Research progress of CRISPR/Cas systems in nucleic acid detection of infectious diseases
Abstract
Infectious diseases are a serious threat to human health, and accurate, rapid and convenient early detection of pathogens is the first step of active treatment. Technologies that detect pathogens have advanced significantly because of the development of fundamental disciplines and the integration of multidisciplinary fields. Among these technologies, nucleic acid detection technology is preferred because of its rapid measurement, accuracy and high sensitivity. The CRISPR/Cas system, consisting of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-associated (Cas), is an adaptive immune system that specifically recognizes, binds and cleaves exogenous invasive nucleic acids. The CRISPR/Cas system is widely found in bacteria and archaea. Researchers have developed nucleic acid detection technologies with single-molecule sensitivity, single-base precision specificity, portability and low cost based on the specific cleavage and trans-cleavage activities of the CRISPR/Cas system. The next generation of in-vitro diagnostics is shifting to nucleic acid technology because this technology shows promise in a wide range of applications in resource-constrained environments. In this review, the development and mechanism of the CRISPR/Cas system are presented together with representative CRISPR/Cas applications in nucleic acid detection. Additionally, the review summarizes future perspectives and trends of the CRISPR/Cas system in nucleic acid detection.
Abbreviations
-
- AuNPs
-
- Au nanoparticles
-
- Cas
-
- CRISPR-associated
-
- crRNA
-
- CRISPR RNA
-
- CRISPR
-
- Clustered Regularly Interspaced Short Palindromic Repeats
-
- dsDNA
-
- double-stranded DNA
-
- G4s
-
- G-quadruplexes
-
- gRNA
-
- guide RNA
-
- ITO
-
- indium-tin-oxide
-
- MB
-
- methylene blue
-
- NTS
-
- non-target strand
-
- PAM
-
- protospacer adjacent motif
-
- PFS
-
- protospacer flanking sequence
-
- POCT
-
- point-of-care testing
-
- PtNP
-
- platinum nanoparticles
-
- reRNA
-
- reporter RNA
-
- RGO
-
- reduced graphene oxide
-
- SA
-
- streptavidin
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2
-
- SERS
-
- surface-enhanced Raman scattering
-
- SPR
-
- surface plasmon resonance
-
- ssDNA
-
- single-stranded DNA
-
- TMB
-
- 3,3′,5,5′-tetramethylbenzidine
-
- TS
-
- target strand
-
- WHO
-
- World Health Organization
1 INTRODUCTION
Infectious diseases pose a serious threat to human well-being. According to the World Health Organization (WHO), the number of deaths caused by infectious diseases accounts for 16.2% of yearly mortality worldwide [1]. In terms of emerging infectious diseases, the Ebola virus swept through Africa's Guinea, Sierra Leone and Liberia in 2014, killing 11 299 people [2]. The Zika virus swept through Latin America in 2015, infecting more than 200 000 people in Brazil. In 2020, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) emerged and has infected approximately 600 million people globally. Foodborne diseases caused by pathogens are prevalent in daily life, and 10% of the world population is diagnosed with foodborne diseases every year [3]. Infectious diseases are often characterized by the sudden and rapid transmission of a pathogen. Thus, convenient, timely and accurate early detection of a pathogen is crucial for effective prevention and treatment. For screening and diagnosing specific diseases, rapid medical detection of the suspected population is required to capture pathogen carriers and patients, and initiate treatment as soon as possible. To detect suspicious samples, a convenient and fast multi-channel detection method is needed to rapidly detect various pathogens that may exist in samples, and thus prevent the spread of the infectious pathogen.
From the conventional plate culture method to enzyme-linked immunosorbent assay [4], immunochromatography [5], nucleic acid amplification technology [6] and molecular hybridization technology, and then to the application of various amplification strategies [7, 8] and biosensing technologies, the detected species of pathogen detection technologies have been reduced from a single colony to a single cell, and even accuracy at the protein and nucleic acid level is available. The detection principles cover chemical reactions, immune reactions, nucleic acid hybridization and the superposition of multiple technologies. Signal conversion and output involve the application of optical, electrical, magnetic and acoustic approaches. With the continuous development of basic disciplines and the integration of multi-disciplinary fields, the detection of pathogens has made significant progress with the development of improved and cost-effective methods. Among them, rapid nucleic acid detection technology has been recognized by the WHO because of its rapid output, accuracy and high sensitivity. During the early stages of the spread of the coronavirus in 2019, nucleic acid amplification technology was recommended by the WHO as the only detection technology for case diagnosis [9].
Although various methods have been developed for nucleic acid detection, most techniques have trade-offs in performance indicators. Among them, the chemiluminescence assay [10] has high sensitivity but involves complex reaction systems and operation steps, whereas nucleic acid hybridization technology [11, 12] has high specificity but insufficient sensitivity. The detection performance of polymerase chain reaction (PCR) [13, 14] is better than hybridization technology but requires a cluster of large or expensive instruments. With emerging infectious diseases and increasing food and environmental contamination incidences, the global demand for point-of-care testing (POCT) is high, especially in underdeveloped countries or resource-limited areas. The CRISPR/Cas nucleic acid detection system has the advantages of single-molecule sensitivity, single-base precision, specificity, simplicity, portability and low cost. This system has gradually become the focus of the next generation of in vitro diagnostic technologies because it can be applied on a large scale in resource-limited environments [15-17].
2 DISCOVERY AND DEVELOPMENT OF CRISPR/CAS SYSTEMS
As early as 1987, Ishino et al. [18], while cloning the coding sequence of the alkaline phosphatase isoenzyme gene in Escherichia coli, unexpectedly discovered a repeat sequence located in the 3′ flanking region of the inhibitor of apoptosis, which consists of five 29-bp-long highly homologous sequence repeats separated by 32-bp-long spacer sequences. Researchers have since found the repeat in various bacterial and archaeal genomes. Mojica et al. [19] named the repeat Short Regularly Spaced Repeats in 2000. In 2002, Jansen et al. [20] officially named this structure Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) with agreement from Mojica. In addition, four CRISPR-related nucleases or helicases have been discovered, collectively termed CRISPR-associated (Cas). Thus, the CRISPR/Cas system was born. In 2005, several studies [21, 22] showed that most spacer sequences are homologous to bacteriophages or conjugative plasmids and that the antisense RNA produced by CRISPR inhibits phage reproduction. CRISPR may also be involved in specific immunity to exogenous DNA. In 2007, Barrangou et al. [23] used a phage-host model system to demonstrate the adaptive immunity of the CRISPR/Cas system, determining that the CRISPR spacer sequence defines the specificity of this immune system and that the Cas protein machinery provides resistance. In 2008, Brouns et al. [24] demonstrated that the transcripts of CRISPR sequences are processed by the Cas protein effector complex into mature CRISPR RNA (crRNA), which specifically guides related Cas proteins to recognize and cleave nucleic acid sequences in phages. In 2011, the mechanism of CRISPR/Cas in the bacterial-acquired immune system was described and includes three stages: adaptation, expression and interference. In the adaptation stage, DNA fragments derived from the phage or plasmid are processed by Cas1, Cas2 and other related proteins and integrated into the CRISPR site between the leader sequence and the first repeat to form spacer sequence units. In the expression stage, the spacer sequence is transcribed into pre-crRNA, which is then processed into mature crRNA with the involvement of the Cas protein effector complex. The crRNA binds to the tracrRNA to form guide RNA (gRNA). When exogenous DNA, such as bacteriophages or plasmids, invades again during the interference stage, crRNA and gRNA direct Cas proteins to cleave exogenous DNA paired with crRNA, preventing the exogenous DNA invasion. The elucidation of this mechanism provides a solid foundation for further application of the CRISPR/Cas system [25, 26].
In 2015, Makarova et al. [27] defined CRISPR/Cas system classification criteria based on whether the effector module of cleaved DNA or RNA has subunit complexes or not and classified CRISPR/Cas systems into two classes: class 1 with multi-subunit crRNA-effector complexes and class 2 with single Cas effector proteins. By 2020, Makarova et al. [28] had divided the identified CRISPR/Cas systems into six types and 33 subtypes, with types I, III and IV belonging to class 1 and types II, V and VI belonging to class 2. Most CRISPR/Cas systems involved in nucleic acid detection technology are class 2 (Table 1).
| Type | II | V | VI | |||||
|---|---|---|---|---|---|---|---|---|
| Effector protein | Cas9 | Cas12a | Cas12b | Cas12c1/2 | Cas12h1 | Cas13a | Cas13b | Cas13d |
| Substrate | dsDNA* | dsDNA/ssDNA* | RNA | |||||
| Domain | HNH & RuvC | RuvC | 2×HEPN | |||||
| PAM*/PFS* | 3′NGG | 5′TTTV | 5′TTN | 5′TG/N | 5′RTR | 3′non-G | 3′non-C | − |
| Trans-cleavage | None | ssDNA | ssRNA | |||||
- Abbreviations: *dsDNA, double-stranded DNA; *PAM, protospacer adjacent motif; *PFS, protospacer flanking sequence; *ssDNA, single-stranded DNA.
3 MECHANISMS OF CRISPR/CAS SYSTEMS FOR NUCLEIC ACID DETECTION
3.1 Cas9
Cas9 is a type II CRISPR/Cas system containing two nuclease domains, HNH and RuvC, and is an endonuclease that specifically cleaves double-stranded DNA (dsDNA) under the guidance of gRNA (Table 1). gRNA contains a crRNA and a tracrRNA. The 3′ end of crRNA is paired with tracrRNA, whereas the 5′ end is paired with the target sequence [29]. Upon invasion of exogenous dsDNA, Cas9 binds to gRNA to form the Cas9/gRNA complex. This complex specifically recognizes the G-rich protospacer adjacent motif (PAM) at the 3′ end of exogenous dsDNA under the guidance of gRNA and forms the Cas9/gRNA/dsDNA ternary complex with exogenous dsDNA. The HNH domain then cleaves the target strand (TS) complementary to crRNA, and the cleavage site is 3 bp upstream of PAM. The RuvC domain cleaves the non-target strand (NTS) that is not complementary to crRNA, and this cleavage site is 3–8 bp upstream of PAM, forming a flat end, and the exogenous dsDNA breaks [30] (Figure 1a). Deactivated Cas9 (dCas9), another form of Cas9, retains a specific sequence binding ability after losing the enzyme activity [31] and is widely used in developing nucleic acid detection technology alongside Cas 9.
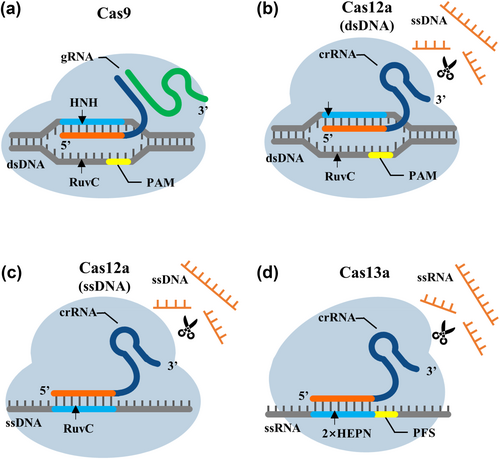
Fundamental differences in CRISPR/Cas nucleic acid detection systems. (a) Mechanism of CRISPR/Cas9. (b) Mechanism of CRISPR/Cas12a recognition of dsDNA. (c) Mechanism of CRISPR/Cas12a recognition of ssDNA. (d) Mechanism of CRISPR/Cas13a.
3.2 Cas12
Cas12 is a type V CRISPR/Cas system and the first Cas protein identified to trans-cleave single-stranded DNA (ssDNA) [32]. Cas12 has over a dozen subtypes, including Cas 12a, Cas 12b and Cas 12i. Different subtypes of Cas12 are guided by different crRNAs or gRNAs and have different PAM specificities. Cas12a recognizes the 5′ TTTV PAM, Cas12b, Cas12i1 and Cas12i2 recognize the 5′ TTN PAM, Cas12h1 recognizes 5′ RTR PAM, and Cas12c2 and Cas12c1/OspCas12c recognize short 5′ TN and 5′ TG PAMs [33-35] (Table 1). Cas12a contains only a single RuvC domain, which binds directly to crRNA of 42–44 nt in length to form Cas12a/crRNA complexes. This complex specifically recognizes the T-rich PAM at the 5′ end of exogenous dsDNA and forms a Cas12a/crRNA/dsDNA ternary complex with exogenous dsDNA, thereby activating the trans-cleavage activity of Cas12a and making Cas12a an ssDNA cleaving enzyme. Any exposed ssDNA in the system is cleaved to 2–4 nt at a specific temperature. Concurrently, the NTS of the target dsDNA unwinds to expose its cleavage site, and only NTS strand breaks stabilize the TS cleavage conformation of the Cas12a complex, ensuring that Cas12a uses a single RuvC domain to complete the ordered cleavage of dsDNA to form a sticky end [36, 37] (Figure 1b). In addition to recognizing target dsDNA, Cas12a recognizes target ssDNA and specifically cleaves near its 22nd position; this process does not require PAM [38] (Figure 1c). Cas12f, formerly known as Cas14, is similarly able to recognize and specifically cleave target ssDNA, again without the need of PAM [39]. Cas12f is relatively short, with approximately 400–700 amino acids, which is only half that of other Cas12 proteins and one-third that of Cas9. Cas12f recognizes target ssDNA more specifically, enabling high-fidelity single-nucleotide polymorphism (SNP) genotyping [40].
3.3 Cas13
Cas13 is a type VI CRISPR/Cas system and includes a Cas protein that trans-cleaves RNA under the guidance of crRNA. Currently, 13 subtypes have been distinguished: VI-A, VI-B, VI-C and VI-D. Among them, Cas13a recognizes a 5′ non-G protospacer flanking sequence (PFS) in nucleotides 13–24 bp downstream of the crRNA binding site. Cas13b requires a double-sided PFS: a downstream 5′ non-C PFS and a NAN/NNA sequence in nucleotides 12–26 bp upstream. Recognizing PFS by Cas13d is not required [41, 42] (Table 1). Cas13a lacks the DNase domain and has two conserved HEPN domains involved in RNA cleavage. Therefore, the Cas13a/crRNA complex specifically recognizes the PFS at the 3′ end of exogenous RNA, forming a Cas13a/crRNA/RNA ternary complex with the exogenous RNA [43]. Among them, the double-stranded RNA formed by crRNA and target RNA causes a conformational change in the HEPN domain of Cas13a and activates the trans-cleavage activity of Cas13a, making Cas13a an RNA-cleaving enzyme that indiscriminately cleaves arbitrarily exposed RNA in the system at a specific temperature [44] (Figure 1d). Researchers [45, 46] have also conducted further studies on the active site, recognition and cleaving mechanism of Cas13, which provides important information for subsequent applications of CRISPR/Cas13.
4 APPLICATION OF CRISPR/CAS SYSTEMS IN DETECTING INFECTIOUS DISEASES
The CRISPR/Cas system is widely used in nucleic acid detection of infectious diseases. Fluorescence response-based CRISPR/Cas sensing systems, electrochemical response-based CRISPR/Cas sensing systems, chromogenic response-based CRISPR/Cas sensing systems and other response-based CRISPR/Cas sensing systems are available (Figure 2).
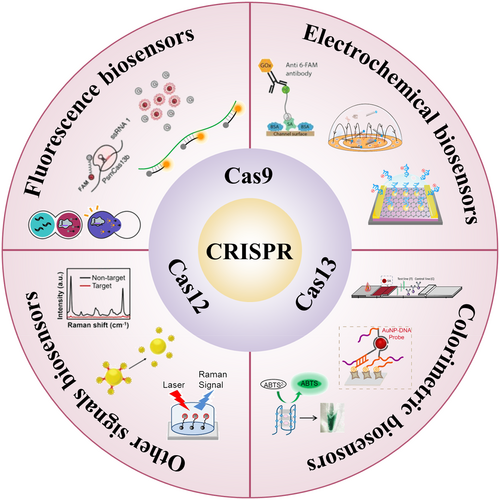
Application of CRISPR/Cas systems in nucleic acid detection of infectious diseases.
4.1 Fluorescence response-based CRISPR/Cas sensing systems
Fluorescent biosensors have the advantages of high sensitivity, short analysis time and simple operation and are widely used in nucleic acid detection. Relatively diverse fluorescent dyes and advanced dye modification technologies can support the demand for reagent materials required for nucleic acid detection. Researchers have combined fluorescent dyes with CRISPR/Cas systems to develop a variety of fluorescence response-based CRISPR/Cas sensing systems that afford better detection performance (Figure 3). The specific cleavage activity of the CRISPR/Cas9 system and the unique trans-cleavage activity of the CRISPR/Cas12 and Cas13 systems are commonly used in the construction of fluorescent biosensors. In particular, based on the characteristic that Cas12/Cas13 become ssDNA/ssRNA cleaving enzymes after activation by target nucleic acids, researchers modified fluorophores and quenchers at both ends of ssDNA/ssRNA and used them as fluorescent probes to report the presence of the target nucleic acid. With ssDNA/ssRNA cleavage, the fluorophore detaches from the influence of the quencher to fluoresce. Depending on the intensity of the fluorescent signal, the qualitative or even quantitative detection of the target nucleic acid can be realized. According to different signal reading methods, the CRISPR/Cas sensing system can be divided into fluorescence instrument reading, smartphone reading and visual observation sensing systems.
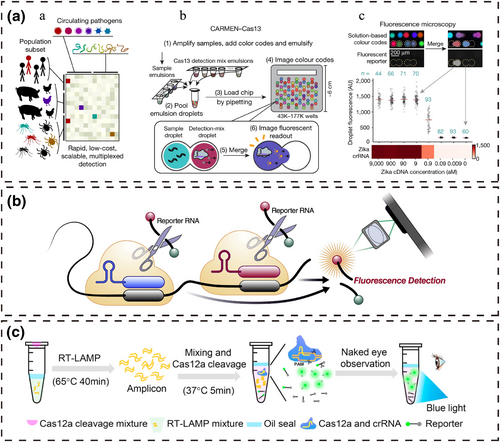
4.1.1 Fluorescence instrument reading
Huang et al. [47] developed CAS-EXPAR, a novel nucleic acid amplification technique that combines Cas9-specific cleavage with EXPAR constant temperature rapid amplification. The CRISPR/Cas9 system recognizes, binds and cleaves the target DNA through PAM to generate specific fragments. The specific fragments are then used as primers to amplify the reaction performed cyclically to generate numerous DNA replicates, which are detected using a real-time fluorescence monitoring system. Compared with conventional nucleic acid amplification techniques, CAS-EXPAR does not require exogenous primers and avoids non-specific amplification of the target. SHERLOCK is a nucleic acid detection system developed by Gootenberg et al. [48] based on Cas13, which can independently detect RNA viruses and mRNA and combine amplification techniques and DNA transcription for DNA detection. This technology can be used to detect viral loads and distinguish different strains. Combined with HUDSON technology [49], direct detection of whole blood, plasma, serum, urine or saliva samples can be achieved. Subsequently, Gootenberg et al. [50] built a quantitative, highly sensitive and multiplexable nucleic acid detection platform, SHERLOCK v2, based on SHERLOCK, which is based on four proteins: LwaCas13a, PsmCas13b, CcaCas13b and AsCas12a. SHERLOCKv2 is limited by the type of Cas protein and its cleavage propensity, which hampers the simultaneous detection of dozens or even more target nucleic acids. In contrast, the CARMEN [51] microwell-array system combines Cas13 and different crRNAs to achieve high-throughput, miniaturized, scalable and self-organizing simultaneous detection of hundreds of pathogens. CARMEN has a wide range of uses for distinguishing viral species, strains and SNPs and can be rapidly expanded to perform high-throughput detection of novel pathogens, which is of great benefit to patients and public health. CARMEN is the first scalable high-throughput detection platform based on the CRISPR/Cas system, which will undoubtedly push the CRISPR/Cas system to new heights in nucleic acid detection.
4.1.2 Smartphone reading
Doudna et al. [52] reported developing an amplification-free CRISPR-Cas13a assay to detect SARS-CoV-2 from nasal swab RNA that can be read using a mobile phone microscope. This research team combined Cas13a with various crRNAs to generate different RNPs that act in series on the same RNA target region and achieved 100 copies/μL sensitivity in under 30 min of measurement time. Subsequently, the number of RNPs was increased to eight and concatenated with Csm6 to achieve higher sensitivity and a faster detection time of RNA targets without amplification [53]. Ning et al. [54] developed an RT-RPA CRISPR-fluorescence detection system assay to sensitively quantify SARS-CoV-2 present in saliva without RNA isolation and adapted this assay to a smartphone-read chip format. The built-in smartphone camera app was used to focus on samples and capture images, removing the need for mechanical focusing and lowering the weight and cost of the device while improving its optical stability and usability.
4.1.3 Visual observation
Wang et al. [55] developed a CHP system for the high-fidelity and sensitive detection of dsDNA by combining the CRISPR/Cas9 system with two isothermal amplification techniques. Cas9/gRNA can specifically recognize and cleave dsDNA, leading to NTS exposure. Exposed NTS binds to the hairpin probe to initiate strand displacement amplification, and the amplification product triggers subsequent rolling circle amplification to amplify long ssDNA. Long ssDNA can be used to open the fluorescent hairpin probe and complete the reporting of target dsDNA. Wang et al. [56] developed a one-pot visual reverse transcription (RT)-LAMP-CRISPR method for ultrasensitive visual detection of SARS-CoV-2, which simplified operations and avoided contamination. The RT-LAMP reagents were incubated at the base of the tube. The CRISPR/Cas12a reaction reagents were added to the lid, which was added to the reaction system by an external force after the completion of the RT-LAMP reaction. In the presence of target DNA, the fluorescent probes are trans-cleaved by Cas12 to generate fluorescence, and the fluorescent signal is visible under blue light.
4.2 Electrochemical response-based CRISPR/Cas sensing systems
The electrochemical biosensor, the earliest type of biosensor, has the advantages of high sensitivity, simple structure, simple operation and low cost. Researchers make full use of these advantages by combining this type of biosensor with the high specificity and accuracy of CRISPR/Cas systems to establish electrochemical response-based CRISPR/Cas sensing systems, which can further enhance the detection performance of the electrochemical biosensor (Figure 4). During system construction, researchers typically establish a chimeric or covalent modification relationship between the electrochemical indicator and DNA/RNA, and then use specific recognition of target nucleic acids and trans-cleavage of ssDNA/ssRNA by Cas to complete the establishment or disruption of the above relationship. The target nucleic acid concentration was reported by comparing changes in the electrochemical signal intensity. Depending on whether the reaction reagent needs to be fixed on the electrode surface, the CRISPR/Cas sensing systems can be divided into immobilized and non-immobilized electrochemical biosensing systems.
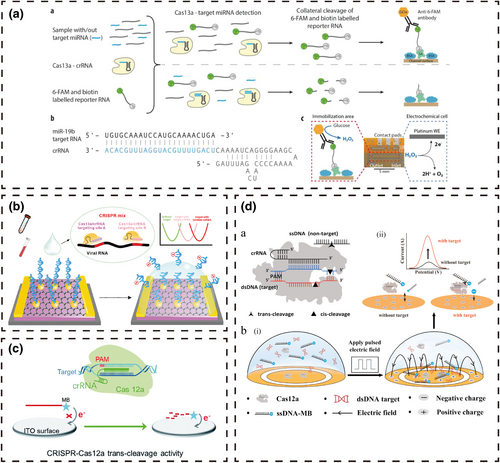
4.2.1 Immobilized electrochemical biosensing systems
Researchers [57-61] covalently modified the electrochemical indicator with agents such as methylene blue (MB) to one end of the DNA or RNA to form an electrochemical probe and fixed the other end of the probe to the electrode surface using the difference in electrochemical signals generated by the physical distance between MB and the electrode surface to report whether the target DNA exists in the reaction system. In contrast, Bruch et al. [62] fixed streptavidin (SA) on the surface of the carrier to link biotin-RNA-fluorescein and then linked anti-fluorescein antibody-glucose oxidase via fluorescein. In the presence of the target RNA, activated Cas13a trans-cleaved the biotin-RNA-fluorescein, releasing glucose oxidase. After cleaning the microchannel, a certain concentration of glucose solution was injected, and the RNA content was determined by measuring the current density generated by H2O2, the oxidation product of glucose. In addition, DNA/RNA electrical signals without electrochemical indicators or glucose oxidase were directly detected. Li et al. [63] modified Au nanoparticles (AuNPs) on the surface of reduced graphene oxide (RGO) and fixed thiolated reporter RNA (reRNA) on the surface of AuNPs for reporting target RNA. The pre-incubated Cas13a/crRNA/RNA ternary complex was then placed onto the surface of the RGO-EFT chip. Using the trans-cleavage activity of Cas13a to cleave the reRNA immobilized on the chip induced p-doping with a positive shift of the Dirac point, with the displacement reflecting the target RNA concentration. Hajian et al. [64] combined catalytically inactivated Cas9 with gRNA and fixed this complex on a graphene field effect transistor. The change in graphene conductivity triggered by the specific binding of these two molecules to target dsDNA reported the presence of the target dsDNA. The label-free nucleic acid detection chip can be used with a handheld reader, and detection can be completed within 15 min with a sensitivity of 1.7 fM.
4.2.2 Non-immobilized electrochemical biosensing systems
Researchers [65, 66] modified electrochemical indicators such as MB at the end of DNA or RNA to make electrochemical probes, which are free in the system. They used the differences in signals of the electrochemical probes caused by steric hindrance and diffusion coefficient changes to report the presence or absence of target DNA in the system. Liu et al. [65] used the trans-cleavage activity of Cas12a on ssDNA combined with the diffusivity difference and electrostatic repulsion of the ssDNA-MB probe on a negatively charged indium-tin-oxide (ITO) electrode to prepare a simple, non-immobilized and homogeneous electrochemical biosensor for detecting human HPV-16. Specifically, the ssDNA-MB probe cannot freely diffuse to the surface of the negatively charged ITO electrode because of the strong electrostatic repulsion, giving rise to a weak electrochemical signal. The presence of target DNA activates the trans-cleavage activity of the CRISPR/Cas12a system, which cleaves the negatively charged ssDNA-MB probe to release MB. Because of higher diffusivity and less electrostatic repulsion, the smaller MB diffuses more readily to the surface of the ITO electrode to enhance the electrochemical response. Li et al. [66] added a pulsed electric field on this basis to increase the distance between the negatively charged ssDNA-MB probe and the electrode surface, reducing the background and enhancing the signal-to-noise ratio. With the help of pulsed electric fields, the detection limit of the reaction system without the assistance of amplification techniques is 1 pM.
4.3 Chromogenic response-based CRISPR/Cas sensing systems
Compared with fluorescence and electrochemical responses, chromogenic response-based CRISPR/Cas sensing systems are more cost-effective and simpler, requiring no precision instruments or supporting equipment. There are numerous ways to build CRISPR/Cas sensing systems with a chromogenic response, including lateral flow chromatography, homogeneous chromogenic and catalytic chromogenic (Figure 5).
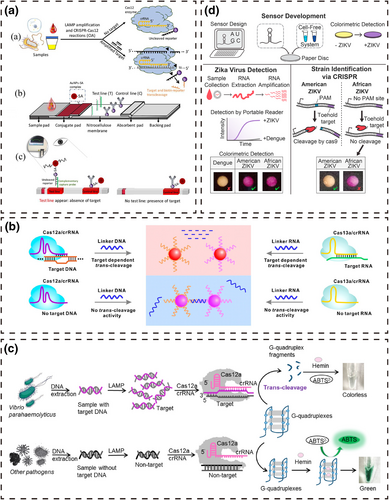
CRISPR/Cas sensing systems based on a chromogenic response. (a) Lateral flow chromatography [72]. (b) Homogeneous chromogenic response [73]. (c) Enzyme mimics that catalyze a reaction to generate a chromogenic response [75]. (d) Enzymes that catalyze a reaction to generate a chromogenic response [77].
4.3.1 Lateral flow chromatography
Lateral flow chromatography based on chromogenic substances such as colloidal gold or latex microspheres has the advantages of simple operation, rapid reaction time and low cost. Researchers have combined lateral flow chromatography with CRISPR/Cas systems to achieve better detection performance and have established various nucleic acid detection systems based on a chromogenic response. Researchers [67-71] combined the amplification technology with the CRISPR/Cas9 system to realize the convenient detection of target DNA based on lateral flow chromatography strips. Initially, target DNA is labeled with biotin, fluorescein isothiocyanate, digoxin or carboxyfluorescein at the end of the amplification primer and then combined with the Cas9/gRNA complex or the Cas9/gRNA complex containing modified groups to form a ternary complex. AuNPs conjugates bind to the target DNA, gRNA or modifier group in the ternary complex to form a quaternary complex, which is then captured by SA, complementary ssDNA or a secondary antibody fixed to the test line, thereby reporting the target DNA by the appearance of a band. Wang et al. [69] incubated biotin-labeled target dsDNA amplification products with Cas9/gRNA to form ternary complexes and placed them on a sample pad. After lateral flow, the stem-loop structure of gRNA in the ternary complexes hybridized with the AuNPs-DNA probes to form quaternary complexes. The quaternary complexes continued to flow to the test line and were captured by fixed SA, while the remaining AuNPs-DNA probes continued to flow to the control line and were captured by the capture probe. Continuous accumulation of AuNPs increases the intensity of the test and control lines, and the presence or absence of the target DNA can be judged by the color indicator. On this basis, Xiong et al. [70] added digoxin-labeled amplification primers to achieve a simultaneous dual-gene diagnosis.
The unique trans-cleavage activity of the CRISPR/Cas12 and Cas13 systems facilitates more novel system construction methods. Mukama et al. [72] placed AuNPs-SA on the conjugate pad to conjugate biotin-ssDNA or biotin in the reaction product and generated AuNP reporter complexes. Single-strand capture probes complementary to ssDNA were fixed at the test line to capture the AuNPs-SA-biotin-ssDNA reporter complexes via base complementation. Biotinylated rabbit polyclonal antibodies immobilized on the control line were used to capture excess AuNPs reporter complexes by binding to SA. The presence of Pseudomonas aeruginosa DNA in the system caused the CRISPR/Cas12a/dsDNA ternary complex to trans-cleave biotin-ssDNA, and the single-stranded capture probe no longer captured the AuNPs-SA-biotin reporter complexes, thus reporting the target DNA as a non-detectable test line.
4.3.2 Homogeneous chromogenic systems
Homogeneous chromogenic systems are simpler than lateral flow chromatography systems. Researchers used the transition of aggregation or dispersion between AuNPs and CRISPR/Cas systems to establish more simple chromogenic response nucleic acid detection platforms. Yuan et al. [73] developed a chromogenic foodborne pathogen detection system based on the trans-cleavage activity of the CRISPR/Cas12a and Cas13a systems and the distance-dependent optical properties of AuNPs-DNA probes. In this strategy, the AuNPs-DNA probes are connected by ssDNA or ssRNA to cause aggregation of the AuNPs-DNA probes, and the solution color is magenta. After low-speed centrifugation, the probe settles to the bottom, and the solution is colorless. When the target nucleic acid is present in the system, the activated Cas12a or Cas13a cleave ssDNA or ssRNA, causing dispersion of the AuNPs-DNA probes and the solution color changes from magenta to red. Moreover, the dispersed AuNPs-DNA probes are less affected by centrifugation, and the solution remains red after centrifugation. On this basis, Zhou et al. [74] greatly improved the signal-to-noise ratio of the detection system by improving the DNA hybridization buffer and were able to detect 6.4 × 102 CFU/mL of Staphylococcus aureus ST398 within 50 min.
4.3.3 Catalytic chromogenic systems
Researchers have combined a catalytic chromogen and the CRISPR/Cas system to achieve chromogenic detection using the catalytic effect of enzymes or enzyme mimics on substrates to report target nucleic acids. Chen et al. [75] proposed a sequence-specific, homogeneous and label-free chromogenic technique for detecting Vibrio parahaemolyticus. The target DNA amplification product activates the trans-cleavage activity of the CRISPR/Cas12a system, allowing this system to arbitrarily cleave G-quadruplexes (G4s) and inhibit its peroxidase mimic activity. The intact G4s conjugate hemin to catalyze ABTS2− to ABTS, and the solution color changes from colorless to green. When the G4s structure loses the activity of the peroxidase mimic, the catalytic process no longer occurs, and the solution is colorless. Based on this principle, Yin et al. [76] replaced the catalytic substrate of G4s with 3,3′,5,5′-tetramethylbenzidine (TMB). The combination of G4s and hemin chloride catalyzes TMB to produce oxTMB under the action of K+, and the solution changed from colorless to blue, enabling the rapid detection of Salmonella SPP.
In addition to the nucleic acid sequences with an enzyme mimic activity, proteases produced by translation can participate in catalytic color development. Collins et al. [77] combined isothermal nucleic acid amplification technology with the toehold switch sensor and the CRISPR/Cas9 system to develop a chromogenic system that distinguishes Zika genotypes with single-base resolution. For the American strain, the CRISPR/Cas9 system cleaves the amplified product to yield short DNA by recognizing the PAM. The short RNA produced by transcription cannot activate the sensor H toehold switch, preventing LacZ production. For the African strain, the CRISPR/Cas9 system cannot effectively recognize the PAM and does not cleave the DNA. The complete RNA produced by transcription activates the sensor H toehold switch to produce the LacZ enzyme, which converts the yellow substance on the paper platform to the purple substance. Thus, the genotype of the Zika virus can be discerned only by the color change on the paper platform. Compared with other detection strategies, the chromogenic response-based CRISPR/Cas12 sensing system can fully use the high sensitivity and specificity of the CRISPR/Cas12 system to convert target nucleic acids into visual signals for output, broadening the application scope.
4.4 Other response-based CRISPR/Cas sensing systems
In addition to the standard fluorescence, electrochemistry and chromogenic sensing systems described above, other response-based CRISPR/Cas nucleic acid detection systems include surface-enhanced Raman scattering (SERS), surface plasmon resonance (SPR) and displacement. CRISPR/Cas sensing systems based on other responses have excellent detection performance and application potential for detecting pathogens (Figure 6).
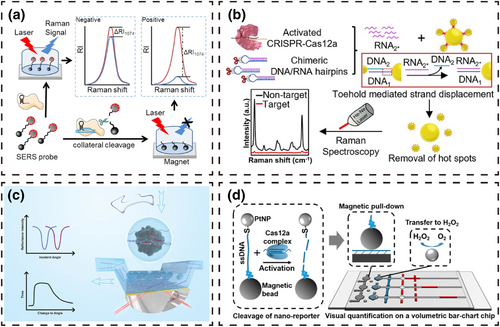
SERS is a spectroscopic technique with ultra-high sensitivity and a fingerprint characteristic map that can achieve single-molecule detection and has been widely used in biochemical analyses. Liang et al. [78] prepared SERS probes by modifying AgNP@4ATP and magnetic beads at both ends of ssDNA and combined the probes with the CRISPR/Cas12a system to develop an amplification-free, sensitive and specific SARS-CoV-2 detection method. When target DNA exists in the system, the activated CRISPR/Cas12a system trans-cleaves the SERS probes to separate AgNP@4ATP and the magnetic beads. The reaction system is then washed several times with the help of an external magnet, and SERS detection is carried out after removing AgNP@4ATP. The Raman signal is weak compared to the system without target DNA. Because steric hindrance of the modified nanomaterials at both ends of ssDNA limits the cleavage of ssDNA by the CRISPR/Cas12a system, Yin et al. [79] designed a SERS probe and a DNA/RNA chimeric hairpin probe to replace direct cleavage of ssDNA by the bulky CRISPR/Cas12a system with a small volume of RNA. AuNPs-DNA1 was used as the core of the SERS probes, and multiple AuNPs-DNA2 coated with 4-mercaptobenzoic acid were linked by complementary DNA pair DNA1/DNA2. Target DNA in the system activates the CRISPR/Cas12a system, which arbitrarily cleaves the DNA/RNA chimeric hairpin probe to release a large amount of RNA. The released RNA completely complements DNA1, separating the AuNPs-DNA2 from the core AuNPs-DNA1 surface and thus lowering the SERS signal.
SPR is a non-destructive technique for monitoring molecular interactions and has been widely used in clinical diagnosis and bioanalysis. Zheng et al. [80] took advantage of the high specificity of the CRISPR/Cas system and the high sensitivity of the SPR sensor to develop a biosensor for real-time quantitative detection of DNA. In this method, a uniform graphene film was grown on the surface of an Au metal layer by the Glaser-Hay coupling reaction, and the graphene film was then further functionalized with the molecular linker 1-pyrenebutyric acid N-hydroxysuccinimide ester to obtain the -NHS active group. The -NHS active group was used to immobilize the dCas9/gRNA complex on the surface of the graphene film. In the presence of target DNA, the dCas9/gRNA complex increases the surface quality of the SPR sensor, resulting in an enhanced refractive index of the gold surface to the same proportion, and the incidence angle also changes. Target DNA is detected by monitoring the change in the angle.
In addition to the above approaches, the displacement response can be used as a signal reporting mode and combined with the CRISPR/Cas system to detect pathogens. Shao et al. [81] reported a visual nucleic acid detection platform combining the CRISPR/Cas12a system, reporter magnetic bead-ssDNA-platinum nanoparticles (PtNP) and magnetic assistance. In this method, the CRISPR/Cas12a system activated by target DNA arbitrarily cleaves the magnetic bead-ssDNA-PtNP reporter to release PtNPs. Magnetic assistance is then used to complete the transfer of PtNPs to catalyze H2O2 to produce O2, which drives the movement of red ink. Quantitative detection of nucleic acids can be achieved according to the displacement length.
5 CONCLUSIONS AND FUTURE PERSPECTIVES
The development and mechanism of CRISPR/Cas systems have been introduced in this review and representative applications of three typical CRISPR/Cas systems, Cas9, Cas12 and Cas13, for detecting nucleic acids from infectious pathogens have been described and summarized (Table 2).
| Responses | Systems | Types | Targets | Amplification | Sensitivity | Time |
|---|---|---|---|---|---|---|
| Fluorescence | CAS-EXPAR [47] | Cas9 | L. monocytogenes | EXPAR | 0.82 aM | 60 min |
| DNA-FISH [82] | dCas9 | MRSA | − | 10 CFU/mL | 60 min | |
| HOLMES [83] | Cas12a | JEV, RPV | PCR | 10 aM | 60 min | |
| DETECTR [84] | Cas12a | HPV16/18 | RPA | aM | 40–70 min | |
| HOLMESv2 [85] | Cas12b | DNA, RNA | LAMP | 10 aM | 60 min | |
| SHERLOCK [48] | Cas13a | Zika, Dengue | RPA | 2 aM | 2–3 h | |
| SHERLOCKv2 [50] | Cas12/13 | DNA, RNA | RPA, RT-RPA | 2 aM | <2 h | |
| Electrochemical | CRISPR-CHIP [64] | dCas9 | DNA | − | 1.7 fM | 15 min |
| E-CRISPR [57] | Cas12a | DNA | − | 50 pM | 60 min | |
| CRISPR/Cas [58] | Cas12a | DNA | − | 30 pM | 60 min | |
| E-CRISPR [59] | Cas12a | L. monocytogenes | RAA | 26 CFU/mL | 75 min | |
| EFE [66] | Cas12a | HPV-16 | − | 1 pM | 60 min | |
| One-for-all [62] | Cas13a | RNA | − | 10 pM | <4 h | |
| CRISPR-FET [63] | Cas13a | HCV | − | 1.56 aM | 30 min | |
| Chromogenic | CASLFA [69] | Cas9 | ASFV | RPA, PCR | 150 copies | 40 min |
| CRISPR/Cas [77] | Cas9 | Zika | NASBA | fM | ~3 h | |
| Bio-SCAN [67] | dCas9 | SARS-CoV-2 | RPA | 6.67 aM | 24 min | |
| CIA [72] | Cas12a | P. aeruginosa | LAMP | aM | 50 min | |
| Cas12a-LFD [86] | Cas12a | ASFV | − | 20 copies | 60 min | |
| CRISPR/Cas [75] | Cas12a | V. parahaemolyticus | LAMP | 9.8 CFU | ~3 h | |
| CRISPR/Cas [73] | Cas12/13 | ASFV | − | 500 fM | 60 min | |
| Other | CRISPR/Cas [87] | dCas9 | S. aureus, | − | fM | 70 min |
| A. baumannii, | ||||||
| K. pneumoniae | ||||||
| CRISPR-eSPR [80] | dCas9 | DNA | 1.3 fM | 5 min | ||
| SERS-CRISPR [78] | Cas12a | SARS-CoV-2 | − | 1 fM | 30–40 min | |
| CRISPR/Cas [79] | Cas12a | DNA | − | 1 aM | 2–3 h | |
| CRISPR/Cas [81] | Cas12a | SNV | − | 0.01% | 40 min |
Compared with traditional nucleic acid detection techniques such as molecular hybridization and PCR, the CRISPR/Cas sensing system has clear advantages in sensitivity and detection time, with significant potential for detecting pathogens. However, the sensitivity of the CRISPR/Cas sensing system depends heavily on the efficiency of nucleic acid amplification technology. Without the assistance of nucleic acid amplification technology, the detection limit of most CRISPR/Cas sensing systems is pM or fM, with detection at the aM level challenging. With the assistance of nucleic acid amplification techniques such as RPA, PCR and LAMP, the detection limit of most CRISPR/Cas sensing systems reaches the aM level. Therefore, CRISPR/Cas sensing systems supplemented with nucleic acid amplification technology are more popular. Compared with the two-step reaction, the one-pot reaction is simple to operate and avoids contamination. However, the one-pot reaction is difficult to carry out because of (i) differing reaction temperatures between the nucleic acid amplification technology step and the reaction with the CRISPR/Cas system, (ii) the influence of reagent components and (iii) interference in the reaction process. Therefore, seeking suitable proteases, replacing interfering substances and reconstructing the appropriate reaction system should resolve existing two-step reaction issues to realize one-pot reactions.
CRISPR/Cas nucleic acid detection has a promising future for simultaneously detecting multiple pathogens. The current multicolor nucleic acid detection systems designed according to the types of Cas proteins and their cleavage propensity cannot meet the demands of high-throughput detection because of the restrictions of Cas proteins [50]. However, multi-compartment nucleic acid detection based on microfluidic chips should remove the limitation of Cas protein types and only require various crRNA sequences to complete the simultaneous detection of numerous pathogens. Furthermore, multiple small-volume CRISPR/Cas reactions can be performed simultaneously using microfluidic chips, yielding higher throughput and lower reagent production costs.
Since the first report of CRISPR/Cas system-based nucleic acid detection technology, most have used fluorescent signals as a response combined with optical instruments for signal detection. Despite the high sensitivity and accuracy of optical detection instruments, implementing these specialized instruments into everyday household use is challenging and prohibitively expensive. In contrast, home electrochemical detection devices represented by blood glucose meters expand the idea of POCT and promote the application of CRISPR/Cas systems in the electrochemical detection field. Electrochemical detection devices perform equally well as optical instruments while offering a simpler, more affordable and portable option. Although many CRISPR/Cas-based electrochemical nucleic acid detection technologies have been reported, most require reporter molecules or other specific substances to be fixed on the electrode surface, inevitably resulting in repeatability issues. Moreover, steric hindrance caused by this operation also affects the efficiency of Cas protein cleavage and the electron conduction rate of the electroactive substance, which affects the precision of the results [62, 65, 66]. In practice, a simple, sensitive and powerful nucleic acid detection system is far superior to an elaborate complex system. Therefore, in electrochemistry and the CRISPR/Cas system, it remains crucial to develop non-immobilized, homogeneous, simple and sensitive nucleic acid detection technologies [65, 66].
AUTHOR CONTRIBUTIONS
Jinying Dong: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Funding acquisition (equal); Investigation (equal); Methodology (equal); Project administration (equal); Resources (equal); Software (equal); Supervision (equal); Validation (equal); Visualization (equal); Writing—original draft (supporting). Yuguang Du: Supervision (equal); Validation (equal). Lei Zhou: Conceptualization (equal); Data curation (equal); Formal analysis (equal); Funding acquisition (supporting); Investigation (equal); Methodology (equal); Project administration (supporting); Resources (supporting); Software (equal); Supervision (supporting); Validation (supporting); Visualization (equal); Writing—review & editing (supporting).
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
Professor Lei Zhou is a member of the iLABMED Editorial Board. To minimize bias, she was excluded from all editorial decision-making related to the acceptance of this article for publication. The remaining author declares no conflicts of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




