangered species with poorly documented population status and geographic distribution.
摘要
zh为确保濒危物种的生存, 生物多样性热点区域的识别对确定保护优先区域及实施策略至关重要。作为全球生物多样性热点地区, 斯里兰卡的爬行动物类群面临严重生存威胁, 其中鬣蜥科 (Agamid lizards) 物种兼具高度濒危性和进化独特性——该地区22种鬣蜥中有19种为岛屿特有种。本研究通过物种分布模型 (Species Distribution Modeling, SDM) 评估了14种濒危鬣蜥的地理分布特征, 并基于生成的地理分布图识别了保护区内外的鬣蜥多样性热点区域。我们综合国际自然保护联盟 (IUCN) 濒危等级、物种出现记录数量及预测分布范围等指标构建了物种保护优先级评价体系。研究结果表明, 鬣蜥多样性热点主要集中于斯里兰卡湿润地带, 其中辛哈拉加森林保护区 (Sinharaja Forest Reserve) 、峰顶荒野保护区 (Peak Wilderness Sanctuary) 和纳克尔斯山脉 (Knuckles Range) 被确定为高优先级保护目标区。尽管所有目标物种的分布区均部分覆盖现有保护区, 但超过40%的高优先级鬣蜥栖息地仍处于无保护状态。本研究倡导推广物种分布模型在热点识别和保护规划中的应用, 尤其适用于地理分布和种群现状数据匮乏的濒危物种。
简明语言摘要
zh斯里兰卡特有的鬣蜥科物种多数仅分布于该热带岛国, 正面临栖息地丧失与气候变化的双重威胁。为保护这些濒危爬行动物, 我们采用机器学习方法——物种分布模型 (Species Distribution Modeling, SDM), 通过栖息地适宜性评估绘制其潜在分布区并确定优先保护区域。研究选取14个受威胁物种的已知分布点数据, 结合多源生物气候环境因子及森林覆盖度, 预测了这些物种在斯里兰卡的栖息地适宜空间格局。结果显示:尽管所有物种在辛哈拉加森林保护区、中央高地及纳克尔斯山脉等现有保护区内均有分布, 但超过40%的最关键栖息地 (特别是中央高地与纳克尔斯山脉的高海拔森林中狭域物种的栖息地) 仍处于无保护状态。研究同时发现, 若实施可持续管理, 农林业系统等人工改造景观亦可成为保护体系的补充。该工作通过揭示斯里兰卡保护区网络的空缺, 为国家保护地扩展提供了科学依据。其中采用的建模方法尤其适用于全球范围内数据匮乏物种的保护规划, 可确保有限资源精准投入最紧迫区域。
Summary
enSri Lanka's unique agamid lizards, many restricted to this tropical island nation, face threats from habitat loss and climate change. To protect these endangered reptiles, we implemented a machine-learning approach, species distribution modeling to map their habitat suitability and identify priority conservation areas. Focusing on 14 threatened species and their known species-presence information, we combined numerous bio-climatic environmental predictors and forest cover to predict their spatial distribution in Sri Lanka based on habitat suitability. Results revealed that while all species have some habitat in protected zones like Sinharaja Forest Reserve, the Central Highlands and the Knuckles Range, over 40% of their most critical habitats remain unprotected. These unprotected areas, often in high-elevation forests of the Central Highlands and Knuckles Mountain Range, are vital for species with tiny ranges. The research also highlighted that some human-modified landscapes, like agroforests, could support conservation if managed sustainably. By pinpointing gaps in Sri Lanka's current network of protected areas, this study provides a roadmap for expanding the country's conservation network. The methods used here, particularly the modeling approach, could help protect other poorly studied species worldwide, ensuring limited resources target the most urgent needs.
-
Practitioner Points
- ∘
Species distribution modeling (SDM) is a powerful tool for identifying conservation gaps/hotpots, including unprotected critical habitats, even for species with limited data, guiding targeted conservation actions.
- ∘
Prioritize wet and montane zones: Key conservation lands like Sinharaja Forest, the Knuckles Range, and Central Highlands require expanded and enhanced protection and habitat connectivity to safeguard Ceylonese endemic agamids.
- ∘
Integrate human-modified landscapes: Secondary forests and agroforests can complement protected areas if restored and managed to support biodiversity, offering a buffer against habitat loss.
- ∘
实践者要点
zh
-
物种分布模型 (SDM) 作为高效工具, 能识别保护空缺/热点 (包括未受保护的关键栖息地), 即使对数据稀缺物种仍可指导针对性保护行动
-
优先保护湿润区与山地生态系统:辛哈拉加森林、纳克尔斯山脉及中央高地等关键区域需扩大保护范围并增强栖息地连通性, 以保障斯里兰卡特有鬣蜥的生存
-
整合人工改造景观:通过生态修复与可持续管理, 次生林与农林复合系统可作为保护区的有效补充, 缓解栖息地破碎化压力
1 Introduction
Accelerated rates of habitat deterioration and natural-resource exploitation continue to imperil biodiversity worldwide (Hanski 2011; Lovejoy 2006). Systematic conservation planning plays an important role in addressing the biodiversity crisis to protect ecosystem integrity and functions (Margules and Pressey 2000), as well as endemic and threatened species (Myers et al. 2000). Establishing Protected Areas (hereafter PAs) that sufficiently represent variable levels of biological organization and spatial extents has become a critical goal for conservation policies (Lovejoy 2006; Margules and Pressey 2000; Pearson 2016). Establishment and maintenance of PAs remain the most widespread and historic countermeasures against biodiversity erosion (Gray et al. 2016). PAs safeguard against the ill-effects of demographic and genetic stochasticity and support the ecological infrastructure necessary for long-term population viability despite anthropogenic stressors (Pressey et al. 2007; Rodrigues et al. 2004). Despite the increase in global PA cover within the past decade, only 17% of the planet surface is currently protected (UNEP-WCMC and IUCN 2020). Filling these gaps via systematic conservation planning requires spatially explicit data on species distribution, but scientific knowledge of geographic patterns of biodiversity remains incomplete (Lovejoy 2006). Both information paucity and conservation gaps are particularly notable in the tropical realm, especially with respect to less charismatic, nongame vertebrates, such as reptiles (Rodrigues et al. 2004).
Sri Lanka, a tropical island in Indian ocean, exemplifies such conservation and information gaps, as PAs in the country are primarily designed for charismatic megafauna and economically valuable ecosystems (Miththapala 2016). Sri Lanka is recognized as one of richest and herpetologically most diverse countries of the world with a high number of threatened species (Böhm et al. 2013), and out of 247 reptile species, 67.2% are endemics (Gibson et al. 2020). Therefore, it is worthwhile examining whether its PAs protect reptile diversity, as well as that of other taxa. In particular, we selected agamid lizards as focal species, for several reasons. First, agamid species are ecologically sensitive and evolutionarily distinct, with 19 of 22 species being endemic to Sri Lanka. They are distributed throughout the island, yet accurate data on their populations and distributions remain limited. Second, a high proportion (72.7%) of Sri Lankan agamid lizards are classified as threatened on the IUCN Red List (MOE 2012). Third, agamids are often used as biodiversity indicators (Ishwar et al. 2003; Megía-Palma et al. 2020). Similarly to other taxa, agamid lizards are threatened by land-use changes and other anthropogenic disturbances (Miththapala 2016), such as habitat loss from agricultural conversion, forest degradation and over-exploitation (Böhm et al. 2013). Overall, identifying conservation targets for reptile diversity and endemism in Sri Lanka may help implement proactive policies to halt habitat modification and curb biological homogenization.
Assessment of species distribution ranges is crucial for identifying conservation targets, which typically requires spatially and temporally extensive field surveys (Pearce and Ferrier 2001). Species Distribution Modeling (hereafter SDM) is useful in mapping the potential geographic range of species, especially in regions where comprehensive field surveys are lacking (Hirzel and Le Lay 2008; Peterson 2001). By analyzing known species occurrence data alongside environmental variables, SDMs can predict species distributions across geographic space and time. Multiple approaches to SDMs exist, including generalized regressions, classification trees, discriminant functions, and machine-learning algorithms (Hirzel and Le Lay 2008; Peterson 2001). In recent years, SDM applications have been commonly used by conservation professionals and have been featured prominently in the scientific literature (Feng et al. 2019). In applications of conservation biology, SDM is a valuable tool and has been applied to a wide range of taxa, from herbaceous plants (Abdelaal et al. 2019) to numerous vertebrate species such as mammals (Razgour et al. 2011), amphibians and reptiles (Pawar et al. 2007). The maps generated by SDMs delineate geographic areas where species are most likely to occur, that is, their potential distributions. When these spatial predictions are compared with maps of existing PAs, we can identify “conservation gaps,” regions of high-priority conservation areas that currently lack formal protection (Ahmadi et al. 2023; Margules and Pressey 2000). Recognizing these gaps is essential for guiding reserve design and establishing conservation priorities. The explicit development and application of SDMs in conservation planning is increasingly recognized as best practice, particularly when detailed occurrence data are limited (Feng et al. 2019; Johnson and Gillingham 2005).
In this study, with the overarching goal of guiding conservation planning for agamid lizards in Sri Lanka using an SDM-based approach, our specific objectives were to (1) estimate the potential distribution ranges of selected agamid lizard species, (2) identify conservation targets based on SDM-generated species ranges, and (3) highlight conservation gaps outside of the existing PAs. We believe that our study will provide insights for future conservation planning in Sri Lanka for agamid lizards and assist conservation authorities to prioritize high-value targets and to efficiently use limited financial and logistic resources.
2 Materials and Methods
2.1 Focal Species
Agamid lizards (Family Agamidae, dragon lizards) in Sri Lanka are both ecologically unique and evolutionarily distinct (Karunarathna et al. 2020; Schulte et al. 2002). Their exceptional degree of endemism, as well as their restricted geographical ranges (Karunarathna et al. 2020), combine to give Sri Lankan agamids a high conservation value, both nationally and globally. Recently, the project “Assess to Plan: Conservation Action Planning for the Snakes and Lizards of Sri Lanka” (Gibson et al. 2020) identified four critically endangered, nine endangered, and two vulnerable agamid species.). However, we excluded Sitana ponticeriana because this species is no longer considered a threatened species according to the latest categorization by the IUCN (www.iucn.org) and we didn't include recently described data deficient species Ceratophora ukuwelai. Hence, we selected 14 agamid species for this study (Table 1).
| Species | Conservation status | Range | Qualitative estimate of abundance |
|---|---|---|---|
| Calotes desilvai Bahir & Maduwage, 2005 | CR | Rakwana Hills | Very Rare |
| Calotes liocephalus Günther, 1872 | EN | Peak Wilderness | Rare |
| Calotes manamendrai Amarasinghe & Karunarathna, 2014 | EN | Knuckles Range | Rare |
| Calotes nigrilabris Peters, 1860 | EN | Horton Plains and Nuwara Eliya areas of Central Highlands | Common |
| Calotes pethiyagodai Amarasinghe, Karunarathna, Hallermann, Fujinuma, Grillitsch & Campbell, 2014 | EN | Knuckles Range | Rare |
| Ceratophora aspera Günther, 1864 | EN | Southwestern wet zone | Uncommon |
| Ceratophora erdeleni Pethiyagoda & Manamendra-Arachchi, 1998 | CR | Rakwana Hills | Rare |
| Ceratophora karu Pethiyagoda & Manamendra-Arachchi, 1998 | CR | Rakwana Hills | Rare |
| Ceratophora stoddartii Gray, 1835 | EN | Central Highlands | Uncommon |
| Ceratophora tennentii Günther & Gray, 1861 | CR | Knuckles Range | Uncommon |
| Cophotis ceylanica Peters, 1861 | EN | Central Highlands | Rare |
| Cophotis dumbara Samarawickrama, Ranawana, Rajapaksha, Ananjeva, Orlov & Ranasinghe, 2006 | CR | Knuckles Range | Rare |
| Lyriocephalus scutatus Linnaeus, 1758 | VU | Wet zone and some inselberg mountains | Uncommon |
| Sitana devakai Amarasinghe, Ineich & Karunarathna, 2015 | VU | North west coastal areas | Common |
- Note: The column “Range” identifies the largest single area specifically occupied by each species. The qualitative estimate of abundance is based on Bahir and Surasinghe (2005), which describes the abundance of this species in sites where it is present.
- Abbreviations: CR, critically endangered; EN, endangered; NT, near threatened; VU, vulnerable.
2.2 MaxEnt-Based Distribution Modeling Approach
The prediction of a species' potential distribution based on georeferenced presence-only records is possible through MaxEnt, a spatially explicit, machine-learning algorithm based on maximum entropy theory (Phillips and Schapire 2004). To estimate the probability distribution of a species across a certain geographical area, MaxEnt produces the least biased solution, that is, the one that maximizes its entropy using information on confirmed associations between the species' georeferenced points and abiotic and biotic geospatial variables (e.g., elevation, climate, hydrological features, and land-use or tree cover data (Elith et al. 2006; Pawar et al. 2007). The resulting SDM is a probability distribution prediction over a geographical area of interest, which generates a scaled potential habitat map, wherein each mapped pixel carries a potential value generated as a function of the predictor variables. Higher values correspond to greater predicted potential distribution for the species of interest. Maxent is robust when modeling with presence-only occurrence data, even for small sample sizes, and has outperformed many alternative conventional techniques (Elith et al. 2006; Pearson et al. 2007) in delivering stable, reliable, and high-accuracy predictions across a range of sample sizes (Wisz et al. 2008). MaxEnt has been adopted across a diverse range of ecological, evolutionary, conservation and biosecurity applications (e.g., to determine environmental drivers of species presence, map current and future distributions, and design protected-area networks).
2.3 Data Sources: Species Distribution Records and Environmental Predictors
To incorporate the environmental heterogeneity of Sri Lanka, and to accommodate a multi-species perspective, we included the entire country as the study region. We obtained georeferenced species occurrences from both peer-reviewed publications and unpublished field observations by field biologists (Table S1) which were collected using random (Greig-Smith 1964) and adaptive sampling (Krebs 1989) methods. To reduce spatial autocorrelation, we identified clusters of occurrence points within the same 1 km2 pixel, the smallest spatial unit in our geospatial environmental space. From each cluster containing multiple points, we randomly selected one occurrence record and removed the rest. This process minimized the influence of spatially autocorrelated data points on model predictions (Tobeña et al. 2016). To apply this method, occurrence points for the selected agamid species were spatially rarefied using SDMtoolbox 2.0 (Brown et al. 2017).
2.4 Species Distribution Modeling
Initially, we used all 19 bioclimatic variables (BIO 01–BIO 19) as climatic predictors of agamid distribution, encompassing annual, monthly, and quarterly climatic trends (http://www.worldclim.org/). These variables include central tendencies (e.g., mean annual temperature), seasonal variations (annual range in temperature and precipitation), and extreme measurements (e.g., mean temperature of coldest quarter), and hence are biologically meaningful proxies of species’ potential distributions (Fick and Hijmans 2017; Hijmans et al. 2005). These bioclimatic predictors are spatially explicit (spatial resolution: 1 km) and derived from monthly rainfall and temperature records for the period of 30 years (1970–2000; Fick and Hijmans 2017; Hijmans et al. 2005). We excluded BIO 8, 9, 18, and 19, as their use in SDMs is discouraged due to spatial artifacts (Booth 2022).
In addition to the bioclimatic variables, we included tree density (Crowther et al. 2015) as a proxy for habitat structure and forest cover (spatial resolution: 1 km). Further, an elevation layer (Digital Elevation Model) was added, which was produced by the Shuttle Radar Topography mission (Farr et al. 2007). For land use, we used current layers at 1 km2 resolution (digitized from 30-m spatial resolution map; https://earthexplorer.usgs.gov/), with 31 land-use types, including natural and anthropogenic habitats (Table S1). To avoid over prediction in the modeling process due to collinearity among predictors, we calculated Pearson correlation tests and removed variables that were highly correlated with others (|r| ≥ 0.7), retaining those variables that have been used in previous SDM studies conducted in Sri Lanka (Wijerathne et al. 2025). The final SDM contained BIO 2, BIO 3, BIO 5, BIO 12, BIO 14, BIO 15, elevation, land use and tree density layer.
Considering the rarity and low coverage of some of the species, we combined several methods for data processing suggested in Jeliazkov et al. (2022). Specifically, we used the SDM + pseudo absence (Barbet-Massin et al. 2012) and bias-corrected SDM approaches (Fourcade et al. 2014). Since our study area is an island, we adopted an unlimited dispersal scenario, which assumes there are no significant dispersal barriers, although we recognize this assumption may not hold true for some species that cannot navigate across valleys between mountain ranges.
Regarding the specifics of the SDM, background points and occurrence data can exhibit geographical bias (Gafna et al. 2023). Multiple methods have been employed in different studies to mitigate this bias (Acevedo et al. 2012; Valavi et al. 2022). We adopted a method similar to that used by Fourcade et al. (2014), generating restricted background points as pseudo absences for all the species to minimize the bias of presence-only data. Initially, we evaluated nine different approaches using a grid graph (Whitford et al. 2024; Supporting Information S1: Figure S1) and assessed performance based on Area Under the Curve (AUC) and Boyce's index, which compares predicted potential distribution with actual occurrences (Hirzel et al. 2006). This evaluation indicated that using 1000 background points with unlimited buffer distance was the optimal approach for SDMs of these species.
We used R package “ENMeval,” specifically designed for optimizing the MaxEnt approach to SDMs (Kass et al. 2021; Muscarella et al. 2014). Various combinations of feature classes were explored including linear, linear-quadratic, hinge, and linear-quadratic-hinge models (Merow et al. 2013; Radosavljevic and Anderson 2014). Regularization multipliers were applied in increments of 0.5, ranging from 0.5 to 4, to control model complexity. To determine the optimal model for each species, we utilized a spatial block cross-validation approach (Roberts et al. 2017). This method involved dividing the data into four blocks, using three for training and one for testing, as outlined by Hirzel et al. (2006) and Valavi et al. (2022). Initially, the model with the lowest average 10.0% omission rate was selected. If multiple models displayed the same omission rate, the one with the highest average validated AUC value was chosen instead (Zhang et al. 2021). Permutation importance (PI) values for all the predictor variables were obtained to evaluate their influence for the model (Schnase et al. 2021). Subsequently, the consequent drop in area under the receiver operating characteristics curve (AUC) was measured (> 0.8 were considered “good”; Fielding and Bell 1997) where a greater drop in AUC signifies greater biological importance of the randomized predictor to the overall model performance (Phillips and Schapire 2004). We used Boyce Index together with the AUC to measure the model performance of the SDMs (Table S2). Using QGIS version 3.24.1 (QGIS.org, 2024), we reclassified the distribution maps into a binary raster maps depicting either presence (1) or absence (0) of the focal species using the maximum training sensitivity and specificity cumulative threshold values obtained through the model, as a standard approach to selecting thresholds of occurrence in the prediction of species (Table S2, Liu et al. 2005).
2.5 Conservation Targets and Gaps
Ranking species based on their conservation priority should account for numerous parameters such as rarity, population trends and density, their current range, and threats. To incorporate these factors, we developed a conservation-priority ranking system, using three main criteria (Table S3): the conservation status in the IUCN Red List 2019 (Gibson et al. 2020; Karunarathna et al. 2020), the number of georeferenced points in the data set, and the total area of the MaxEnt-predicted distribution range. The ranking system ranges between 1 and 5, with 5 representing the highest conservation priority. To determine the final conservation priority for each agamid species, we summed across the three criteria and then reclassified the summed rank into the same 1–5 scale (Table S3).
We then ranked regions of Sri Lanka in their conservation importance. We first excluded fragments < 5 km2 from SDMs since such small habitats are unlikely to sustain minimum viable populations, and thus are inadequate for conservation. Then we superimposed the SDM maps for all species in ArcMap. Geographic areas where the distribution ranges of > 3 species overlapped were considered “conservation targets.” As these conservation targets included protected as well as unprotected landscapes, we superimposed conservation targets with the current PAs of Sri Lanka, to separate areas receiving statutory protection from those without such protection (“conservation gaps”).
Next we ranked PAs in their conservation importance and identified areas for conservation outside of PAs. Based on agamid species present at each PA, we summed species-specific conservation-priority ranks (Table 2) to generate PA-specific conservation priority rankings (Table S4: e.g., a PA with three threatened species each of rank 5, was valued at 15), where PAs with the greatest concentrations of threatened agamid species are valued the highest. Likewise, conservation gaps were defined as areas outside of PAs in which the distribution ranges of at least three agamid species overlapped. Next, by superimposing the SDM results with a land-use layer of Sri Lanka (Land use/land cover image of Sri Lanka using Landsat-7 ETM+C2L1 images acquired on 2017 with 30 m spatial resolution, https://earthexplorer.usgs.gov/), we identified areas outside of PAs with land-cover types suitable for agamid conservation. Referring to published chronicles of agamid autecology (Karunarathna and Amarasinghe 2011; Karunarathna and Amarasinghe 2012; Pethiyagoda and Manamendra-Arachchi 2012), we determined the conservation potential of land-cover types, as is shown in Table S5.
| Species | Code | Red list status | Rank | Occurrence points | Rank | Area of potential distribution (km2) | Rank | Total | Overall rank |
|---|---|---|---|---|---|---|---|---|---|
| Calotes desilvai | cades | CR | 3 | 12 | 5 | 63.6 | 5 | 13 | 5 |
| Calotes liocephalus | calioc | EN | 2 | 15 | 5 | 210.4 | 5 | 12 | 4 |
| Calotes manamendrai | caman | EN | 2 | 17 | 4 | 640.3 | 4 | 10 | 3 |
| Calotes nigrilabris | canig | EN | 2 | 35 | 3 | 457.2 | 5 | 10 | 3 |
| Calotes pethiyagodai | capet | EN | 2 | 21 | 4 | 558.5 | 5 | 11 | 4 |
| Ceratophora aspera | ceasp | EN | 2 | 40 | 2 | 3372.0 | 3 | 7 | 2 |
| Ceratophora erdeleni | ceerd | CR | 3 | 12 | 5 | 38.2 | 5 | 13 | 5 |
| Ceratophora karu | cekar | CR | 3 | 12 | 5 | 37.2 | 5 | 13 | 5 |
| Ceratophora stoddartii | cesto | EN | 2 | 41 | 2 | 2454.7 | 4 | 8 | 2 |
| Ceratophora tennentii | ceten | CR | 2 | 17 | 4 | 307.2 | 5 | 11 | 4 |
| Cophotis ceylanica | cocey | EN | 2 | 17 | 4 | 415.4 | 5 | 11 | 4 |
| Cophotis dumbara | codum | CR | 2 | 16 | 5 | 302.2 | 5 | 12 | 4 |
| Lyriocephalus scutatus | lyscu | VU | 1 | 84 | 1 | 9965.7 | 2 | 4 | 1 |
| Sitana devakai | sidev | VU | 1 | 23 | 4 | 11710.9 | 1 | 6 | 1 |
- Note: Both criterion-specific and final ranks (summed ranks from all criteria) for conservation prioritization ranged between 1 and 5, indicating lowest and highest priorities for conservation.
3 Results
3.1 Importance of Predictor Variables
The average AUC values for all SDMs were over 95% (0.95 ± 0.03), and Boyce Index (0.72 ± 0.21) values indicated satisfactory model performance for all selected agamid species. Among all the predictors, BIO 5 (Maximum temperature of the warmest month) and Elevation had the most influence on the resulting SDMs (Supporting Information S1: Figure S2).
3.2 Predicted Distribution Ranges of Selected Species and Their Conservation-Priority Ranks
According to SDM maps we generated (Figure 1, Table 2), Sitana devakai showed the broadest distribution range (117,10.90 km2), followed by Lyriocephalus scutatus (9965.76 km2), while Ceratophora karu (37.21 km2) and Ceratophora erdeleni (38.2 km2) had the most restricted ranges, exclusively limited to the Rakwana Hills. In addition, Calotes desilvai (63.60 km2) was among the species with the smallest ranges, also restricted to the Rakwana Hills. In contrast, the predicted distribution of Lyriocephalus scutatus covered lowlands as well as the three montane areas of southwestern Sri Lanka. For all the species collectively, the wet zone encompassed 45.0% of the predicted distribution ranges, the intermediate zone covered 17.1%, and the remaining portion of the distribution range was in the dry zone (Supporting Information S1: Figure S3). However, these figures overemphasize the importance of the dry-zone, as only Sitana devakai was widely distributed there. All other species had their primary distribution range within the wet and montane zones (see Figure 1).
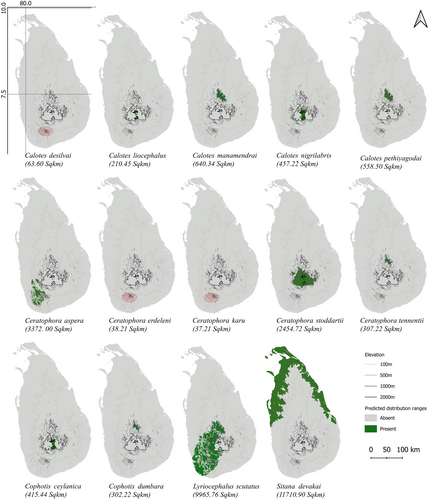
Three species were given the highest priority conservation rank (5; Table 2), with all these species showing the most restricted predicted distribution ranges (< 65 km2) and being classified as CR, according to the IUCN reptile assessment of 2019 (Gibson et al. 2020). Likewise, five species were ranked as high conservation priorities (rank 4). In contrast, several species were ranked as low conservation priorities (two species at rank 1, and two further species at rank 2).
3.3 Conservation Targets and Gaps
3.3.1 Conservation Targets Based on Number of Species Present
We identified three geographically distinct clusters as conservation targets in Sri Lanka (Figure 2). These include the Central Highlands, the Rakwana Hills, and the Knuckles Range. Nested in the southwestern wet zone, and characterized by moist, evergreen montane forest ecosystems, the Rakwana Hills showed both high overall agamid species richness and endemism. The predicted distribution of Ceratophora karu, Ceratophora erdeleni, and Calotes desilvai were restricted to the Rakwana Hills, at elevations > 900 m above mean sea level. In addition, the predicted distribution of Ceratophora aspera included the Rakwana Hills, while also including parts of the lowland (< 900 m) wet zone. The Knuckles Range, a humid montane ecosystem dominated by evergreen rainforests with high vegetation heterogeneity, included the predicted distribution ranges for five threatened agamid species (Calotes manamendrai, Calotes pethiyagodai, Ceratophora tennenti, Cophotis dumbara and Lyriocephalus scutatus). Finally, the distribution of five agamid species included the Central Highlands (> 900 m), with four species (Ceratophora stoddartii, Calotes nigrilabris, Cophotis ceylanica and Calotes liocephalus) restricted to that mountain range (Table 3).
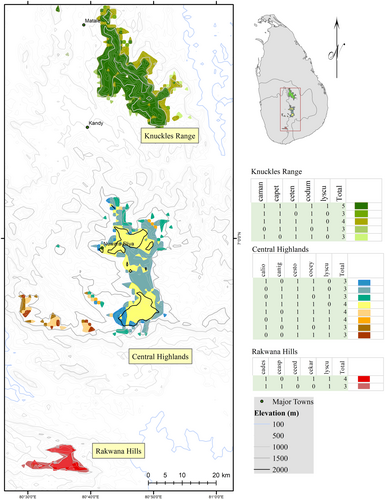
| Region | Total no. of species present | Species confined to mountain range | Maximum species overlapping in one place | Total high-priority conservation area (km2) |
|---|---|---|---|---|
| Knuckles Range | 5 | Calotes manamendrai | 5 | 387.22 |
| Calotes pethiyagodai | ||||
| Ceratophora tennentii | ||||
| Cophotis dumbara | ||||
| Lyriocephalus scutatus | ||||
| Central Highlands | 5 | Calotes liocephalus | 5 | 418.99 |
| Calotes nigrilabris | ||||
| Ceratophora stoddartii | ||||
| Cophotis ceylanica | ||||
| Lyriocephalus scutatus | ||||
| Rakwana Hills | 5 | Calotes desilvai | 4 | 63.34 |
| Ceratophora erdeleni | ||||
| Ceratophora aspera | ||||
| Ceratophora karu | ||||
| Lyriocephalus scutatus |
- Note: The total number of species present within the three regions are shown, although all the species are not evenly distributed throughout the regions.
3.3.2 Conservation Targets Inside PAs, and Their Prioritization
Conservation targets, defined as regions with high species richness of agamids (> 3 species), were restricted to the higher elevations (> 900 m) of the Central Highlands and other mountainous regions of southwestern Sri Lanka (Figure 2). A substantial proportion (53%) of these targets is already within a designated PA (Figure 3). PAs covered 461.33 km2 of these target distribution range of threatened agamids, without Sitana devakai (this species was not included in the conservation target analysis because of its discontinuous distribution). Many protected areas > 900 m elevation also were highly ranked (with a score of more than 10) as conservation priorities (Table S4). Among all PAs we analyzed, Sinharaja Forest Reserve in the Rakwana Hills (> 900 m elevation range of eastern Sinharaja) gained the highest total priority rank (18), as it encompassed the distribution of five threatened agamid species (Table S4). Numerous PAs in the Central Highlands, particularly those located at the higher elevations (> 1500 m, e.g., Peak Wilderness, Samanala Adawiya, Piduruthalagala, Kandapola, Hakgala, and Horton Plains) also received high ranks (Table S3). With a richness of five threatened agamid species, the Knuckles Range also ranked highly among the PAs, receiving a total score of 16.
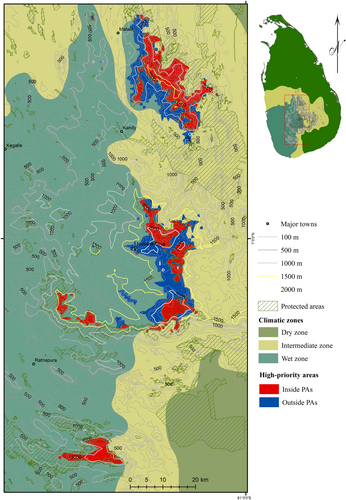
3.3.3 Conservation Gaps Outside PAs
Located within the Central Highlands and Knuckles Range, conservation targets that amount to an area of 403.32 km2 lie outside the PAs (Figure 3 and Supporting Information S1: Figure S4). In contrast, all conservation targets found in the Rakwana Hills were found within PAs, and no more than two of the selected agamid species were found in unprotected conservation targets there. Aside from the areas west of the Knuckles Range and a few habitat patches along the Central Highlands, the remaining unprotected conservation targets are located above 1200 m elevation (Supporting Information S1: Figure S4). Much of the Central Highlands, as well as the eastern slopes of the Knuckles Range, offer sufficiently large, contiguous, high-priority habitats for agamid conservation, though these areas are currently unprotected. These unprotected conservation targets include a small portion (16.07 km2) of highly fragmented habitats (< 5 km2) attached to PAs. However, much (387.25 km2) of these unprotected high-priority habitat patches are sufficiently large in spatial extent (> 5 km2) and are found along the PA boundaries (Supporting Information S1: Figure S5).
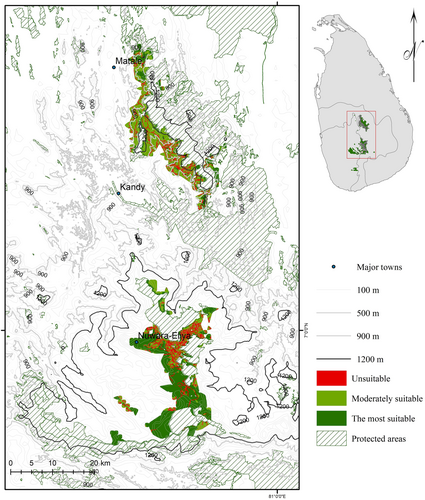
3.3.4 Conservation Targets After Land-Cover Analysis
After conducting the land-use analysis (Figure 4), nearly 172.61 km2 of conservation target areas outside of PAs were identified as suitable for threatened agamid conservation, being composed of isolated patches of forests, scrublands and agroforests with high agamid diversity (Table S5). This corresponds to nearly 42.8% of the total acreage of conservation targets outside the PAs. In contrast, 230.71 km2 of the conservation targets outside of PAs corresponded to habitat areas moderately or highly unsuitable for the focal species.
4 Discussion
4.1 Distribution-Range Predictions and Species-Level Conservation Prioritization
Our study marks a cornerstone in Sri Lanka's systematic conservation planning where we produced SDMs for multiple species of conservation interest to estimate their current distribution, thereby identifying conservation target areas, including conservation gaps. We identified that nearly ~45% of agamid conservation targets remain unprotected, requiring immediate conservation action. These findings align with global-scale conservation gap analyses for reptiles, which indicate that a high proportion of conservation priority areas remains unprotected (Cox et al. 2022; Rodrigues et al. 2004a; Rodrigues 2004b).
Overall, our predicted distribution ranges exceeded historical estimates from previous publications for both widely distributed and range restricted species. For example, historical range sizes for Lyriocephalus scutatus and Ceratophora aspera were estimated at 700 km2 and 800 km2, respectively (Bahir and Surasinghe 2005), while our analyses suggest ranges of 3300 km2 and 9000 km2. Similarly, historical estimates for range-restricted species such as Calotes desilvai, Ceratophora karu, and Ceratophora erdeleni were < 10 km2, whereas our models predict ranges of 30–60 km2. Recent studies (Gibson et al. 2020) suggest that, contrary to historical records and natural history accounts (Deraniyagala 1955), geographical relicts such as Lyriocephalus scutatus and Ceratophora aspera may have had healthy, stable, widely distributed populations across the mid-elevations and lowlands of the wet zone. Our predicted distributions are also corroborated by recent surveys, in which these threatened agamid species were found in isolated secondary forest fragments, forest plantations, and home gardens (Karunarathna and Amarasinghe 2011). Structural complexity of vegetation, cool-moist microclimatic features, and food availability have been identified as the critical determinants for the occupancy of range-restricted agamids (Jayasekara et al. 2018; Somaweera et al. 2015). Novel ecosystems such as secondary and degraded forests and abandoned monocrop plantations that characterize Sri Lanka's wet zone are utilized by threatened and endemic fauna for at least part of their life cycle (Pethiyagoda 2012). Our study adds credence to the importance of these novel ecosystems as shelters for native biodiversity, although not as replacements for primary forests.
The species that we found to be conservation priorities have also been highlighted as conservation targets in previous, location-specific studies (Jayasekara et al. 2018; Somaweera et al. 2015), as well as in island-wide conservation assessments (Bahir and Surasinghe 2005). Moreover, the agamid species we ranked for high-priority conservation also have phylogenetic and evolutionary uniqueness. For instance, the endemic, horned-lizard genus Ceratophora reveals multiple, independent evolutionary events involving their rostral appendage (Johnston et al. 2013; Schulte et al. 2002). Likewise, the endemic Calotes have undergone insular radiation (Macey et al. 2000).
4.2 Prioritization of Conservation Target Areas
We identified forests in both wet and intermediate zones as critical targets for protecting threatened agamid species. These tropical rain forests are critical for conservation of endemic reptiles (Erdelen 1988), amphibians (Surasinghe 2009), freshwater fish (Pethiyagoda 1994), woody plants (Gunatilleke and Ashton 1987) and the avifauna of Sri Lanka (Abeyarama and Seneviratne 2017). Among these, the montane forests of the Central Highlands, the Knuckles Range, and the Rakwana Hills are particularly notable for their biodiversity (Gunatilleke, Pethiyagoda, et al. 2017). The elevation gradient characteristic of these regions begets a climatic gradient, which then shapes differential vegetation layering of the forest. The resulting environmental heterogeneity is largely responsible for the high overall faunal diversity characteristic to these mountainous regions. Ecological distinctiveness of these regions has been used to recognize “key biodiversity areas” at the global scale, based on taxonomic uniqueness, exceptional biodiversity and endemism, evolutionary and phylogenetic phenomena, and rarity of habitat types (Olson and Dinerstein 1998; Eken et al. 2004). These recognitions highlight the vulnerability and irreplaceability of the conservation targets identified in our study, both of which are fundamental criteria in systematic conservation planning (Margules and Pressey 2000). Thus, the conservation targets we identified for agamids may provide multiple benefits, encompassing a range of taxa, safeguarding globally and regionally rare ecoregions, and preserving unique biogeographic histories and evolutionary processes.
Beyond their high endemism, the conservation targets we identified face numerous anthropogenic threats. Except for small tracts in inaccessible terrains (e.g., high elevations, steep slopes), both lowland and montane rainforests have historically been cleared for commercial-scale export agriculture and selectively logged for plywood and hardwood timber for decades (Perera 2001). Although logging is now banned in PAs, these ecosystems continue to bear the legacy of past exploitation (Perera 2001). Furthermore, amidst the growing human population, forest cover of the wet and intermediate zone has dwindled to less than a tenth of its original cover and the remaining forests suffer from severe fragmentation (Aukema et al. 2017). Even today, illicit resource extractions (timber felling, non-timber forest products extraction, mining, poaching, and collecting for international pet trade) continue despite strict legislative frameworks (Gunatilleke, Pethiyagoda, et al. 2017). Enigmatic forest diebacks have also been reported from montane forests, which may alter unique forest structural complexity and microclimatic features rendering these habitats less suitable for environmentally sensitive agamids (Ranasinghe et al. 2007).
Our predicted critical agamid habitats are not immune to the adversities of climate change. Altitudinal shifts of species (i.e., moving to higher elevations) seeking wet and cool climatic conditions have been documented worldwide (Neate-Clegg and Tingley 2023; Parmesan and Yohe 2003; Rubenstein et al. 2023; Walther et al. 2002). Several critically endangered agamids (e.g., Ceratophora erdeleni, Calotes desilvai, Ceratophora karu) are already confined to higher elevations as substantiated through our SDMs. Species with restricted high-altitude ranges are at the greatest risk of extinction (Aukema et al. 2017; Parmesan and Yohe 2003; Tobeña et al. 2016; Wijerathne et al. 2025). Increased drought frequency, altered moisture regimes, reduced water availability, and elevated temperatures pose significant threats to the ecological integrity of optimal agamid habitats (Kottawa-Arachchi and Wijeratne 2017). Climatic anomalies are already evident in Sri Lanka (Burt and Weerasinghe 2014). If these anomalies continue to intensify, they will significantly impact Sri Lanka's agamid species through various ecological changes (Dayananda et al. 2021). These imminent threats underscore the high-priority we have assigned to both the wet and intermediate zones.
4.3 Effectiveness of SDMs in Conservation Planning
The potential distribution ranges we have predicted reflect physiological and behavioral adaptation of a species to abiotic and environmental conditions. The underestimation of geographic ranges in previous studies may result from imperfect detection or spatial biases during field surveys, leading to false negatives (Nichols et al. 2008; Otto and Roloff 2011). In addition, it is both logistically infeasible and prohibitively expensive to cover all potential habitats via conventional surveys, which can still underestimate the true distribution range (MacKenzie and Nichols 2004; Wilson et al. 2005). Since MaxEnt delivers reliable predictions despite small field samples (Costa et al. 2010), the SDM approach is of great value when targeting such species for protection (Abdelaal et al. 2019; Guisan and Thuiller 2005; Remya et al. 2015).
The SDM-based predictions we made indicate agamid predicted current geographic distribution, not their long-term persistence. In practice, large portions of species' distribution ranges are suboptimal for species persistence. Due to inadequate management actions, even PAs can suffer from diminished ecological integrity (Rodrigues et al. 2004). Therefore, the increased distribution range sizes of agamids we reported does not warrant downgrading their conservation status. The long-term suitability of heavily modified and disturbed, suboptimal habitats for threatened and endangered agamids remains questionable (Erdelen 2012). For instance, compared to primary and natural forests, agroecosystems of Sri Lanka's wet zone are dominated by common agamid species, whereas the occurrence of highly specialized, threatened and endemic species are infrequent (Gamage et al. 2011). These suboptimal habitats are also prone to biological invasions, frequent human disturbances (Perera 2001), and extractions (Chokkalingam et al. 2001) that may alter critical habitat structure, rendering them less suitable for conservation-dependent agamids. Subsidized predation by human commensals or competition against disturbance-tolerant generalists can also challenge long-term persistence of sensitive agamid species (Oro et al. 2013). Nonetheless, with sustainable management actions, these suboptimal habitats can offer opportunities to promote conservation.
4.4 Limitations of the Study
Our study has several limitations commonly encountered in SDM studies. For example, SDMs may lack microhabitat information (McCoy and Bell 1991), and neglect the effect of various biotic interactions, such as competition and predation (Dunne et al. 2002), which can lead to inaccurate predictions. Another prevalent issue is the reliance on coarse resolution data, which may overlook critical habitat features (Guisan and Thuiller 2005). Also, a lack of updated data layers, such as land use and vegetation, hinders the modeling efforts, and a deficiency of occurrence data poses a significant challenge in Sri Lanka, particularly for rare species restricted to specific geographic patches. Thus, future research should focus on exploring specific microclimatic layers for SDMs and also recommended to follow new sampling and modeling technique to enhance the clarity of research.
Another potential issue is over-prediction, which is a well-documented issue that can lead to inflated estimates of species distributions (Mendes et al. 2020). One major assumption of our SDM is unlimited dispersal within the island; however, climatic, habitat, and anthropogenic factors can constrain dispersal. Certain species, endemic to specific montane regions such as the Knuckles Range and Rakwana Hills, have historically struggled to move across unfavorable low-elevation habitats that separate these montane areas; traversing such areas may be even more difficult now due to habitat loss and fragmentation. Small populations may die out due to demographic stochasticity or anthropogenic disturbance, and then this lack of dispersal may mean that they are not colonized again. Hence, it is very important to follow-up our modeling with more ground-truthing, and our maps (e.g., Figure 4, showing high diversity agamid target areas outside of protected areas) can guide such surveys.
4.5 Recommended Conservation Actions for Conservation Targets
Habitat loss due to human settlements, agriculture, and natural resource overexploitation are the most impactful threats to reptiles (Böhm et al. 2013), especially in continental islands like Sri Lanka. Existing conservation lands we ranked with high-priority (Sinharaja and the Rakwana Hills, the Knuckles Range) can be targeted in management plans. But there is an inadequacy of conservation actions outside conventional PAs in Sri Lanka (Bambaradeniya et al. 2004; Erdelen 2012). Addressing such challenges is a critical necessity for agamids and other taxa of conservation interest (e.g., mammals, Fernando and Ranasinghe 2009; Nekaris et al. 2015, and avifauna, Goodale et al. 2014).
The sizable proportion (44.6%) of high-priority conservation gaps we identified calls for strategizing conservation outside PAs. With effective restoration (e.g., introduction of native flora, invasive species removal, species re-introductions), abandoned croplands, forest plantations (Ashton et al. 2001), and mixed-crop eco-agricultural landscapes will provide opportunities for agamid conservation (Scherr and McNeely 2008). The conservation gaps we identified can also be managed as reserve buffers, wildlife corridors, or components of the landscape matrix, thereby enhancing landscape-scale conservation and habitat connectivity (Pressey et al. 2007). While conventional land protection is crucial for agamid conservation, Sri Lankan authorities must extend management efforts beyond PA boundaries to address anthropogenic stressors, such as habitat degradation, fragmentation, and human-wildlife conflict. A landscape-scale approach, including buffer zones, wildlife corridors, and habitat restoration, will improve connectivity between protected areas, mitigate ecological isolation, and enhance long-term species survival (Lovejoy 2006; Pressey et al. 2007). As PAs in the wet and intermediate zones are severely fragmented, landscape-scale connectivity is essential to rekindle metapopulation dynamics and gene flow in isolated habitat fragments (Chokkalingam et al. 2001; Erdelen 2012; Miththapala 2016). Lowland rainforests that mostly remain as scattered small fragments (> 100 ha) with varying degrees of protection benefit from landscape-scale connectivity and reserve buffers to ameliorate edge effects.
The rare and endemic flora and fauna provides biodiversity conservation potential (Gunatilleke et al. 2005; Gunatilleke, Gunatilleke, et al. 2017), ecosystem values (Surasinghe et al. 2020), and carbon sequestration (Senadheera et al. 2019), and is therefore compatible with holistic, multi-purpose conservation. Reptiles broadly mirror distribution patterns of other taxa and are sensitive to habitat degradation, and thus paint a representative view of biodiversity, albeit being greatly overlooked in reserve selection and design (Böhm et al. 2013; Roll et al. 2017). Therefore, we propose threatened, endemic reptiles as a biodiversity surrogate for systematic conservation planning in Sri Lanka. Our taxon-specific approach is comparable to similar efforts applied for other taxa, such as Important Bird Areas (Fishpool and Evans 2003), Endemic Bird Areas (Brooks et al. 2017), and Important Plant Areas (Darbyshire et al. 2017) which led to successful on-ground conservation actions at variable spatial scales. Furthermore, the fine-grained, spatially explicit approach we used to identify conservation targets can assist Sri Lanka's natural resource managers and conservation officials in expanding existing PAs, designating new conservation areas, enhancing legislative protection for current PAs, and reforming land management policies with a focus on conservation.
5 Concluding Remarks
Tropical wet evergreen lowland rainforests and moist montane and submontane evergreen forests are critical for conservation of the range-restricted, threatened agamid lizards of Sri Lanka. These forest ecoregions are irreplaceable, ecologically distinct, and vulnerable to anthropogenic pressures, and thus must be prioritized in systematic conservation planning. Yet high-priority agamid habitats in Sri Lanka span across protected and unprotected landscapes, and therefore conservation actions must also include novel ecosystems such as secondary and regenerating forests. Thus, we recommend a landscape-scale, holistic approach that accounts for habitat connectivity, science-based management, and forest restoration for agamid conservation in Sri Lanka. As pressure mounts on natural resources due to anthropocentric demands, effective management of human-modified landscapes is imperative for agamid conservation, which presents a daunting task for Sri Lankan conservation authorities. A broad range of eco-evolutionary and socioeconomic factors that are temporally and spatially dynamic will define and contextualize the ecological integrity and future status of conservation targets. As geographic patterns in biodiversity, species vulnerabilities, and anthropogenic legacies vary in time, the prospects for biodiversity protection should identify and accommodate spatial and temporal heterogeneity in conservation needs and opportunities. Biodiversity threats such as climate change and environmental pollution will add complexity to future conservation planning. Predictive tools such as SDMs that account for both natural and anthropogenic dynamics will be increasingly useful to make scientifically informed conservation decisions.
Author Contributions
Iresha Wijerathne: conceptualization, methodology, software, formal analysis, writing – original draft. Suranjan Karunarathna: investigation, data curation, validation. Thilina Sursinghe: writing – original draft, writing – review and editing, validation. Dulan R. Vidanapathirana: investigation, validation, data curation, visualization. Kanishka Ukuwela: investigation, validation, data curation. Sriyani Wickramasinghe: writing – review and editing, conceptualization, resources. Chaya Sarathchandra: visualization, software. Jagath Gunatilake: resources, visualization, project administration. Aiwu Jiang: writing – review and editing, visualization, resources. Eben Goodale: writing – review and editing, visualization, validation. Suranjan Fernando: conceptualization, methodology, supervision.
Acknowledgments
Our special thanks to Bud Chapmen and Channaka Jayathilake, Janaki Manori, Malika Gunawardhana, Pavithra Panduwawala and Shyamala Nirmani for their technical assistance to the project. ILW is sincerely grateful to her family for encouragement, and also thanks the Idea Wild team for providing field equipment. Finally, our sincere gratitude to the reviewers and editorial board of the journal for valuable comments and suggestions for improving the manuscript. Iresha Wijerathne appreciates the support of a Chinese Government Scholarship from the Chinese Scholarship Council (CSC-2021SLJ009558).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




