Fat storage and drought tolerance in a seasonally-adapted primate: Implications for modeling the effects of animal responses to global climate change
灵长类动物在季节性适应下的脂肪储存和耐旱性:模拟动物应对全球气候变化影响的启示
Editor-in-Chief & Handling Editor: Ahimsa Campos-Arceiz
Abstract
enGlobal warming is changing habitats and affecting biodiversity, and is expected to exacerbate aridification in many regions. Animals and plants in seasonally dry tropical forests often exhibit adaptations to cope with seasonal resource limitation. However, whether these adaptations will facilitate drought tolerance or increase drought vulnerability is unclear. Here, we combine long-term individual-based data on phenology, morphometrics, and demographics to investigate how drought impacts the food resources, health, reproduction, and behavior of a population of Verreaux's sifaka (Propithecus verreauxi), a critically endangered lemur inhabiting dry deciduous forests in Madagascar. Between December 2010 and May 2023, the population experienced 3 years of severe drought (2016, 2017, 2022). During green periods in severe drought, the availability of high-quality sifaka foods (young leaves, fruit) was significantly reduced and fruit tree mortality increased. This reduced availability of fruit persisted in the year after a drought, despite typical rainfall. Yet surprisingly, we found no negative effects on body condition or commonly-used metrics for reproductive success during drought years or years following a drought. Instead, sifaka exhibited significantly higher levels of subcutaneous body fat during severe droughts. We observed little change in sifaka behavior between drought and non-drought periods. However, they were more likely to lick dew during severe drought, and spent significantly less time feeding on young and mature leaves. They also significantly increased their time feeding on flowers and fruits, despite the reduced abundance of fruit in the habitat. Together, our results suggest that increased consumption of water-rich fruit and flowers during severe droughts could facilitate physiological mechanisms that help sifaka cope with water scarcity, including fructose-mediated fat storage, metabolic water production, and water conservation. These results provide new insights into how critically endangered animals may respond to climate change, suggesting that behavioral and physiological adaptations to seasonal resource limitation may buffer some mammals from the effects of severe drought or other extreme weather events.
摘要
zh全球变暖正在改变栖息地并影响生物多样性,预计还将加剧许多地区的干旱化。季节性干旱热带森林中的动植物通常会表现出适应性,以应对季节性资源限制。然而,这些适应性是否会促进耐旱性亦或是降低对干旱的防御性还不清楚。在本文中,我们结合了基于个体的物候学、形态计量学和种群统计学的长期数据,阐释了干旱如何影响马达加斯加干燥落叶林栖息地内的极度濒危狐猴——维氏冕狐猴(Propithecus verreauxi)种群的食物资源、健康、繁殖和行为。在2010 年12月至2023年5月期间,维氏冕狐猴种群在2016 年、2017 年和 2022 年分别经历了三次严重的干旱。在严重干旱的绿期,优质食物(嫩叶、果实)的供应量显著减少,果树的死亡率也不断增加。尽管降雨量达到通常水平,在干旱后的一年里,水果供应减少的情况依然存在。然而,令人惊讶的是,我们发现在干旱期间或干旱后的年份里,维氏冕狐猴的身体健康状况或常见的繁殖成功率指标并没有受到负面影响。相反地,在严重干旱期间,维氏冕狐猴的皮下脂肪含量表现出明显升高。进一步,我们观察到,在干旱和非干旱时期,维氏冕狐猴的行为几乎没有变化。这可能是因为在严重干旱期间,它们更有可能通过舔食露水,从而明显减少在嫩叶和成熟树叶上取食的时间。尽管栖息地中的果实数量减少,但它们取食花朵和果实的时间却明显增加。总之,我们的研究结果表明,在严重干旱期间增加对富含水分的果实和花朵的 取食,可能有助于促进维氏冕狐猴应对缺水的生理机制,包括果糖介导的脂肪储存、代谢产水和节水。这些结果为极度濒危动物如何应对气候变化提供了新的视角,表明对季节性资源限制的行为和生理适应可能会使一些哺乳动物免受严重干旱或其他极端天气事件的影响。【审阅:周聪】
Plain language summary
enDroughts are projected to intensify with climate change, posing significant challenges for plant and animal communities. Seasonally dry tropical forests typically experience long dry seasons, which may make resident animals more vulnerable if the dry season is prolonged by drought. However, our study on Verreaux's sifaka (Propithecus verreauxi), a critically endangered lemur inhabiting seasonally dry forests in western Madagascar, revealed surprising resilience during severe droughts. Despite reduced food availability, sifaka showed minimal changes in health, survival, reproduction, or behavior. Instead they exhibited an unexpected adaptation–increased subcutaneous body fat during drought, which could potentially be used to generate metabolic water similar to desert mammals. Sifaka also sought out water-rich and sugar-rich foods like fruits and flowers during droughts, despite their scarcity. Our findings, representing data collected over 13 years (including three severe droughts), suggest that behavioral and physiological traits that evolved to cope with typical dry seasons may buffer sifaka from the effects of drought. This adaptive strategy of storing fat and selective foraging during drought could be vital for their survival in a changing climate. By understanding how these lemurs cope with extreme events, we gain insights into broader strategies for conservation in the face of escalating climate challenges.
简明语言摘要
zh预计干旱将随着气候变化而加剧,给动植物群落带来重大挑战。季节性干旱的热带森林通常会经历漫长的旱季,如果旱季因干旱而延长,可能会置定居型动物于不利的境地。然而,我们对栖息在马达加斯加西部季节性干旱森林中的一种极度濒危狐猴——维氏冕狐猴(Propithecus verreauxi)的研究表明,在严重干旱期间它们具有很强的恢复能力。尽管食物供应量减少,但维氏冕狐猴在健康、生存、繁殖或行为方面的变化极小。相反,它们表现出了一种意料之外的适应能力--在干旱期间增加皮下脂肪,这可能有助于产生与沙漠哺乳动物类似的代谢水特征。尽管水果和鲜花等富含水分和糖分的食物稀缺,但维氏冕狐猴在干旱期间也会寻找这些食物。我们的研究结果表明,为应对典型旱季而进化出的行为和生理特征可能会使维氏冕狐猴免受干旱的影响。这种在干旱期间储存脂肪和选择性觅食的适应性策略可能对它们在应对不断变化的气候环境中生存至关重要。通过了解维氏冕狐猴如何应对极端干旱事件,对我们深入了解在面对不断升级的气候挑战时提供了更广泛的保护策略。
Practitioner points
en
-
In a seasonally dry tropical forest in western Madagascar, focal trees had increased mortality and reduced abundances of ripe fruit and young leaves during severe drought. The year after severe drought was also characterized by reduced fruit abundance.
-
During severe drought, sifaka exhibit little change in body condition, reproductive output, infant survival, or behavior. However, they do spend more time feeding on water-rich fruit and flowers, are more likely to lick dew, and surprisingly exhibit increased subcutaneous body fat.
-
The physiological and behavioral adaptations that sifaka exhibit for dealing with resource scarcity during typical dry seasons may facilitate drought tolerance in this critically endangered species.
实践者要点
zh
-
在马达加斯加西部季节性干旱的热带森林中,在严重干旱期间,目标树木的死亡率增加,成熟果实和幼叶的数量减少。严重干旱后一年内的果实数量也有所减少。
-
在重度干旱期间,维氏冕狐猴的身体状态、繁殖能力、幼体存活率或行为变化不大。然而,它们会花费更多的时间采食富含水分的果实和花朵,也更有可能舔食露水,而且令人惊讶的是,它们的皮下脂肪有所增加。
-
在典型的干旱季节,维氏冕狐猴表现出的应对资源匮乏的生理和行为适应可能促进了这一濒危物种的耐旱性。
1 INTRODUCTION
Droughts are extreme environmental events that pose a severe threat to human health and agriculture (Ebi et al., 2021; Hixon et al., 2021). They are also hazardous to natural ecosystems, impacting animal and plant survival, health, and reproductive success (Campos et al., 2020; Clifton, 2010; Corlett, 2016; Ferreira et al., 2019; Maxwell et al., 2019). Historic climate data and modeling studies suggest that global warming is contributing to increases in the intensity and frequency of drought events in many regions, including Madagascar (Dai, 2013; IPCC, 2019; Rabezanahary Tanteliniaina & Andrianarimanana, 2024). These increased drought events are predicted to negatively impact ecosystem biodiversity and may have particularly dire consequences for already threatened or endangered species (Aguirre-Gutiérrez et al., 2020; Maxwell et al., 2019). A growing body of research suggests that species differ in their vulnerability to drought, even when inhabiting the same environment (Aguirre-Gutiérrez et al., 2020; Campos et al., 2020; Ferreira et al., 2019). For example, a study in Kruger National Park in 2015/2016 found significantly higher mortality and lower birth rates during drought in the white rhinoceros, but no effects of drought on mortality and birth rates in the sympatric black rhinoceros (Ferreira et al., 2019; le Roex & Ferreira, 2021). Thus, identifying features that can contribute to drought tolerance is critical for assessing species vulnerabilities and targeting conservation action (Aguirre-Gutiérrez et al., 2020; Rymer et al., 2016).
Several studies have investigated adaptations to drought in arid-dwelling mammals (e.g., desert and semi-desert species; Gedir et al., 2020; Rocha et al., 2021; Rymer et al., 2016), however, species in seasonally dry tropical forests (SDTFs) have received relatively less attention. The nature of SDTFs nonetheless suggests that mammals in these habitats may be particularly important models for identifying traits contributing to drought tolerance or vulnerability. SDTFs receive 250–2000 mm of rainfall annually, but are characterized by a high degree of seasonality, experiencing both a rainy wet season (receiving 80%+ of annual rainfall) and a prolonged dry season (5+ months) with little to no rainfall (Allen et al., 2017; Stoner & Timm, 2011). While arid habitat mammals cope with low levels of rainfall year-round, species in SDFTs must pivot between bountiful wet seasons and resource-limited dry seasons. SDTF-dwelling mammals exhibit a variety of physiological and behavioral adaptations that allow them to address this challenge. Mouse lemurs (Microcebus murinus) in SDTFs in Madagascar, for example, use torpor to conserve water during the dry season (Schmid & Speakman, 2009). Many SDTF mammals are dietary generalists and shift diets between the wet and dry seasons (Hemingway & Bynum, 2005; Stoner & Timm, 2011; van Schaik et al., 1993). Similarly, species change movement patterns (e.g., decreasing or increasing home range or travel) or activity patterns (e.g., shifting between diurnal and nocturnal activity) between seasons (Curtis & Rasmussen, 2006; Hemingway & Bynum, 2005; Stoner & Timm, 2011). These seasonal adaptations may facilitate drought tolerance in SDTF mammal species. Alternatively, SDTF-dwelling mammals may be more vulnerable to drought because they are already at their physiological limit in coping with a harsh environment (e.g., Allen et al., 2017).
In this study, we test these two competing hypotheses using a population of Verreaux's sifaka (Propithecus verreauxi), gregarious diurnal lemurs from SDTF in western Madagascar. Verreaux's sifaka live in habitats with low mean annual rainfall (~500–850 mm), nearly all of which falls within just 2 months (Lawler et al., 2009; Lewis & Axel, 2019; Sagar et al., 2021). Green vegetation, which is central to the herbivorous sifaka diet (Koch et al., 2017; Lewis & Kappeler, 2005; Norscia et al., 2006), returns approximately 1 month after the rains (Lewis & Axel, 2019). As medium-sized (~3 kg) folivores inhabiting SDTFs, Verreaux's sifaka thus experience highly seasonal fluctuations in the availability of food and water (Lewis & Kappeler, 2005). Studies have identified several adaptations in sifaka to cope with these cyclical resource fluctuations, including physiological (e.g., highly seasonal reproduction, concentrated urine) and behavioral traits (e.g., dietary flexibility, seasonally reduced activity, utilizing dew as a key water resource) (Gnanadesikan et al., 2022; Lewis & Kappeler, 2005; Norscia et al., 2006; Rudolph et al., 2019). Despite these adaptations, sifaka nevertheless suffer substantial declines in body mass and body fat during the dry season (Lewis & Kappeler, 2005), which may suggest that they are at their physiological limit in coping with resource limitation. Previous studies present potentially conflicting results regarding their ability to recover when disruptions to the typical green-up further strain their already depleted reserves. In one population, severe drought was found to severely negatively impact sifaka health, fertility, and survival (Richard et al., 2000, 2002). However, another sifaka population exhibited no detrimental effects following a 2009 cyclone that caused substantial forest damage and resulted in reduced green-up the following year (Lewis & Axel, 2019; Lewis & Bannar-Martin, 2012; Lewis & Rakotondranaivo, 2011). Importantly, sifaka's bet-hedging life history strategy, which stabilizes fitness over a long rather than a short time frame due to environmental variability (for review of bet-hedging theory see Simons, 2011), suggests an adaptation to the frequent extreme environmental events in Madagascar (Lewis & Axel, 2019; Richard et al., 2002; Wright, 1999).
Here, we exploit a unique collection of long-term data on rainfall, plant phenology, morphometrics, population demographics, and behavior from the Ankoatsifaka Research Station in Kirindy Mitea National Park (KMNP) to investigate how drought events in a SDTF impact sifaka health, survival, reproductive success, and behavior. KMNP experienced 3 years of severe drought over a 13-year period (Dec 2010 to May 2023). If seasonal adaptations facilitate drought tolerance in sifaka, we predicted that health measures (e.g., subcutaneous body fat, body mass index), infant survival, or births do not significantly differ between drought and non-drought periods. We also predicted that sifaka exhibit behavioral shifts during drought events to increase water consumption (e.g., increased licking of dew, increased feeding on water-rich foods) and conserve energy (decreased travel and increased resting). In contrast, if sifaka are more vulnerable to drought, we predicted that measures of health, infant survival, and births decline during or immediately following drought events.
2 METHODS AND MATERIALS
2.1 Study site and species
We studied the effects of drought on a population of Verreaux's sifaka at Ankoatsifaka Research Station (20°47′17″S, 44°10′08″E) in KMNP, a dry deciduous forest/succulent woodland habitat in southwestern Madagascar encompassing 150,000 ha (Figure S1; Burgess et al., 2004; Lewis & Axel, 2019). The research station, founded in 2006, is a 1 km2 section of dry deciduous forest with a grid system of trails every 25 m, and a mean annual rainfall of 850 mm and average temperature of 24°C (Lewis & Axel, 2019; Rasambainarivo et al., 2014). Behavioral, morphometric, and demographic data have been collected on a subset of the Verreaux's sifaka population residing within this 1 km2 area since 2007. While these diurnal lemurs are primarily folivorous, fruit can be seasonally important in their diets (Lewis & Kappeler, 2005; Veilleux et al., 2016). They are primarily arboreal and live in small cohesive social groups of 2–16 individuals (Leimberger & Lewis, 2017; Sussman et al., 2012).
2.2 Rainfall data and defining drought periods
We calculated total monthly rainfall for December 2010 to November 2023 from daily precipitation data manually recorded from rain gauges at the field station. We defined each “year” as beginning in December and ending in November, such that the beginning of each year coincides with the wet season when trees become flush with new leaves [i.e., “seasonal year”, following Lewis & Axel (2019) and Axel et al. (2024)]. On average, 92% of annual rainfall occurs in the wet season (Dec–Mar), with little to no rain in the dry season (Apr–Nov) when leaves are in senescence and being shed.
There are a number of approaches for identifying drought (e.g., Palmer Drought Severity Index, Standardized Precipitation Evapotranspiration Index), however, these metrics require serially complete data for precipitation and temperature that were not available at KMNP for the entire study period. Here, we focused on precipitation-based metrics, consistent with recent work suggesting that drought in semi-arid eastern African regions like KMNP is primarily related to deficits in rainfall (Kew et al., 2021). First, we used two common indicators, Percent of Normal Precipitation and Standardized Precipitation Index (PNP and SPI, respectively; WMO & GWP, 2016), to identify abnormally dry years. While these indicators typically need at least 30 years of rainfall data, only 13 years (Dec 2010–Nov 2023) were available at KMNP and these years completely overlap the study period. To compensate for this constraint, we calculated PNP relative to both the mean annual rainfall at KMNP and to an 87-year average at the Morondava weather station 70 km north of KMNP (Sorg et al., 2003). We calculated SPI for the 13 years of annual rainfall data at KMNP using the SPEI package (Beguería & Vicente-Serrano, 2023) in R version 4.3.1. Despite these limitations, all three metrics consistently identified the same seasonal years—2016, 2017, 2022—as experiencing abnormally low rainfall (Table S1). Each of these years received less than 60% of normal rainfall, suggesting severe to extreme drought (Svoboda et al., 2002). We also categorized the severity of the droughts in these 3 years using the simplified rainfall index (RIS) developed by Swain et al. (2021). This index compares observed rainfall to long-term averages to categorize the severity of wet and drought periods as extremely wet, severely wet, moderately wet, near normal, moderate drought, severe drought, or extreme drought (Table S1). We calculated RIS using the Morondava weather station average (767 mm). According to this metric, 2016, 2017, and 2022 were “severe drought”, while the other years in the study period were either “near normal” (2011–2014, 2018–2021) or “extremely wet” (2015, 2023).
2.3 Phenological data and analysis
Detailed phenological data were collected on known sifaka feeding trees (identified during behavioral observations) in the 1 km2 Ankoatsifaka Research Station grid system beginning June 2010. Every month, the health of each tree was evaluated (dead vs. alive), and data were collected on the relative presence of five major sifaka food products: young leaves, mature leaves, flowers, ripe fruit, and unripe fruit. The presence of these plant parts was quantified in five ranks: 0 (absent), 1 (1%–25% present), 2 (26%–50% present), 3 (51%–75% present), and 4 (76%–100% present). For all trees confirmed to have died, we excluded any subsequent observations of that tree after the first call of death. If a tree initially called “dead” had plant parts in subsequent months, we considered it living and recorded data on its health and the relative presence of food products.
We restricted our analyzes to observations collected between Jan 2011 and May 2023 to match the available rainfall data. Note that phenology data were not available for Dec 2011–May 2012. The dataset included 21,531 monthly observations of 256 trees/lianas and 68 species, with each tree/liana being sampled for 84.1 ± 42.6 observation months. On average, 168.2 trees (±30.9) were sampled each observation month. For each ordinal response variable (relative abundance of young leaves, mature leaves, flowers, ripe fruit, unripe fruit observed in a tree for a particular month), we performed two cumulative link mixed models (CLMMs) with a logit function using the ordinal package in R (Christensen, 2022) and compared the two models using two-tailed likelihood ratio tests (LRTs). The null model included a fixed effect of resource period and random effects of tree identity nested in tree species, observation month, and observation year. We categorized resource period as either “green” (Dec-May) or “yellow” (Jun–Nov), corresponding to the period of phenological green-up or senescence/shedding, respectively (Axel et al., 2024; Lewis & Axel, 2019). Previous work found these resource periods to be important in sifaka life history (Lewis & Axel, 2019). We used “seasonal year” (Dec–Nov) rather than calendar year because it more accurately reflects the rainfall and phenological cycle. The alternative model included drought (yes/no) and an interaction between drought and season (season × drought). We repeated the set of CLMMs to investigate whether there were phenological changes in the year immediately following a drought compared to years of typical rainfall. For this “year after” analysis, we excluded data from 2011 and all drought years (2016, 2017, 2022). Finally, we quantified the number of trees that died each year and used a one-tailed Fisher exact test to evaluate effects of drought on tree mortality.
2.4 Morphometric data and analysis
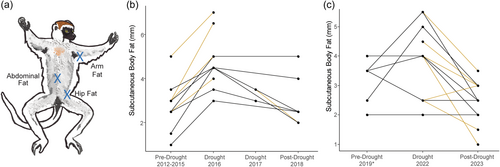
Beginning in 2006, sifaka have been captured and sedated for morphometric data collection over a 5-week period each dry season (Jun–Jul) as part of the long-term Sifaka Research Project (Figure S2). During each capture, measurements were collected on body length (top of crown to base of tail) with measuring tape, body mass, and subcutaneous body fat. Body mass was measured using a hanging scale and a basket (Figure S3), and subcutaneous body fat was measured in mm at four locations (scapular, arm, abdominal, hip) using a skinfold caliper. Note that annual captures were not conducted in 2020 and 2021 due to the COVID-19 pandemic. Capture and collection protocols are described in Rasambainarivo et al. (2014) and were performed in accordance with protocols approved by the University of Texas at Austin Institutional Animal Care and Use Committee (protocol numbers: 08110301, AUP-2011-00143, AUP-2014-0036, AUP-2017-00152, and AUP-2020-00143). Following sedation via remote injection using a blowpipe or CO2 rifle to deliver darts with Zoletil® or Telazol®, animals were caught in a large cotton sheet and processed in the forest near the location of the capture. Animals were placed in cotton sacks in the shade to reduce stressful stimuli and returned to their known social group or the location of the capture after full recovery. This study complied with all established IACUC guidelines and Malagasy law.
To evaluate effects of drought on sifaka health, we restricted our morphometric data to captures between 2011 and 2023 (years with available rainfall data). This dataset included 73 individuals, with an average of 2.2 ± 1.5 captures per individual. All individuals were either subadult (3–4 years) or adult (≥5 years). We quantified body condition for each individual at each capture with three metrics: subcutaneous body fat, mass, and body mass index (BMI). Subcutaneous body fat was calculated as the sum of the scapular, arm, abdominal, and hip skinfold fat measurements (Lewis & Kappeler, 2005). We calculated BMI as mass (kg)/(tail-crown length (cm)/100)2, with tail-crown length averaged over all adult captures for an individual. For subadults, we used the tail-crown length measured during that capture for BMI calculation. Body mass and subcutaneous body fat have been linked to reproductive success in Verreaux's sifaka (Lewis & Kappeler, 2005; Richard et al., 2000).
We used linear mixed effects models (LMMs) to investigate the associations between these measures of sifaka health and the presence of drought (yes/no), while controlling for other factors that might affect body condition, including sex and pregnancy status (coded as male, nonpregnant female, pregnant female) and age class (subadult/adult). For each health measure, we performed eight models: (1) null model with no fixed factors; (2) sex/pregnancy as only fixed factor; (3) age class as only fixed factor; (4) drought as only fixed factor; (5) sex/pregnancy + age class; (6) sex/pregnancy + age class + drought; (7) sex/pregnancy + drought; and (8) sex/pregnancy × drought + pregnancy status + age class. The last model allowed for an interaction between sex/pregnancy and drought to investigate whether drought differentially affects males and females. All models included random effects of individual identity and year to control for variation between individuals and years. We identified the best fit model using Akaike Information Criterion (AIC) values and evidence ratios (Burnham et al., 2011) and used two-tailed LRTs to compare similar models (ΔAIC < 2). AIC values were estimated using the native AIC function in R, while the GLMMs were implemented using the lme4 (Bates et al., 2015) and LmerTest (Kuznetsova et al., 2017) packages. While we noted singularity warnings for BMI and body mass analyzes because of near zero variance for random effect of year, when we reran the models excluding year (removing the singularity warning), the best fit models identified by AIC did not change.
2.5 Reproduction data and analysis
As part of a long-term, longitudinal study of this population (RJ Lewis, unpublished data), the Sifaka Research Project endeavors to census all sifaka in the 1 km2 Research Station every month. We used these demographic census data collected monthly for our social groups to investigate the effects of drought on two measures of reproduction: reproductive output and infant survival to the first year (Richard et al., 2002; Veilleux et al., 2016). Sifaka are seasonal breeders, conceiving in the wet season, birthing in early to mid-dry season, and weaning in the following wet season (Figure S2; Lewis & Kappeler, 2005). We defined reproductive output as whether a reproductively mature (subadult or adult) female had given birth in a given year (yes/no). A female was categorized as having given birth if she was observed with an infant in the mid-dry season or if a pregnancy was palpated during morphometric captures in June/July (even if she was not observed with an infant later). Infant survival to the first year was also a yes/no variable based on whether an infant was observed after September 1 of the year following its birth.
For each reproduction measure, we fitted binomial generalized linear mixed effects models (GLMMs) with a log-link function and random effects of mother's identity and year. The null model for both reproduction measures included age class (subadult/adult) as the sole fixed effect. For reproductive output, we performed two additional models: one including drought during the conception period (yes/no) as a fixed effect in addition to age class, and one including drought in the seasonal year before conception (yes/no) as a fixed effect in addition to age class. Similarly, for infant survival, our three drought models included either drought during the infant's birth year (including its conception/gestation), during its weaning period, or in the year before its conception. We noted singularity warnings for some models due to near zero variance of mother's identity, but the results were identical when we reran the models excluding this random effect. We compared each of the drought models to the null model using two-tailed LRTs in R. For all analyzes testing for effects of drought in the year before conception, we excluded data from before 2011 because our rainfall data are only available beginning in the 2011 wet season.
2.6 Behavioral data and analysis
To investigate effects of drought on sifaka behavior, we used focal instantaneous sampling, where the activity of the focal animal is recorded every 10 min. Every hour, observers switch to a different focal animal within the group, with observations typically lasting the full day. For analysis, we restricted data to only include mature individuals (adult/subadult). We focused on behavioral data collected the 6 months before morphometric data collection in June (Dec–May), which corresponds to the “green period” when trees at KMNP are flush with new leaves (Lewis & Axel, 2019). These behaviors are likely to directly impact the morphometric measurements collected in June/July. For each focal individual, we calculated the number of focal point samples per focal month of observation in each activity category, as well as the total number of point samples per focal month. The final dataset included 89,302 point samples (14,884 observation hours) collected over 1230 days and representing 806 focal months (221 drought, 585 typical rainfall) across 12 years (2011–2023). The dataset includes data for 58 individuals, with each individual sampled for an average of 13.9 ± 11.3 focal months, and an average of 15.1 ± 4.6 individuals sampled per year.
We performed two sets of analyzes. First, we investigated the effect of drought on overall time budgets, focusing on the major activity categories that we predicted could be impacted by drought: feed/forage, lick, rest, social (including grooming and play), travel, and rest. For each of these major activities, we performed GLMMs with a zero-inflated negative binomial distribution using the count of sampling points in that activity per month as the response variable, fixed effects of sex (male/female), age class (subadult/adult), and drought (yes/no), an offset factor of total number of sampling points per month, and random effects of focal identity, month, and seasonal year (Dec–Nov). For each activity response variable, we also performed a null model GLMM with the same parameters as the test model excluding the drought factor and compared the drought and null models using two-tailed LRTs.
Next, we investigated the effect of drought specifically on feeding time budgets, focusing on the major food categories that may be impacted by drought: flowers, fruit, seeds, young leaves, and mature leaves. Fruit feeding observations did not discriminate between ripe and unripe fruit and thus represent generalized fruit feeding, while feeding on seeds was defined as explicitly discarding the fleshy pulp to consume the fruit's seed (Veilleux et al., 2016). We also performed an analysis of “sugar” consumption, which combined fruit and flower feeding observations. We performed the same set of GLMMs for the feeding categories as for the activity categories above, except the offset variable was the total number of sampling points spent feeding. We compared null and test models using two-tailed LRTs. All zero-inflated negative binomial GLMMs were implemented using the glmmTMB package in R (Brooks et al., 2017).
3 RESULTS
3.1 Drought effects on food availability
The presence of severe drought had major effects on the phenology and health of focal sifaka food trees and lianas at KMNP. While we observed typical seasonal variation in the relative abundance of sifaka food items (Figure 1 and Table S3), the relative availability of young leaves, mature leaves, and ripe fruit was significantly impacted by the presence of severe drought. However, this effect appears primarily to impact green period (Dec–May) phenology, as evidenced by the significant interaction between resource period and drought (Table S3). Specifically, in the green period, severe drought was associated with reduced abundances of ripe fruit (χ22 = 69.72, p < 0.0001) and young leaves (χ22 = 10.83, p = 0.004) in focal trees/lianas. Conversely, focal plants exhibited relatively greater abundance of mature leaves during drought months compared to non-drought months (χ22 = 107.2, p < 0.0001). Drought was not significant in explaining variation in the relative abundance of unripe fruit (χ22 = 3.41, p = 0.1822) or flowers (χ22 = 1.31, p = 0.518). Additionally, tree/liana mortality was significantly higher in years of severe drought (one-tailed Wilcoxon rank sums test, W = 4.5, p = 0.044).
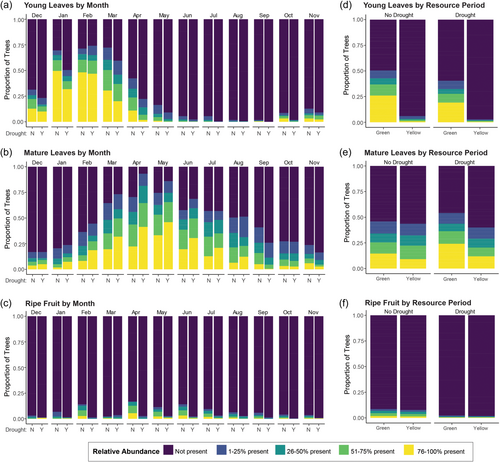
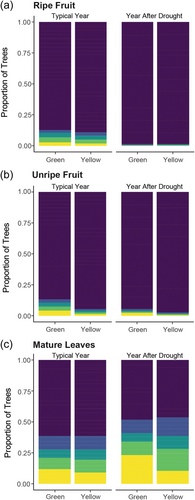
Some studies have reported reduced fruit biomass and increased tree mortality in the 1–2 years following a drought (Campos et al., 2020; Corlett, 2016). At KMNP, the experience of a severe drought the year prior was associated with reduced relative abundance of ripe (χ22 = 47.84, p < 0.0001) and unripe fruit (χ22 = 23.43, p < 0.0001), particularly in the green period (Figure 2a,b and Table S3). There was a tendency for increased relative abundance of mature leaves (χ22 = 5.36, p = 0.068) in the green period following a drought year (Figure 2c). No effects were observed for young leaves (χ22 = 3.36, p = 0.187) or flowers (χ22 = 3.43, p = 0.180). Tree mortality was not significantly higher in the years following a drought than in other non-drought years (W = 7.5, p = 0.50).
3.2 Drought effects on body condition
Although we observed effects of sex/pregnancy status and age on sifaka body condition, we observed no evidence of declining body condition in severe drought (Table 1). For body mass, the best fit model included only sex/pregnancy status and age class. Overall, pregnant females were larger than males (p = 0.030) and nonpregnant females (p < 0.0001), but males and nonpregnant females did not differ (p = 0.874) (Figure S4). Adults also had significantly higher body mass than subadults (p < 0.0001). The best fit model for BMI included only age class, with subadults exhibiting significantly lower BMIs than adults (p = 0.0002 and Figure S4). However, the best fit model for subcutaneous body fat included both sex/pregnancy status and drought (Table 1 and Figure S4). Pregnant females and males did not differ in subcutaneous fat levels, but both had significantly more fat than nonpregnant females (p = 0.032 and 0.001, respectively). Surprisingly, sifaka exhibited higher levels of subcutaneous fat (p = 0.004) during measurements in severe drought years relative to non-drought years. The model suggests that subcutaneous fat increases on average 49.4% (standard error 12.8%) during droughts.
| Modelsa | Body mass | BMI | Total fatb | ||||
|---|---|---|---|---|---|---|---|
| AIC | ΔAIC | AIC | ΔAIC | AIC | ΔAIC | ||
| m1 | Sex/pregnancy only | −53.10 | 62.92 | 513.94 | 11.50 | 465.10 | 7.34 |
| m2 | Age class only | −111.66 | 4.36 | 502.43 | 0.00 | 470.13 | 12.36 |
| m3 | Drought only | −30.47 | 85.54 | 515.19 | 12.76 | 461.88 | 4.11 |
| m4 | Sex/pregnancy + age class | −116.01 | 0.00 | 507.08 | 4.65 | 466.80 | 9.03 |
| m5 | Sex/pregnancy + age class + drought | −109.85 | 6.16 | 509.93 | 7.50 | 459.60 | 1.84 |
| m6 | Sex/pregnancy + drought | −47.29 | 68.72 | 517.08 | 14.65 | 457.77 | 0.00 |
| m7 | Sex/pregnancy × drought + age class | −98.15 | 17.87 | 506.42 | 3.98 | 463.40 | 5.64 |
| m8 | Null model | −37.04 | 78.97 | 511.89 | 9.46 | 469.448 | 11.68 |
- Abbreviation: BMI, body mass index.
- a Models include random effects of individual sifaka and year. We used evidence ratios (ΔAIC) and LRTs to identify the best fit model (Burnham et al., 2011). Best fit models are in bold.
- b For total fat, the addition of age class to m5 did not significantly improve model fit over m6 (χ21 = 1.55, p = 0.212), and age class was not a significant factor in m5 (p = 0.206).
We explored the drought-fat connection further in two ways. First, we used LMMs to examine the effects of drought separately for each of the four skinfold measures that contribute to subcutaneous body fat (Figure 3a, scapular, abdominal, arm, and hip fat). For three measures (scapular, abdominal, arm), the best fit models included drought year and exhibited increased fat values in severe drought (Table S4). For hip fat, the best fit model was the null model. Next, we examined paired subcutaneous fat data for 23 adult sifaka assessed either before/during or during/after a drought in the 2016–2017 and 2022 severe droughts (Figure 3b,c). Note that 12 individuals were assessed before, during, and after a drought. For the 18 measured before/during a drought, we observed significant changes between pre-drought and drought estimates of subcutaneous fat (paired Wilcoxon signed rank test, V = 4, p = 0.001), but not for BMI (V = 31, p = 0.106) or body mass (V = 33.5, p = 0.139) (Figure S5). Our dataset included 20 drought/post-drought pairs (including two individuals sampled during and after the 2016–17 and 2022 droughts). Again, we found that drought estimates of subcutaneous fat were significantly higher than post-drought estimates (drought mean = 3.92, post-drought mean = 2.60; V = 150.5, p = 0.0005), but neither BMI (V = 57, p = 0.587) nor body mass (V = 45.5, p = 0.083) changed significantly (Figure S5). Together, these results suggest that sifaka subcutaneous fat exhibits a different response to severe drought than other body condition measures.
3.3 Drought effects on reproductive success
Given previous reports of drought impacts on reproduction and infant mortality in SDTF mammals (Campos et al., 2020; Gould et al., 1999; Richard et al., 2002), we next investigated the effects of the 2016, 2017, and 2022 severe droughts on two measures of reproduction: reproductive output and infant survival to the first year. We found that on average 81 ± 15% of females were pregnant or gave birth each year. Not surprisingly, age class had a significant effect on pregnancy (p = 0.001) in GLMMs; subadults were less likely to be pregnant or give birth each year. However, drought did not have a significant effect on reproductive output. Including drought during the conception year or the year before conception did not significantly improve model fit over age class alone in LRTs (χ21 = 0.10, p = 0.750 and χ21 = 0.10, p = 0.755, respectively). This result does not change when we restrict analyzes to only adult females (conception year: χ21 = 0.005, p = 0.942; year before conception: χ21 = 0.20, p = 0.654).
We also saw no effect of drought on infant survival through the first year. Eighty-eight sifaka infants were born at KMNP between 2010 and 2022. On average, 49 ± 28% of infants each year survived to their first birthday. We found no significant effect of drought during an infant's birth year (χ21 = 0.10, p = 0.750), its weaning period (χ21 = 0.29, p = 0.589), or during the year before its conception (χ21 = 0.62, p = 0.430) on survival to its first birthday.
3.4 Drought effects on sifaka behavior
We first investigated whether sifaka shift the amount of time they spend engaging in different major activities between green periods (Dec–May) experiencing severe drought and typical rainfall. While we observed age and sex effects on some aspects of sifaka time budgets (Table 2), we found very little evidence of increased energy conservation during drought, contrary to our predictions. Specifically, no significant drought effects were found on any of the major behavioral activities, suggesting that sifaka do not change how much time they spend engaging in social behavior, travel, rest, or feeding/foraging when confronted with severe drought. While we also found no significant effect of drought on time spent licking dew from branches and leaves, we noted that licking was a very rare behavior overall—only 73 instances were recorded over 14,884 observation hours. If we compare the number of focal observation months in which licking occurred rather than the count of licking point samples, significantly more months in which dew licking occurred were in severe drought (one-tailed Fisher's exact test, p = 0.033), suggesting that sifaka do lick dew more during drought.
| Activity | LRTsa | Direction of significant effectsb | |||
|---|---|---|---|---|---|
| Sex (male) | Age class (subadult) | Drought (yes) | |||
| Activity time budget | Feed/forage | χ12 = 0.06, p = 0.808 | – | ||
| Lick | χ12 = 2.08, p = 0.149 | ||||
| Rest | χ12 = 0.37, p = 0.545 | – | |||
| Social | χ12 = 0.23, p = 0.631 | + | + | ||
| Travel | χ12 = 0.83, p = 0.364 | ||||
| Feeding time budget | Fruit | χ12 = 2.39, p = 0.122 | |||
| Flowers | χ12 = 0.33, p = 0.565 | ||||
| Seeds | χ12 = 1.96, p = 0.161 | ||||
| Fruit + flowers | χ12 = 4.08, p = 0.043 | + | |||
| Mature leaves | χ12 = 4.11, p = 0.043 | – | |||
| Young leaves | χ12 = 6.97, p = 0.008 | – | |||
- Abbreviation: LRT, likelihood ratio test.
- a LRT statistics for drought versus null model including only sex and age class. Bolded models include a significant drought effect.
- b Direction of significant effects in best fit models. When drought is not significant, direction and significance of effect reflects null model. Reference categories are female, adult, and no drought. Symbols depict when males, subadults, or drought has a significant effect in the model, either a positive effect (+) or a negative effect (−). Blanks indicate no significant effect of the factor.
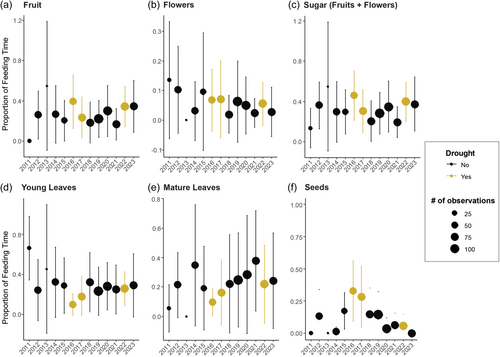
Similarly, we found that drought did not significantly impact most aspects of sifaka green period feeding time budgets (Table 2). Although the proportion of time spent feeding on fruit, flowers, and seeds appears slightly higher in drought compared to neighboring years (Figure 4a,b,f), these differences were not significant after controlling for focal identity, number of observations, month, and seasonal year (Dec–Nov) when examined individually (Table 2). However, when we combined fruit and flowers into a single “sugar” feeding category, sifaka were significantly more likely to spend time feeding on fruits/flowers during drought (Figure 4c). Specifically, they were 39% more likely to spend time feeding on these products (incidence rate ratio (IRR) = 1.39, 95% confidence intervals (CI): 1.03–1.86). By contrast, sifaka were significantly less likely to spend time feeding on leaves (Figure 4d,e) during drought, with drought months characterized by a 30% decrease in the likelihood of feeding on mature leaves (IRR = 0.70, CI: 0.51–0.95) and 47% decrease on young leaves (IRR = 0.53, CI: 0.35–0.80).
4 DISCUSSION
SDTFs represent the second largest type of tropical forest and exhibit high levels of plant and animal biodiversity and endemism (Miles et al., 2006; Stoner & Timm, 2011). These forests are also highly threatened (Miles et al., 2006) and expected to experience more frequent and severe climate change-induced droughts in the coming years (Allen et al., 2017; Siyum, 2020). Nevertheless, SDTFs have historically received less attention than tropical rainforests in conservation and climate change studies (Allen et al., 2017; Siyum, 2020; Stoner & Timm, 2011), particularly in how their animal and plant communities respond to extreme weather events. Here, we investigated how severe droughts have impacted the ecology, health, reproductive success, and behavior of Verreaux's sifaka, a critically endangered mammal from SDTFs in western Madagascar. Our results suggest that pre-existing adaptations to seasonal resource limitation help facilitate drought tolerance in sifaka despite decreased food availability.
4.1 Severe drought affects SDTF phenology
Our long-term survey of sifaka food trees and lianas found that severe droughts significantly impact phenology at the Ankoatsifaka Research Station in KMNP, particularly during the green resource period (Dec–May). During severe drought stress, trees/lianas were less likely to have water-rich products (e.g., young leaves, ripe fruit) and more likely to have mature leaves compared to green periods with typical rainfall. Plant mortality was also significantly higher in years of severe drought. Our results suggest that these drought effects persist even after typical rainfall has returned. Trees and lianas exhibited relatively lower ripe and unripe fruit availability in the year following a severe drought compared to other non-drought years, consistent with reports from Central American STDFs (Campos et al., 2020). Studies investigating the phenological effects of natural droughts and throughfall exclusion experiments tend to focus on tropical rainforests rather than SDTFs (Allen et al., 2017). Most studies have also excluded Madagascar, which has high levels of biodiversity and vascular plant endemism (Goodman & Benstead, 2005). Plant responses to water stress vary considerably between species (de Sousa Leite et al., 2022), and our results suggest that SDTFs in Madagascar can provide valuable data for understanding how SDTF plant communities cope with drought events.
4.2 Sifaka as a mammal model of drought resilience?
Contrary to previous studies of detrimental drought effects on survival and reproduction in other species (Campos et al., 2017, 2020; Maxwell et al., 2019; Richard et al., 2002), we found that sifaka at KMNP appear relatively unaffected by severe drought events. Reproductive output was not significantly different between drought and non-drought years, which is consistent with the sifaka “bet-hedging” life history strategy (Richard et al., 2002). Additionally, no detrimental effect on infant survival or measures of body condition was found. Instead, sifaka exhibited an unexpected and consistent increase in subcutaneous body fat during periods of severe droughts. Additionally, we observed only limited shifts in sifaka time budgets between severe drought and non-drought green periods, with no evidence of an increase in energy conservation behaviors. As predicted, licking dew was more common during drought months. Sifaka were less likely to spend time feeding on young leaves, consistent with their reduced availability in the habitat. They were also less likely to feed on mature leaves, despite a greater relative abundance during drought months. Instead, sifaka appear to increase the time spent feeding on fruit and flower products during drought. Given the reduced availability of water-rich resources, these results suggest that sifaka at KMNP are intentionally seeking out water-rich foods (such as fruit, flowers, nectar) during drought periods. Consumption of water-rich foods is a common adaptation to water scarcity in arid-adapted mammals (Donald & Pannabecker, 2015; Rocha et al., 2021; Rymer et al., 2016). Desert rodents, for example, prefer water-rich foods (Donald and Pannabecker, 2015), and some shift their diets to include more water-rich foods in drier months (Nagy, 1988). While this adaptation has been less well explored in SDTF mammals, similar shifts to water-rich foods have been reported for SDTF bats (Stoner & Timm, 2011) and koalas (Davies et al., 2014).
The increase in fruit and flower consumption during severe drought green periods may actually facilitate additional physiological mechanisms to cope with water scarcity. For example, fructose metabolism stimulates fat storage (Johnson et al., 2020; Lê & Tappy, 2006), which could partially explain the higher subcutaneous fat levels observed in the dry season during drought years. Many arid-adapted species produce metabolic water by metabolizing body fat (Donald & Pannabecker, 2015; Johnson et al., 2016; Rocha et al., 2021; Williams et al., 2001). While this adaptation has not been previously reported for SDTF species, we hypothesize that sifaka use metabolic water production to cope with water scarcity during typical dry seasons. Prior work found that sifaka lose 63%–76% of their subcutaneous fat between wet and dry seasons (Lewis & Kappeler, 2005; Lewis, 2004). Because total food intake does not noticeably differ between seasons (Koch et al., 2017; Norscia et al., 2006; Veilleux et al., 2016) and body mass only decreases 13%–18%, this high seasonal loss of subcutaneous fat could reflect dry season metabolic water production. Additionally, fructose metabolism stimulates the production of arginine vasopressin (Johnson et al., 2020; Wolf et al., 1992) which causes water reabsorption in the kidneys and more concentrated urine (Johnson et al., 2016). Highly concentrated urine is an adaptation for water conservation in arid-dwelling mammals (Donald & Pannabecker, 2015; Rocha et al., 2021; Rymer et al., 2016), and has been observed in sifaka at KMNP (Gnanadesikan et al., 2022). We hypothesize that these adaptations, which may have evolved to help sifaka to cope with seasonal water scarcity in SDTFs, also help buffer them from the negative effects of drought.
Interestingly, our results conflict with those of Richard and colleagues (2000, 2002), who found decreased body mass, reproductive output, and infant survival in Verreaux's sifaka at Beza Mahafaly Special Reserve in southern Madagascar during 3 consecutive years of moderate to extreme drought. But, in addition to involving a more severe and prolonged drought event than our study (Table S2), the plant and animal communities at Beza differ substantially from those at KMNP. Beza primarily consists of deciduous gallery forest dominated by tamarind trees and xeric spiny thicket habitats, and experiences 22% lower average annual rainfall compared to KMNP (Richard et al., 2000, 2002). Thus, Beza may offer fewer or less nutritious food resources for sifaka during severe drought. The animal community at Beza also includes another medium-sized frugivorous diurnal primate (ring-tailed lemur, Lemur catta) which can directly compete with sifaka for fruit (Yamashita, 2002), particularly in times of scarcity. While sifaka at KMNP are sympatric with cathemeral red-fronted brown lemurs (Eulemur rufifrons), work at nearby Kirindy Forest (~52 km away) suggests the two species avoid direct competition during times of scarcity by exploiting different temporal niches, plant species, and canopy levels (de Winter et al., 2013). Sifaka at both sites also share their habitat with folivorous sportive lemurs (Lepilemur ruficaudatus at KMNP, Lepilemur leucopus at Beza), which are nocturnal and much smaller (<1 kg), and do not directly compete for food resources. Nevertheless, the sifaka population at Beza exhibited lower mortality rates during the drought than sympatric ring-tailed lemurs (Gould et al., 1999; Richard et al., 2002), suggesting that sifaka physiological adaptations may still have provided some buffer.
5 CONCLUSION
Climate change is predicted to exacerbate the frequency and severity of extreme weather events in tropical regions, including cyclones and droughts (Dai, 2013; IPCC, 2019; Maxwell et al., 2019), and will likely affect SDTFs like KMNP (Aguirre-Gutiérrez et al., 2020; Allen et al., 2017; Siyum, 2020). Several methods have been developed to assess species vulnerability to climate change (Foden et al., 2019; Pacifici et al., 2017), however, they rely on an accurate understanding of a species's physiological traits and sensitivity to environmental perturbation. Our results suggest that some mammals in SDTFs exhibit physiological adaptations to water scarcity which can buffer them from the negative effects of severe drought. Previous discussions of physiological adaptations in SDTF-dwelling mammals have generally been limited to the ability to delay reproduction, the production of concentrated urine, and rare instances of torpor/hibernation (Stoner & Timm, 2011). We suggest that other adaptations, like fat storage for metabolic water production and “bet-hedging” life history strategy, are present in SDTF mammals and can help these species cope with future droughts. These adaptations may also increase resilience to other extreme weather events—previous work at KMNP, for example, found that sifaka subcutaneous fat and reproductive output were unaffected by a severe cyclone in 2009 that damaged 86% of the feeding trees (Lewis & Rakotondranaivo, 2011). Future work is needed to explore the mechanisms underlying these physiological adaptations for water scarcity and their distribution across SDTF mammals to allow more accurate assessments of species vulnerabilities.
Additionally, our results highlight the importance of considering a species within its broader ecological community when evaluating drought vulnerability. Variation in plant community composition between locations within a species distribution may differentially affect food availability during severe droughts, while the variation in the presence of animal competitors can affect access to available foods. Thus, not only can different species within a habitat vary in drought vulnerability due to physiological traits (Campos et al., 2020; Ferreira et al., 2019), but a single species may be vulnerable to drought at one site in its range but drought tolerant at another.
Finally, although our results suggest that Verreaux's sifaka and other SDFT-dwelling mammals may be more drought tolerant than current models suggest, climate change is not the only threat facing SDTFs. Deforestation and other human activities severely impact SDTFs (Miles et al., 2006), and have led to an estimated ≥80% population decline in sifaka over the last 30 years (Louis et al., 2020). At KMNP, tropical dry forest was reduced by more than a quarter (27%) between 2008 and 2023 (Romanello, 2024). Human activities (including slash and burn agriculture, logging) can change the water available in an ecosystem, and hence, the water content of foods critical for SDTF-dwelling mammals. Combined with poaching (Thompson et al., 2023) and rapid reductions in overall habitat, these anthropogenic disturbances have resulted in Verreaux's sifaka IUCN rating shifting from “Vulnerable” to “Critically Endangered” over the last 14 years (Louis et al., 2020). Consequently, traits such as fat storage may help to mediate some of the negative effects of climate change, but they are unlikely to stop the impending extinctions facing many SDTF-dwelling mammals.
AUTHOR CONTRIBUTIONS
Carrie C. Veilleux: Conceptualization; data curation; formal analysis; investigation; methodology; software; visualization; writing—original draft; writing—review and editing. Stacey R. Tecot: funding acquisition; investigation; methodology; writing—review and editing. Rebecca J. Lewis: conceptualization; data curation; funding acquisition; investigation; methodology; project administration; resources; supervision; writing—review and editing.
ACKNOWLEDGMENTS
We would like to thank the Sifaka Research Project assistants at the Ankoatsifaka Research Station, whose work provided the data analyzed here. We also thank MICET and the University of Antananarivo for facilitating the fieldwork, and Madagascar National Parks and the Madagascar government for permission to collect these data. We are grateful to Clara Scarry and Ann Revill for discussion, and two anonymous reviewers for their valuable suggestions. These data were financed by the University of Texas at Austin, the National Science Foundation Directorate for Social, Behavioral and Economic Sciences (BES #1719654, BES #1719655), the Leakey Foundation, Primate Conservation, Inc., and multiple private donors.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Animal capture and collection protocols were performed in accordance with protocols approved by the University of Texas at Austin Institutional Animal Care and Use Committee (protocol numbers: 08110301, AUP-2011-00143, AUP-2014-0036, AUP-2017-00152, and AUP-2020-00143). This study complies with all established IACUC guidelines and Malagasy law.
Open Research
DATA AVAILABILITY STATEMENT
All data and code used to generate figures in the main text are available on Github at https://github.com/ccveilleux/Veilleux_et_al_2024/. This includes summary data on the relative abundances of young leaves, mature leaves and fruit during the study period, total body fat measures for individuals sampled before/during and/or during/after a drought event, and summary data of monthly feeding behavior. Code for the statistical analyzes in the main text is also available on Github. Individual tree-based and lemur-based raw data is available upon request from R. Lewis.




