A case of central nervous system infection with Scedosporium boydii
Zhen Cai and Dongchao Pan contribute equally to this work and share the co-first authorship.
Jidi Fu and Fen Qu share the co-corresponding authorship.
Abstract
Scedosporium boydii is considered an opportunist fungal pathogen in immunocompromised patients. It also causes serious and fatal central nervous system infections. In this study, a man with brain abscesses infected with S. boydii was diagnosed using multiple methods, including microscopy, culture combined with Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MOLDI-TOF MS), internal transcribed spacer sequencing and Metagenomic next-generation sequencing (mNGS). This successful diagnosis highlights the importance of using a variety of techniques to identify and treat infections caused by this dangerous fungus.
Abbreviations
-
- CNS
-
- central nervous system
-
- CSF
-
- cerebrospinal fluid
-
- ITS
-
- internal transcribed spacer
-
- mNGS
-
- metagenomic next-generation sequencing
-
- MOLDI-TOF MS
-
- matrix-assisted laser desorption ionization time-of-flight mass spectrometry
1 INTRODUCTION
The Scedosporium genus is widely found in soil, sewage, and contaminated water and can cause infections in both healthy people and immune-impaired patients [1]. The genus Scedosporium comprises two clinically significant species, Scedosporium apiospermum and Scedosporium boydii, which have a global distribution. Sites infected by Scedosporium spp. include the joints, eyes, lungs, sinuses, bones, and brain [2, 3]. Patients with Scedosporium central nervous system (CNS) infections usually have a compromised immune system s or traumatic events such as near-drowning accidents [3]. CNS infections by Scedosporium are associated with high mortality rate (>75%), as its diagnosis and treatment are difficult [4]. Therefore, the rapid and accurate diagnosis of Scedosporium infections is critical for clinical outcomes.
Microscopy, culture, and histopathological examinations are conventional methods of laboratory diagnosis. It is hard to directly identify Scedosporium from specimens such as BALF or cerebrospinal fluid (CSF) because the amounts of microbes are too low to detect, and Scedosporium is hard to morphologically distinguish from other filamentous fungi such as Aspergillosis. The morphology of Aspergillosis and Scedosporium is similar, with 45-degree angle branching fungal elements [5]. Moreover, these methods are time-consuming, and even delay the diagnosis of infections caused by Scedosporium by up to 28 days [6]. MOLDI-TOF MS is a rapid identification method that shortens the time of strain identification to a few minutes. However, this method also relies on obtaining pure cultured colonies. mNGS and internal transcribed spacer (ITS) sequencing have emerged as culture-independent methods and take only 24–48 h for rapid diagnosis directly from specimens [7].
2 CASE REPORT
A 53-year-old man presented with headache, intermittent fever, vomiting, and nausea for 1 month after getting tired and wet. Moreover, he had neck stiffness at the same time. Before he was admitted to our hospital, he was suspected of having fungal and bacterial meningitis in another hospital. He was admitted to our hospital on 25 June 2021. An MRI image showed the presence of a brain abscess (Figure 1a). Laboratory examination results were as follows. WBC: 10.46 × 109/L↑, RBC: 3.10 × 1012/L↓, HB: 92 g/L↓, HCT: 29.3%↓, MCHC: 314.0 g/L↓, NEU: 7.93 × 109/L↑, NEU: 75.8%↑, LYM%: 10.7%↓, CRP: 272 mg/L↑, PCT: 0.29 ng/mL↑, ESR: 88↑. The appearance of his CSF was cloudy. The concentration of chlorine in his CSF was 134.0 mmol/L, protein in CSF was 0.80 g/L↑; and glucose in CSF was 3.93 mmol/L. Based on all the images and laboratory findings, the patient was suspected of having CNS infections.
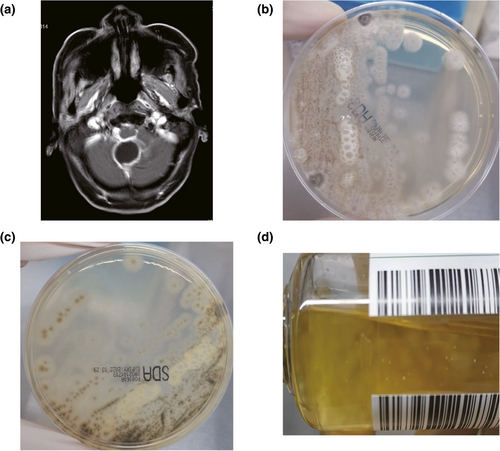
(a) MRI features of brain abscess. (b) Filamentous fungus colony on the front side of SDA. (c) Filamentous fungus colony on the reverse side of SDA media. (d) Fungus in the blood culture bottle.
To find infectious microbes in the CSF, laboratory examinations, including microscopy, and culture, were performed to identify infectious microbes in the CSF. CSF examinations were performed after lumbar puncture. No organisms were found in the CSF by microscopic examination with Gram, acid-fast, or ink staining. The results of different culture methods, including culture with SDA medium, blood medium, and blood bottles, were also negative. On June 30, the patient underwent surgery to remove his brain abscess, during which pyogenic fluids from the CSF were collected and cultured using different methods. After 4 days of culture, filamentous fungi were cultured from one sample of CSF, with a growth of grayish colonies on the SDA medium (Figure 1b), and with dark grayish colonies on the reverse side of the SDA (Figure 1c). After 39.36 h of culture, one blood culture bottle of CSF was positive, showing visible fungus colonies (Figure 1d).
A filamentous fungus colony was picked and stained with cotton blue. Branching septate hyphae with single-celled ovoid conidia were found under the microscope (Figure 2a). We preliminarily identified this strain as Scedosporium genus. MALDI-TOF was used to further confirm the identification of this fungus, MALDI-TOF was used. The results revealed that this strain was S. apiospermum, with a score of 8.134 (Figure 2b) (Note: score 8.134 indicates the genus level confidence). ITS sequencing with the universal fungal primers Its1 and Its4 of this fungus showed that it had 100% identity with S. boydii, but not S. apiospermum (E value: 0.0). Furthermore, on day 2 after surgery, mNGS of the CSF sample revealed the presence of S. boydii (56 reads).
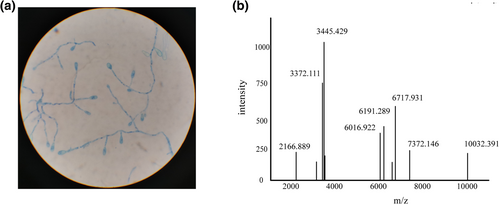
(a) Microscopic description showing branched septate hyphae with single-celled ovoid conidia. (b) MALDI-TOF spectra of Scedosporium boydii.
From June 25 to July 4, tigecycline and fluconazole were used, based on the previous hospital's suspicion of fungal and bacterial meningitis. On June 30, surgery was performed to remove the patient's brain abscess, and a drainage tube was placed for external ventricular drainage. After verification of S. boydii as the causative pathogen on July 5, voriconazole was used intravenously, and piperacillin-tazobactam was used to control his pneumonia. On July 9, piperacillin-tazobactam was replaced with tigecycline, as carbapenem-resistant Klebsiella pneumoniae were cultured from the CSF. On July 19, to control the S. boydii and Klebsiella pneumoniae infection, the encephaloceles were flushed with polymyxin and voriconazole. During the treatment, another two surgeries were conducted to remove his brain abscess on July 30 and August 9. After approximately 2 months of treatment, there were no microbes cultured from the CSF, and the patient's temperature was normal. On 26 August, he was discharged for personal reasons (Figure 3).
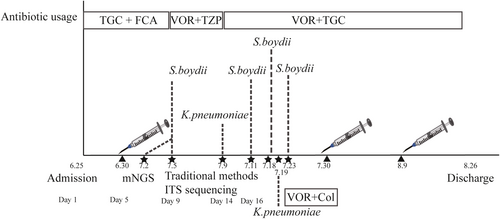
Daily treatment course used for the patient. Bars represent the different antibiotic usage regimes. Syringes represent the time of three surgeries. Dotted lines indicate the identification of microbes. Col, polymyxin; FCA, fluconazole; TGC, tigecycline; TZP, piperacillin-tazobactam; VOR, voriconazole.
3 DISCUSSION
S. boydii and S. apiospermum are two of the most frequently isolated species in the genus Scedosporium [8]. Sedosporium infects various organs, such as the lung (27%), skin (23%), eyes (8%), and CNS (13%) [9]. It is crucial to diagnose Scedosporium CNS infections quickly and accurately, as different types of filamentous fungal infections require different treatment regimens. Delayed treatment can worsen the prognosis, leading to a high mortality rate.
In this case, we took about 10 days to identify Sedosporium as the causative pathogen in the CSF with culture combined with MALDI-TOF methods. Culture with SDA or blood culture bottles efficiently increased the positive rates. Morphology examinations and MALDI-TOF results indicated S. apiospermum may have been the causative microbe, but ITS and mNGS sequencing finally helped us to diagnose S. boydii as the CNS infection agent [10]. S. apiospermum and S. boydii are two important species of Sedosporium. They are not easily identified with MALDI-TOF or microscopy methods. Thus, molecular methods such as the ITS sequence and mNGS are more suitable for identifying these two species. Some reports have suggested that it is better to use mNGS combined with traditional culture for fungal diagnosis than traditional culture alone [7, 11]. Compared with traditional culture methods, mNGS shortens the time of identification to only 2 days, which highly emphasizes the importance of mNGS in the early diagnosis of infections.
Apart from the methods mentioned above, beta-D-glucan assay (BG) is also widely used to identify fungal infections, especially in invasive candidiasis and aspergillosis. A cell wall component of many fungi, (1, 3)-β-D-glucan is detected by BG directly from serum or CSF. A result of more than 80 pg/mL indicates fungal infection. To date, there have been a few reports that BG can also be effective in diagnosing Sedosporium infections, even in CSF [12-15]. Thus, the use of BG should be encouraged as an early tool in invasive fungal disease (IFD) diagnosis.
Because of the limited experimental conditions, we did not perform an antifungal susceptibility test. Based on the literature, S. boydii has characteristic innate resistance to amphotericin B [16, 17], and is susceptible to voriconazole, miconazole, and posaconazole but resistant to fluconazole and flucytosine in vitro [6]. Posaconazole reportedly performed well in the treatment of a man with S. apiospermum brain abscesses [18]. However, most references indicated that the early use of voriconazole combined with surgical debridement is critical in cases of suspected scedosporiosis because it greatly decreases the fatality ratio [2, 19].
Here we present a case of a brain abscess caused by S. boydii and highlight the importance of employing both molecular and traditional methods for early identification.
AUTHOR CONTRIBUTIONS
Zhen Cai: Data curation (lead); investigation (equal); writing—original draft (equal). Dongchao Pan: Data curation (equal); investigation (equal); project administration (equal); resources (equal); writing—review and editing (equal). Ronghua Geng: Formal analysis (equal); methodology (equal); project administration (equal). Jidi Fu: Project administration (equal); supervision (supporting). Fen Qu: Project administration (equal); supervision (equal).
ACKNOWLEDGMENTS
We thank Professor Heping Xu for assisting in identifying S. boydii as the causative agent of the infection.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
The study protocol was approved by the Ethics Committee of Aviation General Hospital (approval number HK2022-28), and it was compliant with the Helsinki Declaration of 1975, as revised in 2008.
INFORMED CONSENT
The patient provided written informed consent at the time of entering this study.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




