Gut microbiota dynamics of adult and sub-adult sika deer during reintroduction
重引入过程中成年和亚成年梅花鹿肠道微生物的动态变化
Wentao Zhang and Feifei Yang contributed equally to this study.
Editor-in-Chief & Handling Editor: Binbin Li
Abstract
enPrey populations significantly influence the distribution of top predators. The sika deer (Cervus nippon), a key prey species for the Amur tiger in Northeast China, plays a critical role in the recovery and dispersal of Amur tiger populations. Reintroduction is a pivotal strategy for restoring prey populations, but it presents challenges, especially in terms of adaptation to the natural environment during the natural training process before animals are released. In this study, we sampled six adult and six sub-adult sika deer and employed high-throughput sequencing of the 16S ribosomal RNA gene to investigate changes in gut microbial diversity, composition, and function during natural training. The results showed that adult sika deer had higher gut microbiota diversity compared to sub-adults. However, as natural training progressed, the gut microbial diversity in sub-adults approached that of adults. Additionally, beneficial, fiber-digesting bacteria associated with adaptation to the natural environment tended to increase during nature training in both adult and sub-adult sika deer, while pathogenic bacteria tended to decrease. We also compared the metabolic function of the gut microbiota in adult and sub-adult sika deer, which showed that the carbohydrate metabolic function of both adults and sub-adults decreased significantly with natural training, declining more rapidly in sub-adults. Conversely, the lipid metabolic function in sub-adults increased significantly with natural training. Overall, a period of nature training is necessary before reintroducing animals to their natural habitats, and sub-adult sika deer, in particular, exhibit greater adaptability to environmental changes as reflected by their gut microbiota dynamics. These findings offer valuable insights for the reintroduction of sika deer and other ungulates. We recommend incorporating natural training in reintroduction programs and prioritizing sub-adult animals for reintroduction.
摘要:
zh猎物种群丰度是影响顶级捕食者分布的最重要因素。梅花鹿作为东北虎的主要猎物之一,对东北虎种群的恢复和扩散起着至关重要的作用。重引入是恢复猎物种群的一种有效方式,但也面临着许多问题,尤其是在自然训练过程中对自然环境的适应问题。在这项工作中,采集了6只成年梅花鹿和6只亚成年梅花鹿的样本,使用16S核糖体RNA (rRNA) 基因的高通量测序来确定成年和亚成年梅花鹿在自然训练过程中肠道微生物多样性、组成和功能的变化过程和差异。结果表明,成年梅花鹿肠道微生物的多样性高于亚成年,但随着训练的进行,亚成年梅花鹿的微生物多样性接近成年的多样性水平。此外,在成年和亚成年梅花鹿的训练过程中,与适应自然环境有关的有益纤维消化菌呈上升趋势,而致病菌呈下降趋势。通过比较成年和亚成年梅花鹿肠道微生物的代谢功能,发现成年和亚成年梅花鹿的碳水化合物代谢功能随着训练的进行而显著下降,亚成年的下降速度更快,而亚成年的脂质代谢功能随着自然训练的进行显著增加。总之,在将动物重引入到自然环境之前,进行一段时间的自然训练是很有必要的,这对亚成年梅花鹿更有积极意义,因为它们的肠道微生物能够更好地应对强烈的环境变化。这些结果为重引入梅花鹿或其他有蹄类动物提供了重要的理论依据。本研究建议,在以后的重引入工作中应增加这种自然训练过程,考虑选择亚成年个体。 【翻译:张文韬】
Plain language summary
enThe sika deer is a primary prey species of the Amur tiger, and the availability of prey is an important factor affecting the distribution of top predators. Reintroduction programs are an effective way to restore prey populations, but a significant challenge is ensuring the reintroduced animals can adapt to their natural environment. In this study, we examined the changes and differences in gut microbiota structure and function in six adult and six sub-adult sika deer during natural training before their reintroduction. The results showed that gut microbiota diversity was higher in adult sika deer than in sub-adults, but as training progressed, sub-adults approached adult levels. In addition, beneficial fiber-digesting bacteria, which are associated with better adaptation to the natural environment, tended to increase during training in both adult and sub-adult sika deer, while pathogenic bacteria tended to decrease. A comparison of gut microbiota metabolic functions revealed that carbohydrate metabolism declined significantly in both groups during training, with a more rapid decline observed in sub-adults. Conversely, lipid metabolism functions of sub-adults increased significantly during the natural training period. These findings suggest that natural training before reintroduction is key and that sub-adult individuals may be better suited to reintroduction programs due to their adaptability.
简明语言摘要
zh梅花鹿是东北虎的主要猎物之一,猎物数量是影响顶级捕食者分布的重要因素。重引入是恢复顶级捕食者猎物种群的有效方式,但在此过程中动物能否适应自然环境是个问题。在这项研究中,比较了重引入前自然训练过程中6只成年和6只亚成年梅花鹿肠道微生物结构和功能的变化和差异。结果表明,成年梅花鹿的肠道微生物多样性高于亚成年,但随着训练的进行,亚成年的肠道微生物多样性接近成年的多样性水平。此外,在成年和亚成年梅花鹿的训练过程中,与适应自然环境有关的有益纤维消化菌呈上升趋势,而致病菌呈下降趋势。对比肠道微生物的代谢功能发现,成年和亚成年梅花鹿的碳水化合物代谢功能随着训练显著下降,亚成年的下降速度更快,并且亚成年的脂质代谢功能随着自然训练而显著增加。因此,在将动物重引入到自然环境之前进行自然训练非常重要,建议在后续的重引入工作中应选择亚成年个体。
Practitioner points
en
-
During nature training, the structure and function of the gut microbiota in sub-adult sika deer can quickly align with those of adults, indicating their adaptability to the natural environment.
-
Nature training is necessary before animals are reintroduced into the wild, with a recommendation to prioritize sub-adult individuals for reintroduction due to their enhanced adaptability.
实践者要点
zh
-
在自然训练过程中,亚成年梅花鹿的肠道微生物群结构和功能可迅速接近成年梅花鹿。
-
动物重引入野外环境之前,有必要开展自然训练,建议选择亚成年个体。
1 INTRODUCTION
Reintroduction is an effective strategy for protecting endangered species. Numerous reintroduction projects involving endangered species have been implemented worldwide, including those involving red deer (Cervus canadensis) (Guo et al., 2022), Javanian bison (Bos javanicus) (Chaiyarat et al., 2020), black bears (Ursus thibetanus) (Clark et al., 2002), and giant pandas (Ailuropoda melanoleuca) (Shan et al., 2014; Yang et al., 2018). However, the average success rate of these reintroductions remains low, indicating a need for further research and analysis to improve outcomes (Dallas & Warne, 2023; Fischer & Lindenmayer, 2000; Seddon et al., 2012; Tang et al., 2020). One contributing factor to these low success rates may be conditions associated with captivity: limited space to move around, high population densities, and suboptimal hygiene can lead to increased levels of pathogenic bacteria in the gut microbiota of captive animals, potentially compromising their health and adaptability upon release (Kedia et al., 2021; Lecorps et al., 2021; Li, Hu, et al., 2018; Mosca et al., 2016).
Gut microbiota play a crucial role in maintaining the health and development of animal organisms (Tremaroli & Bäckhed, 2012). The host provides the stable environment necessary for the survival of these microbiota while relying on them to break down complex carbohydrates and other nutrients that cannot be directly absorbed, establishing a complex and dynamic balance (Yeoman et al., 2011). There are many factors that influence the diversity of the intestinal flora, such as diet (De Filippo et al., 2010; Turnbaugh et al., 2006), age (Mariat et al., 2009; Zhang, Liu, et al., 2018), seasonal changes (Xue et al., 2015), and habitat environment (Clayton et al., 2016; Kohl, Skopec, et al., 2014; Mckenzie et al., 2017). When animals transition from captivity to the wild, they must adapt to intense changes, such as increased food variety, expanded range of activity, complex social networks, and the absence of veterinary intervention. These changes can result in significant differences in the diversity, composition, and function of their gut microbiota (Guo et al., 2022; Trevelline et al., 2019).
The sika deer (Cervus nippon), once abundant in the forested areas of China, has experienced a dramatic decline in its wild population due to increased human disturbance and illegal poaching. As a result, it is now classified as a Class I protected species in China and serves as one of the primary prey species of the Amur tiger (Panthera tigris altaica) (Gu et al., 2018). In captivity, sika deer are typically fed a diet high in carbohydrates and proteins, whereas in the wild, they rely on fiber-rich plants to meet their nutritional needs. If these deer are released directly into the wild without preparation, their gut microbiota may struggle to adapt in time to the sudden shift in environmental conditions and nutritional changes, which could ultimately lead to mortality (Trevelline et al., 2019). Therefore, natural training is essential before reintroduction into the wild. This training allows the deer to gradually adjust to the wild environment, develop a gut microbial system capable of digesting new foods, detoxify plant secondary metabolites accumulated from plants (Kohl, Weiss, et al., 2014; Miller et al., 2014; Pratchett and Jones, 1991), reduce the number of pathogenic bacteria, and ultimately increase their chances of successful adaptation.
Previous studies have focused on the analysis of differences in gut microbiota between captive and wild populations (Borbón-García et al., 2017; Clayton et al., 2016; Ning et al., 2020; Trevelline et al., 2019), as well as between herbivores of different ages, such as sika deer (Li, Wang, et al., 2018), foals (Costa et al., 2016), goats (Jiao et al., 2016), and cattle (Uyeno et al., 2010). These studies provide a solid theoretical foundation for the success of reintroduction projects. However, there is limited research focused on designing a period of natural training to assess how animals adapt to natural environments before reintroduction. For wild sika deer, the high abundance of fiber-digesting bacteria in the gut is an adaptation to the high-fiber diet typical in their natural environment (Guan et al., 2017). Therefore, during the natural training of captive populations, particular attention should be paid to promoting the growth of fiber-digesting bacteria. In this context, we define fiber-digesting bacteria and beneficial bacteria, which support the health of sika deer, as favorable for adaptation to the natural environment, while pathogenic bacteria, which are detrimental to their health, are considered unfavorable. To test these concepts, we conducted natural training by reducing the amount of dried food and adjusting the size of the rearing environment. During this process, we explored changes and differences in the diversity, composition, and function of the intestinal microbes of sika deer of different ages. We proposed three hypotheses: (1) Sub-adult sika deer exhibit lower gut microbiota diversity than adult sika deer but gradually approach adult levels with natural training; (2) Natural training reduces the abundance of pathogenic bacteria detrimental to adaptation while increasing the abundance of beneficial/fiber-degrading bacteria conducive to adaptation to the natural environment; (3) In the early stages of natural training, adult sika deer have higher levels of functional metabolic pathways than sub-adults, but sub-adults are expected to rapidly approach adult levels.
2 MATERIALS AND METHODS
2.1 Sample materials
In August 2021, we selected 12 captive sika deer (C. nippon hortuloru) for reintroduction, consisting of six adults and six sub-adults. After conducting disease screenings and genetic purity tests, the deer were transported from a breeding facility in Ning'an, Mudanjiang City, Heilongjiang Province, to the training ground at Heilongjiang Xiaobeihu National Nature Reserve for natural training.
The Heilongjiang Xiaobeihu National Nature Reserve, located in the middle of Zhang Guang Cai Ling, Ning'an City, Heilongjiang Province, serves as the historical habitat of the sika deer. The reserve is geographically positioned between 128°33′07”-128°45′48“E and 44°03′16”-44°18′59 N. It stretches 28 km from north to south and 17 km from east to west, covering a total area of 20,834 hectares. A training ground, approximately 12 ha in size, was established within the reserve in an area with abundant natural water sources and minimal human interference. The area was secured with a metal fence featuring a 5 × 5 cm mesh and standing 3−3.5 m tall, further reinforced with a layer of nylon rope netting ranging from 1 to 1.5 m in height. Trees were used to support the fence, with deeply buried tree poles filling larger gaps between the outer trees. The training ground was divided into two sections: an isolation area and a semi-freedom enclosure area. All the sika deer were initially kept together. From Day 1 to 20, the deer were kept in the isolation area to manage potential emergencies caused by environmental changes; this period was defined as isolation time, during which clean river water was artificially provided for drinking. From Day 21 to 68, the deer were kept in the semi-free area, with access to natural river water; this period was defined as semi-free time. The diet consisted mainly of corn grain, wheat bran, and soybean meal, with the nutrient composition following the guidelines of Xiong et al. (2020) (detailed in Supporting Information S1: Table 1). To prevent chronic acidosis, Mongolian oak (Quercus mongolica) branches and leaves were also provided twice daily. Detailed information about the natural training design and other related aspects are illustrated in Figure 1.
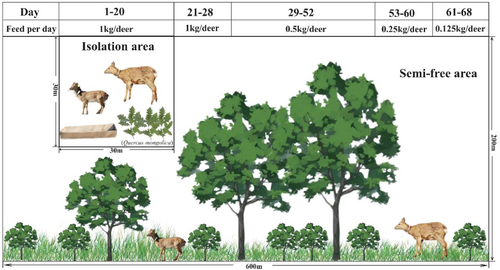
A total of 94 fresh fecal samples were collected during the natural training process. Each sika deer was individually numbered, and efforts were made to collect fecal samples from each deer at different feeding stages. The groups and the number of samples collected are shown in Supporting Information S1: Table 9. Samples were collected within 2 min of defecation, ensuring minimal delay and contamination. During the collection process, researchers directly observed the deer, recorded the number and date of the sample, and ensured that each sample was accurately linked to the corresponding individual. Disposable gloves were used for sample collection, with gloves being changed between each collection to prevent cross-contamination. After collection, the samples were stored in an ultra-low temperature freezer (−80°C), and experiments were performed as soon as possible. Detailed information about the age structure, sample numbers, and sex of the deer are presented in Supporting Information S1: Table 2–3.
2.2 DNA extraction
Total community genomic DNA extraction was performed using the E.Z.N.A™ MagBind Soil DNA Kit (Omega, M5635-02), following the manufacturer's instructions. To verify the quality and concentration of the extracted DNA, we used a Qubit 4.0 (Thermo), ensuring that the extracted DNA met the necessary criteria for subsequent analysis.
2.3 16S ribosomal RNA (rRNA) gene amplification by PCR
Our target was the V3–V4 hypervariable region of the bacterial 16S rRNA gene. Polymerase chain reaction (PCR) was started immediately after the DNA was extracted. The 16S rRNA V3–V4 amplicon was amplified using 2 × Hieff® Robust PCR Master Mix (Yeasen, 10105ES03). Two universal bacterial 16S rRNA gene amplicon PCR primers (polyacrylomide gel electrophoresis purified) were used: the amplicon PCR forward primer (CCTACGGGNGGCWGCAG) and the amplicon PCR reverse primer (GACTACHVGGGTATCTAATCC). The reaction was set up as follows: template DNA (10 ng/µL) 2 µL; amplicon PCR forward primer (10 µM) 1 µL; amplicon PCR reverse primer (10 µM) 1 µL; 2 × Hieff® Robust PCR Master Mix (Yeasen, 10105ES03) (total 30 µL). The plate was sealed, and PCR was performed in a thermal instrument (Applied Biosystems 9700) using the following program: one cycle of denaturing at 95°C for 3 min, first five cycles of denaturing at 95°C for 30 s, annealing at 45°C for 30 s, elongation at 72°C for 30 s, then 20 cycles of denaturing at 95°C for 30 s, annealing at 55°C for 30 s, elongation at 72°C for 30 s and a final extension at 72°C for 5 min. The PCR products were checked using electrophoresis in 2% (w/v) agarose gels prepared in TBE buffer (tris, boric acid, ethylene diamine tetraacetic acid) and stained with ethidium bromide. Visualization was carried out under UV light.
2.4 16S gene library construction, quantification, and sequencing
We used Hieff next generation sequencing ribosomal database project™ DNA Selection Beads (Yeasen, 10105ES03) to purify the free primers and primer dimer species in the amplicon product. Samples were delivered to Sangon BioTech for library construction using universal Illumina adaptors and indices. Before sequencing, the DNA concentration of each PCR product was determined using a Qubit® 4.0 Green double-stranded DNA assay and it was quality-controlled using a bioanalyzer (Agilent 2100). Depending on coverage needs, all libraries can be pooled for one run. The amplicons from each reaction mixture were pooled in equimolar ratios based on their concentration. Sequencing was performed using the Illumina MiSeq system (Illumina MiSeq), according to the manufacturer's instructions.
2.5 Sequence processing, operational taxonomic unit (OTU) clustering, representative tags alignment, and biological classification
After sequencing, the paired-end reads were assembled using PEAR software (version 0.9.8) based on the overlap and fastq files were processed to generate individual fasta and qual files, which could then be analyzed by standard methods. The resulting effective tags were clustered into OTUs at a similarity threshold of ≥97% using Usearch software (version 11.0.667). Chimeric sequences and singleton OTUs (with only one read) were removed, after which the remaining sequences were sorted into their respective samples based on OTU assignments. The tag sequence with the highest abundance was selected as a representative sequence within each cluster. Bacterial OTU representative sequences were classified by blasting from the ribosomal database project.
2.6 Statistical analysis
Species accumulation curves were plotted using the R package “vegan” to assess the adequacy of sampling. Bacterial diversity was determined through sampling-based OTU analyses and expressed using Shannon and Chao1 indices, both calculated with the “vegan” package (Oksanen et al., 2020). The Wilcoxon rank sum test was used to compare the alpha diversity of adult and sub-adult sika deer at each stage of forage change. Constrained principal coordinates analysis (CPCoA) based on Bray-Crutis distances was conducted, with statistical significance assessed via permutational multivariate analysis of variance (PERMANOVA) (p < 0.05) (Oksanen et al., 2015). For each stage of feed change, the distribution of the gut microbial phyla and genera in adult and sub-adult sika deer was mapped using the R package “ggplot2.” A linear mixed model (LMM) was used to evaluate changes in gut microbial diversity, composition, and metabolic function over time. In this model, gut microbial diversity, composition, and metabolic function served as response variables, with rearing date as the explanatory variable. The individual ID of each sika deer was included as a random effect to account for individual variation (Van Doormaal et al., 2015). The LMM was fitted using the R package “YawMMF” (Zhang et al., 2020). Model testing was performed using the R package “MuMIn” to calculate the marginal and conditional R-squared values of the model. All analyses were conducted in R version 4.3.2.
2.7 Function prediction
Functional prediction analysis of bacterial communities was conducted using PICRUSt (v1.1.4) software, by comparing the existing 16S rRNA gene sequencing data with a microbial reference genome database (Douglas et al., 2019) of known metabolic functions, enabling the prediction of bacterial metabolic functions based on known metabolic pathways.
3 RESULTS
3.1 Overview of the sequencing data
A total of 6,551,447 high-quality reads were obtained after quality control of 94 sika deer fecal samples (Mean ± SD: 69,696 ± 15,698), with an average sequence length of 412 ± 1 (Mean ± SD). We used these reads for subsequent bacterial community diversity analyses, including Alpha-diversity indices such as Shannon and Chao1 (Supporting Information S1: Table 4). Rarefaction analysis was performed based on the Shannon diversity index, and the results showed that with the increase of the sequencing amount, the curve gradually plateaued, indicating that the sequencing depth basically met the requirements for subsequent analysis (Supporting Information S1: Figure 1).
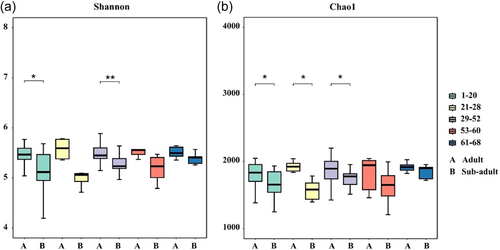
3.2 Gut microbial diversity of adult and sub-adult sika deer
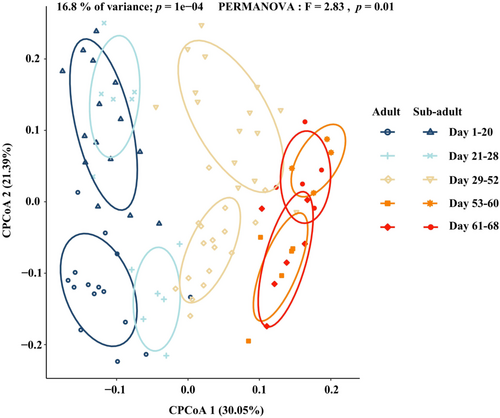
Our results showed that the Alpha-diversity of gut microbiota in adult and sub-adult sika deer fluctuated with the progression of natural training. On Days 1−20, adult sika deer had significantly higher Shannon and Chao1 indices than sub-adults. In the semi-free stage, adult sika deer continued to show significantly higher Shannon indices than sub-adults on Days 29−52, and significantly higher Chao1 indices on Days 21−52. However, after Day 53, no significant differences were observed between adults and sub-adults across all indices (Figure 2a,b). We employed LMMs to analyze the trends in Shannon and Chao1 indices, using sika deer ID as a random factor and training days as a fixed factor. The results showed an increasing trend in the Shannon and Chao1 indices of sub-adult sika deer, while no significant changes were observed in the adult sika deer. The results of the model are presented in Table 1, and the results of the model validation are shown in Supporting Information S1: Table 5. To explore differences in gut microbiota composition between adult and sub-adult sika deer at various stages of natural training, we performed CPCoA analysis based on Bray-Crutis distance. The analysis revealed that CPCoA1 accounted for 30.05% and CPCoA2 accounted for 21.39% of the total gut microbiota variation between adult and sub-adult sika deer. The large degree of separation between adult and sub-adult sika deer from Day 1−20 of natural training indicated that their gut microbiota compositions differed considerably, but with natural training, the gut microbiota compositions of adult and sub-adult sika deer showed a trend toward convergence (Figure 3; PERMANOVA: p < 0.05).
| Age structure | Alpha diversity index | Covariates | Coefficients | Confidence interval | p Value | |
|---|---|---|---|---|---|---|
| (2.5%−97.5%) | ||||||
| Adult | Shannon | Intercept | 5.382 | 5.209 | 5.553 | <0.001 |
| Training day | 0.002 | −0.002 | 0.006 | 0.220 | ||
| Chao1 | Intercept | 1803.552 | 1672.739 | 1935.014 | <0.001 | |
| Training day | 1.228 | −1.017 | 3.485 | 0.286 | ||
| Sub-adult | Shannon | Intercept | 4.99 | 4.753 | 5.223 | <0.001 |
| Training day | 0.005 | 0.001 | 0.009 | 0.017 | ||
| Chao1 | Intercept | 1540.544 | 1385.284 | 1692.871 | <0.001 | |
| Training day | 3.701 | 0.828 | 6.5 | 0.014 | ||
3.3 Gut microbial composition and differences between adult and sub-adult sika deer
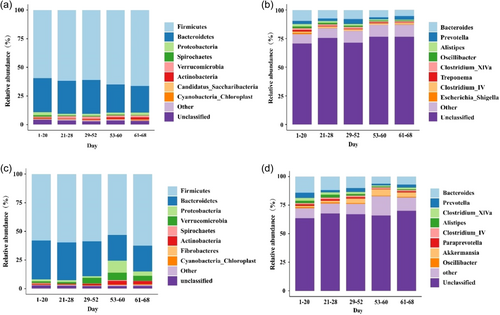
We identified a total of 31 phyla, 56 classes, 69 orders, 159 families, and 383 genera within the gut microbiota community from the 94 fecal samples of sika deer. The relative abundance of gut microbiota between adult and sub-adult sika deer is shown in Figure 4. Our analysis revealed the core phyla common to both adult and sub-adult sika deer were Firmicutes and Bacteroidetes, collectively accounting for more than 80% of the microbiota. During natural training, gut microbiota that play a crucial role in the adaptation of both adult and sub-adult sika deer to the natural environment include Firmicutes, Verrucomicrobia, and Actinobacteria at the phylum level, and Roseburia and Akkermansia at the genus level. We applied LMMs to analyze adult and sub-adult sika deer gut microbiota conducive to adaptation to the field environment, using feeding days as a fixed effect and sika deer ID as a random effect. The model results are shown in Table 2, with the model validation detailed in Supporting Information S1: Table 6. Our results showed that, among the gut microbiota favorable for adaptation, the relative abundance of all microbes showed an increasing trend in both adult and sub-adult sika deer, with the exception of Firmicutes in sub-adults, which showed a nonsignificant increasing trend. Sub-adult sika deer exhibited a higher rate of increase in these beneficial gut microbiota than adults, except for Firmicutes.
| Age structure | Microbes | Covariates | Coefficients | Confidence interval | p Value | |
|---|---|---|---|---|---|---|
| (2.5%−97.5%) | ||||||
| Adult | Firmicutes | Intercept | 57.996 | 52.404 | 63.636 | <0.001 |
| Training day | 0.106 | 0.024 | 0.189 | 0.015 | ||
| Verrucomicrobia | Intercept | 1.083 | 0.428 | 1.735 | 0.003 | |
| Training day | 0.016 | 0.002 | 0.031 | 0.029 | ||
| Actinobacteria | Intercept | 0.256 | −0.093 | 0.606 | 0.167 | |
| Training day | 0.017 | 0.009 | 0.025 | <0.001 | ||
| Roseburia | Intercept | 0.426 | 0.105 | 0.757 | 0.023 | |
| Training day | 0.011 | 0.006 | 0.017 | <0.001 | ||
| Akkermansia | Intercept | 0.584 | 0.097 | 1.068 | 0.022 | |
| Training day | 0.014 | 0.003 | 0.026 | 0.016 | ||
| Sub-adult | Firmicutes | Intercept | 57.564 | 54.152 | 61.003 | <0.001 |
| Training day | 0.025 | −0.052 | 0.106 | 0.529 | ||
| Verrucomicrobia | Intercept | 0.451 | −1.807 | 2.717 | 0.694 | |
| Training day | 0.083 | 0.026 | 0.136 | 0.004 | ||
| Actinobacteria | Intercept | −0.225 | −0.937 | 0.479 | 0.535 | |
| Training day | 0.042 | 0.025 | 0.059 | <0.001 | ||
| Roseburia | Intercept | 0.756 | 0.255 | 1.256 | 0.005 | |
| Training day | 0.016 | 0.004 | 0.029 | 0.014 | ||
| Akkermansia | Intercept | 0.231 | −1.86 | 2.275 | 0.826 | |
| Training day | 0.071 | 0.021 | 0.122 | 0.009 | ||
Gut microbiota that are unfavorable to the adaptation of adult and sub-adult sika deer to their natural environment include Spirochaetes at the phylum level, and Alistipes, Treponema, Oscillibacter, and Clostridium_XlVa at the genus level. LMMs were used to analyze the gut microbiota of adult and sub-adult sika deer that are unfavorable for adaptation to the field environment, with days of feeding as a fixed effect and sika deer ID as a random effect. The model results are shown in Table 3, and the model validation is shown in Supporting Information S1: Table 7. The results indicated that among the gut microbiota unfavorable for adaptation to the field environment, Spirochaetes, Treponema, and Oscillibacter declined more rapidly in the gut microbiota of adult sika deer than in sub-adults, with no significant trend of decline observed for Spirochaetes and Treponema in sub-adults. Alistipes and Clostridium_XlVa in the gut microbiota of sub-adult sika deer showed a faster rate of decline than in adults, with no significant decreasing trend in Clostridium_XlVa in the adult sika deer.
| Age structure | Microbes | Covariates | Coefficients | Confidence interval | p Value | |
|---|---|---|---|---|---|---|
| (2.5%−97.5%) | ||||||
| Adult | Spirochaetes | Intercept | 1.329 | 0.939 | 1.703 | <0.001 |
| Training day | −0.016 | −0.023 | −0.009 | <0.001 | ||
| Alistipes | Intercept | 2.195 | 1.784 | 2.596 | <0.001 | |
| Training day | −0.019 | −0.028 | −0.011 | <0.001 | ||
| Treponema | Intercept | 1.314 | 0.924 | 1.688 | <0.001 | |
| Training day | −0.016 | −0.022 | −0.009 | <0.001 | ||
| Oscillibacter | Intercept | 1.71 | 1.507 | 1.913 | <0.001 | |
| Training day | −0.015 | −0.019 | −0.01 | <0.001 | ||
| Clostridium_XlVa | Intercept | 1.441 | 1.17 | 1.714 | <0.001 | |
| Training day | −0.003 | −0.009 | 0.003 | 0.356 | ||
| Sub-adult | Spirochaetes | Intercept | 0.604 | 0.204 | 0.995 | 0.009 |
| Training day | −0.006 | −0.013 | 0.001 | 0.127 | ||
| Alistipes | Intercept | 2.31 | 1.783 | 2.822 | <0.001 | |
| Training day | −0.021 | −0.032 | −0.011 | <0.001 | ||
| Treponema | Intercept | 0.592 | 0.191 | 0.982 | 0.01 | |
| Training day | −0.006 | −0.013 | 0.002 | 0.139 | ||
| Oscillibacter | Intercept | 1.166 | 0.935 | 1.393 | <0.001 | |
| Training day | −0.01 | −0.014 | −0.005 | <0.001 | ||
| Clostridium_XlVa | Intercept | 2.848 | 2.247 | 3.448 | <0.001 | |
| Training day | −0.024 | −0.038 | −0.01 | 0.001 | ||
3.4 Analysis of differences in metabolic functions between adult and sub-adult sika deer
Based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database, functional changes in the gut microbiota of adult and sub-adult sika deer during natural training (KEGG pathway level 2) were analyzed to investigate how such alterations in microbiota structure would affect functional pathways (Supporting Information S1: Table 10). Carbohydrate metabolism and lipid metabolism were selected as secondary metabolic pathways contributing to adaptation to the natural environment. LMMs were used to analyze changes in these metabolic functions in adult and sub-adult sika deer, with days of feeding as a fixed effect and sika deer ID as a random effect. The model results are shown in Table 4, and the model validation is shown in Supporting Information S1: Table 8. The model results revealed that the functional pathways of carbohydrate metabolism in the gut microbiota of both adult and sub-adult sika deer decreased significantly. In contrast, the functional pathways of lipid metabolism in the gut microbiota of both adult and sub-adult sika deer showed an increasing trend, although this was not significant in adults (p = 0.337).
| Age structure | Metabolism | Covariates | Coefficients | Confidence interval | p Value | |
|---|---|---|---|---|---|---|
| (2.5%−97.5%) | ||||||
| Adult | Carbohydrate metabolism | Intercept | 10.067 | 10.006 | 10.127 | <0.001 |
| Training day | −0.001 | −0.003 | 0.000 | 0.046 | ||
| Lipid metabolism | Intercept | 2.838 | 2.800 | 2.876 | <0.001 | |
| Training day | 0.000 | 0.000 | 0.001 | 0.337 | ||
| Sub-adult | Carbohydrate metabolism | Intercept | 10.170 | 10.088 | 10.254 | <0.001 |
| Training day | −0.003 | −0.005 | −0.001 | 0.002 | ||
| Lipid metabolism | Intercept | 2.754 | 2.690 | 2.819 | <0.001 | |
| Training day | 0.003 | 0.002 | 0.005 | <0.001 | ||
4 DISCUSSION
An important reason for the low success rate of reintroduction efforts is the inability of the animals' gut microbiota to quickly adapt to the drastic changes in diet and other environmental factors when transitioning from captivity to the wild (Guo et al., 2022; Lecorps et al., 2021; Trevelline et al., 2019). In response to this challenge, our study implemented a natural training regimen for sika deer before their reintroduction in Heilongjiang Xiaobeihu Nature Reserve. The study analyzed changes in gut microbial diversity, composition, and function in both adult and sub-adult sika deer during this training, demonstrating the existence of distinct adaptation processes. Natural training, in effect, altered the gut microbial structure of adult and sub-adult sika deer in a way that favored their adaptation to the natural environment.
4.1 Gut microbial diversity in adult and sub-adult sika deer
Most studies on gut microbiota rely on fecal samples, which reflect the overall status of microbial communities in the intestinal tract (De La Torre et al., 2019; Su et al., 2018; Zhang, Shi, et al., 2018). Research has shown that the primary factors influencing gut microbiota diversity in mammals are their living environment and diet (Henriques et al., 2020; Ley et al., 2008; Youngblut et al., 2019). Increased microbial diversity is associated with improved host health, function, and stability (Bestion et al., 2017; Dallas & Warne, 2023; Lozupone et al., 2012; Mckenney et al., 2018; Tap et al., 2015).
Beta diversity reflects the differences between sample groups (Knights et al., 2011). CPCoA revealed significant differences in the gut microbiota between adult and sub-adult sika deer (Figure 3; PERMANOVA: p < 0.05). However, as natural training progressed, the gut microbiota of adult and sub-adult sika deer exhibited converging trends, suggesting that natural training indeed influences gut microbiota diversity, allowing sub-adult gut microbiomes to rapidly approach those of adults.
Alpha diversity indices were significantly higher in adult sika deer compared to sub-adults from Days 1 to 52, but these differences diminished as natural training progressed. The model results showed that the Alpha diversity of gut microbiota in sub-adults tended to increase significantly with training, while there was no significant change in adults. These results suggest that in natural environments, where most plants have not been previously foraged by sika deer and where sika deer rearing farms have brick floors (limiting exposure to soil), gut microbial diversity increases as both adult and sub-adult forage and explore their new surroundings (Dallas & Warne, 2023; Diaz et al., 2023). Adult sika deer maintain a high level of dynamic gut microbial diversity to counterbalance the possible adverse effects of long-term captivity before nature training (Li, Wang, et al., 2018). In contrast, sub-adult sika deer initially displayed lower diversity, which rapidly increased to near adult levels as they adapted to significant environmental and dietary changes during natural training.
4.2 Gut microbiota in adult and sub-adult sika deer: Favorable/unfavorable for adaptation to the natural environment
At the beginning of natural training, the sika deer were fed a diet rich in carbohydrates, proteins, fats, and amino acids (Supporting Information S1: Figure 1). As the training progressed, the amount of concentrated feed was gradually reduced, requiring the sika deer to consume large amounts of fiber-rich plants to obtain the nutrients needed for survival in the wild. The gastrointestinal tract of ruminants can ferment starch and sugars from fibrous plant material, but it cannot produce the necessary fiber-degrading enzymes on its own; these enzymes must be produced by the bacteria colonizing their intestines (Hu et al., 2017). Firmicutes and Roseburia are important fiber-digesting bacteria that increase with the intake of fiber-rich foods (Mirande et al., 2010; Leth et al., 2018). They encode three major cellulose-digesting enzymes—endoglucanase, β-glucosidase, and cellulose 1,4-beta-cellobiosidase (Wang et al., 2019)—which break down fiber into volatile fatty acids that are available to the host. Roseburia, in particular, is an important butyrate-producing bacterium in Firmicutes. Butyrate has wide-ranging anti-inflammatory and metabolic regulatory roles in various disease models (Canfora et al., 2015; Mcnabney and Henagan, 2017; Tamanai-Shacoori et al., 2017).
During natural training, we also observed an increase in the relative abundance of Verrucomicrobia and Actinobacteria, both of which are bacteria beneficial to the organism. Verrucomicrobia is found in humans mainly as Akkermansia, a common inhabitant of the human gut, accounting for about 1−3% of the total gut microbiota (Almeida et al., 2020). Akkermansia is considered a next-generation beneficial microbe (Cani, 2017; Cani et al., 2022; Zhai et al., 2019) with important roles in maintaining gut health, regulating host metabolism and immune response (Macchione et al., 2019; Naito et al., 2018), and protecting against cardiovascular diseases (Li et al., 2016). Its products can provide energy for ruminants, and Akkermansia may act as an energy sensor, being abundant during caloric deficits and scarce during energy surpluses, reflecting a co-evolutionary mechanism for energy absorption when available (Chevalier et al., 2015). Akkermansia levels have been shown to be elevated in malnourished mice (Preidis et al., 2015). The relative abundance of Akkermansia tends to increase as the amount of feed is gradually reduced during natural food training.
Actinobacteria are mainly distributed in organic matter-rich and slightly alkaline soils and in the gastrointestinal tract of herbivorous animals, aiding digestion. The lactic and acetic acid produced by Actinobacteria can regulate gut pH, inhibit the proliferation of harmful bacteria, maintain intestinal health, and slow down gut aging (Di Gioia et al., 2014; Yang et al., 2020). In a study of goats transitioning from a forage-based diet to a high-grain diet, the abundance of Actinobacteria tended to decrease as dietary starch content increased (Grilli et al., 2016). This finding aligns with our study, which showed that the abundance of Actinobacteria increased as the amount of forage in the sika deer diet increased and the amount of edible starch decreased.
Long-term captive environments can increase the risk of disease in animals, leading to problems such as excessive obesity (Ley, 2010; Vallianou et al., 2019) or gut inflammation (Mosca et al., 2016), which can threaten survival and reduce reintroduction success (Dallas and Warne, 2023). During natural training, we found a tendency for a decrease in Spirochaetes, Alistipes, Treponema, Oscillibacter, and Clostridium_XlVa in the gut microbiota of adult and sub-adult sika deer. These are pathogenic bacteria mostly associated with inflammation (Abdel-Moein et al., 2015; Feng et al., 2015; Frosth et al., 2023; Kim et al., 2020; Zhou et al., 2018), and they have been linked to the development of osteoporosis (Wei et al., 2021), various types of cancers (Parker et al., 2020; Yin et al., 2023; Zhang et al., 2019), chronic kidney disease (Kim et al., 2020), and certain psychiatric disorders (Jolivet-Gougeon & Bonnaure-Mallet, 2018; Naseribafrouei et al., 2014; Yang et al., 2021).
Overall, our results indicate that the beneficial/fiber-digesting bacteria in the gut microbiota of sika deer showed an increasing trend during natural training, while pathogenic bacteria showed a decreasing trend. This suggests that natural training can help sika deer adapt to a high-fiber diet in the natural environment by enhancing the presence of beneficial bacteria and reducing pathogenic bacteria, thereby promoting better health and adaptability to the natural environment.
4.3 Metabolic pathways of gut microbial functions in adult and sub-adult sika deer
A previous study showed that carbohydrate metabolism, lipid metabolism, and other processes are the main functions of the gut microbiota in tigers (Jiang et al., 2020). During natural training, as the variety of available diets decreases, sika deer must consume more food rich in hard-to-digest fiber to maintain the nutrients and energy required for survival. This shift leads to a decrease in carbohydrate metabolism in adult and sub-adults as they adjust to digesting and breaking down their fibrous diets. Moreover, adult sika deer are more competitive for food than sub-adults, which was observed during the natural training; adults would drive away sub-adults when food was scarce to secure more nutrients for themselves. Lipid metabolism involves the biosynthesis and degradation of lipids such as fatty acids, triglycerides, and cholesterol (Schoeler & Caesar, 2019). Short-chain fatty acids, which are bacterial metabolites produced by gut microbiota through the anaerobic fermentation of fibers, serve as major energy sources. These include acetate, propionate, and butyrate, which play roles in immunity, inflammation, and the regulation of lipid metabolism (Dalile et al., 2019; Hu et al., 2018; Zhang et al., 2023). Natural training for sika deer takes place between August and October, so sub-adult sika deer require a low-fat, low-sugar, high-fiber diet to maximize lipid metabolism, allowing them to store more energy in preparation for impending food shortages and the cold winter in the wild. This is consistent with previous studies that showed gut microbiota in mice increases intestinal absorption, energy homeostasis, and fat burning during cold periods (Ufarté et al., 2015). In addition, these changes improve digestive efficiency and SCFA production in ruminants living in cold environments and at high altitudes (Earle et al., 2015; Geva-Zatorsky et al., 2015). However, these metabolic changes may also correspond to a reduced abundance of microbes related to host immune function and an increased risk of disease (Cummings et al., 1987; Hooper et al., 2002; Rooks and Garrett, 2016).
5 CONSERVATION IMPLICATIONS
To improve the reintroduction success rate of sika deer and other captive ungulates, we put forward the following recommendations based on the findings of this study: (1) Breeders often raise large numbers of sika deer in a limited space and feed them high-sugar and high-fat diets to maximize economic benefits. These practices can increase the potential pathogenicity of the animals, which is not conducive to successful reintroduction. This issue can be mitigated by rationally planning breeding numbers, ensuring a clean environment, and increasing the fiber content of the feed. Such measures will improve the overall health of the animals in captivity, creating a healthier and more viable breeding stock for reintroduction efforts. (2) Before releasing captive animals into the natural environment, it is recommended to implement a period of natural, localized training. This allows the animals to establish a stable gut microbiota that is better adapted to the natural environment and the new food composition. Such training is likely to improve the success of reintroduction by helping animals acclimate more effectively to their new surroundings. (3) Adult sika deer tend to have more stable gut microbiota diversity, composition, and function during natural training, which develops under long-term captive conditions to cope with possible adverse effects of such environments. In contrast, the gut microbiota of sub-adult sika deer, though less stable initially, can rapidly approach adult levels after natural training, demonstrating good plasticity. Therefore, we recommend prioritizing sub-adults when reintroducing ungulates, as they may adapt more effectively to the wild. These findings could be essential for the successful reintroduction of large ungulates in future conservation efforts.
AUTHOR CONTRIBUTIONS
Guangshun Jiang designed this research; Wentao Zhang, Feifei Yang, Jiale Sun, Yanhui Guan, Shixian Guo, and Jixu Sun contributed to the data collection work; Data analysis was mainly carried out by Wentao Zhang, Feifei Yang, Heng Bao, Jiale Sun, Wannian Cheng, Shiyu Chen and Nathan J. Roberts; Wentao Zhang wrote the first draft of the manuscript; Feifei Yang, Heng Bao, and Nathan J, Roberts revised and improved it. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
We appreciate the fieldwork support of the Administration of Heilongjiang Xiaobeihu National Nature Reserve. We appreciate the members of the team who took part in the data collection for their contributions. This research was supported by National Key R&D Program of China (2023YFF1305000), National Natural Science Foundation of China (NSFC 32100392) and the Fundamental Research Funds for the Central Universities (2572022DS04).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




