Single cell metabolic phenome and genome via the ramanome technology platform: Precision medicine of infectious diseases at the ultimate precision?
[Corrections added on 24 May 2023, after first online publication: 1. Expansion of CAST-R has been corrected in the Abbreviations section. 2. Microbial dingle-cell has been corrected as Microbial single-cell in the figure 1 caption.]
Abstract
Due to the limitations of existing approaches, a rapid, sensitive, accurate, comprehensive, and generally applicable strategy to diagnose and treat bacterial and fungal infections remains a major challenge. Here, based on the ramanome technology platform, we propose a culture-free, one cell resolution, phenome-genome-combined strategy called single-cell identification, viability and vitality tests and source tracking (SCIVVS). For each cell directly extracted from a clinical specimen, the fingerprint region of the D2O-probed single cell Raman spectrum (SCRS) enables species-level identification based on a reference SCRS database of pathogen species, whereas the C-D band accurately quantifies viability, metabolic vitality, phenotypic susceptibility to antimicrobials, and their intercellular heterogeneity. Moreover, to source track a cell, Raman-activated cell sorting followed by sequencing or cultivation proceeds, producinging an indexed, high coverage genome assembly or a pure culture from precisely one pathogenic cell. Finally, an integrated SCIVVS workflow that features automated profiling and sorting of metabolic and morphological phenomes can complete the entire process in only a few hours. Because it resolves heterogeneity for both the metabolic phenome and genome, targets functions, can be automated, and is orders-of-magnitude faster while cost-effective, SCIVVS is a new technological and data framework to diagnose and treat bacterial and fungal infections in various clinical and disease control settings.
Abbreviations
-
- AST
-
- antimicrobial susceptibility
-
- CAST-R
-
- clinical antimicrobial susceptibility test ramanometry
-
- FCM
-
- flow cytometry
-
- FlowRACS
-
- flow-mode Raman-activated cell sorter
-
- ID
-
- identification
-
- IRCA
-
- intra-Ramanome correlation analysis
-
- MIC
-
- minimal inhibitory concentration
-
- MIC-MA
-
- minimal inhibitory concentration via metabolic activity
-
- mNGS
-
- metagenomic next-generation sequencing
-
- (MP-G)n
-
- single cell metabolic phenome and genome
-
- MS
-
- mass spectrometry
-
- pDEP-DLD-RFC
-
- positive dielectrophresis induced deterministic lateral displacement-based Raman Flow Cytometry
-
- RACS
-
- Raman-activated cell sorting
-
- RACS-seq
-
- Raman-activated cell sorting coupled to sequencing
-
- RAGE
-
- Raman-activated gravity-driven cell encapsulation
-
- SCIVVS
-
- single cell identification, viability and vitality tests and source tracking
-
- scRACS-seq
-
- single-cell RACS-seq
-
- SCRS
-
- single cell Raman spectrum
-
- SRS
-
- stimulated Raman scattering
1 INTRODUCTION
Among human diseases, infectious diseases rank third in mortality and first in morbidity. The major contributors are approximately two dozen bacterial, fungal, and viral species that account for two-thirds of deaths from infections. Moreover, the alarming rise of antimicrobial resistance globally poses another challenge, and enormous casualties and economic output are at risk because of the worldwide emergence and spread of drug-resistant pathogens [1]. Diagnosis, antimicrobial agent administration, and source tracking for these pathogens are pivotal to combat such infections. For a given specimen collected from the patient, the properties of the pathogens that underlie timely diagnosis and effective treatment usually include (a) the total live bacteria count, (b) identification (ID) of ingredient organisms, (c) organism-resolved viability, vitality, and antimicrobial susceptibility (AST), and (d) the source and spreading the route of the pathogen. However, because of the limitations of existing approaches, a rapid, sensitive, accurate, comprehensive, and generally applicable framework to assess these parameters remains a major challenge, and its solution is a top priority in the battle against infectious diseases [2, 3].
2 TECHNOLOGICAL CHALLENGES IN DIAGNOSING AND TREATING INFECTIONS
Specifically, (a) the total live cell count, which measures the overall viability of cells [4], can be determined by culture-based approaches such as plate counting and agar diffusion. However, they can be insensitive, laborious, and time consuming [5]. Moreover, plate counting by the number of colonies instead of cells [6] is usually unable to account for cells that are sub-lethally damaged, injured, inhibited, dormant, or inactive [7]. Culture-independent methods, such as polymerase chain reaction combined with ethidium monoazide or propidium monoazide, can also be used to count live/dead cells [4] based on the prevention of DNA amplification in dead cells, but the high reagent cost, complexity of operation, and inaccuracy due to variation in DNA extraction efficiency have limited their clinical application [8].
(b) For pathogen ID of clinical samples [9], metagenomic next-generation sequencing (mNGS) can be employed for culture-free ID, but it is costly and fails to distinguish between live and dead bacteria [10]. Thus, for accurate and reliable pathogen ID, culture-based isolation is recommended as the gold standard [11]. However, the cultivation of pathogens from specimens can be a slow technically challenging process that requires days or even weeks, and usually needs highly trained laboratory personnel because of the fastidious growth conditions of many pathogens, such as Helicobacter pylori and Mycobacterium tuberculosis, and is frequently confounded by the presence of commensal flora in clinical specimens and inappropriate transport conditions [12]. Therefore, rapid, low cost, and reliable methods for pathogen ID directly from clinical samples are required.
(c) For ASTs, existing methods interrogate either genotypic or phenotypic features of the microbial response to drugs. Phenotypic ASTs, which are the gold standards to guide the drug administration, are usually based on pure cultures and measurement of the population's drug response by either growth or metabolic vitality [13, 14]. However, this approach can be technically demanding because of the aforementioned challenges underlying bacterial isolation. Moreover, such ASTs can be very slow with a turnaround time of usually days or at least 1 week even in state-of-the-art laboratories [12, 15]. Genotypic ASTs such as mNGS, which are based on the detection of mutations in drug resistance genes, can be faster because it circumvents isolation and culture [15-18]. Unfortunately, such genotype-based predictive ASTs are appropriate only for a very limited range of antimicrobials; that is, only when genotypic mutations underlying resistance to a particular drug are known. In fact, any undiscovered or new drug-resistant mutation mechanisms would likely result in false negatives [19, 20]. Because of these challenges, tracking the dynamic change in antimicrobial resistance along the regimen of antimicrobial administration or recognition of microbes that are drug resistant in situ in a clinical or environmental sample has been exceedingly difficult.
(d) To source track an infectious agent, crucial information on its origin or the spreading route can be derived from the full genome sequence. Moreover, to thoroughly probe drug resistance mechanisms, a complete and accurate genome sequence is pivotal because the genome-wide genetic mutations underlying AST phenotypes provide a mechanistic explanation for the occurrence and origin of drug resistance. However, whole-genome sequencing typically needs a pure culture of sufficient biomass, which can require days or weeks to produce [16]. Approaches such as mNGS directly from clinical samples circumvent time-consuming culture. However, the ability to reconstruct genome-wide mutation maps of target genomes is frequently hindered by the difficulty in accurately and fully assembling metagenomic reads [21]. Conversely, single cell sequencing of the target bacterium, which by definition reveals the genetic heterogeneity of a biopsy sample at the deepest level, would be the ultimate solution. However, clinical deployment of single cell sequencing directly from infected biopsy samples has been hindered by the inability to precisely and readily obtain a target bacterial cell from a clinical sample, owing to the microscopic size of bacterial cells and the challenge for reliable ID of individual bacterial cells in a microbial community under a microscope. Another hindrance to single cell sequencing is the difficulty in producing a high-coverage genome sequence for each cell because of the bias in amplifying genomic DNA and contamination issues [22, 23].
Practical considerations in a pathogen diagnosis and treatment workflow also include throughput, automation, and cost. In addition to the methodological hurdles in each task above, a major challenge is to establish an integrated and automated platform that completes the various tasks in a streamlined and efficient manner. Presently, distinct instruments are usually employed for each task, such as culture plates and incubators for live cell counting, mass spectrometry for ID, flow cytometry for viability assessment, culture-based monitoring of microbial growth or metabolism for AST, and DNA sequencers for source tracking. Consequently, integration and automation of workflows have become quite difficult. Therefore, an integrated platform that can complete multiple or even all of these tasks simultaneously is highly advantageous because it would facilitate workflow automation and reduce operating costs.
3 ESTABLISHING THE RAMANOME TECHNOLOGY PLATFORM TO PROFILE THE METABOLIC PHENOME AND GENOME AT SINGLE CELL RESOLUTION
To overcome these challenges, we propose the ramanome concept to profile the metabolic phenome and genome of pathogens directly from clinical samples at single-cell resolution. Raman spectroscopy is a scattering technique that relies on inelastic scattering of photons, where excitation of molecular bonds results in energy shifts of laser photons and thus a frequency change of the incident light. Therefore, a chemical compound with a defined set of molecular bonds has a specific Raman spectrum, while a single cell Raman spectrum (SCRS) depicts the overall profile of cellular metabolites, which captures the metabolic state of the cell at a particular instance. A ramanome or meta-ramanome is the collection of SCRS, one from each cell, sampled from a cell population or community [24, 25], which represents a single-cell-resolution metabolic phenome that corresponds to the overall metabolic functions for the cellular system at that given instance (Figure 1). Notably, each SCRS can have >1500 Raman peaks with each peak or combination of peaks potentially representing a metabolic phenotype. Therefore, a ramanome is rich in information. Moreover, within a ramanome, even when the genomes of the cells are identical, the SCRS of the cells can be very distinct. Such intercellular heterogeneity of the metabolic phenome, as depicted by SCRS here, is an inherent feature of cellular systems that is biologically significant (e.g., can derive a network of intracellular metabolite interconversions [25]).
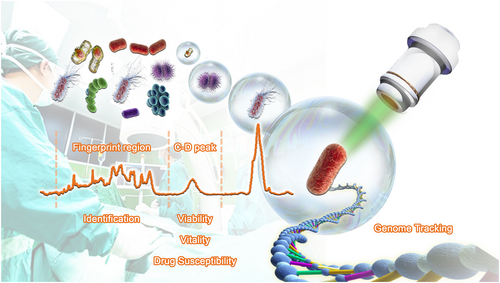
Microbial single-cell approach that combines the metabolic phenome and genome to diagnose and treat infections at the ultimate resolution of one pathogen cell. From a single cell Raman spectrum (single cell Raman spectrum [SCRS]; shown as a brown curve) acquired from a specific cell by a Raman microspectrometer, the fingerprint region is used for rapid species identification by searching against a reference SCRS database of frequently encountered human pathogens and symbionts, while the C-D peak is used to measure the viability, vitality, and susceptibility to a particular antimicrobial at the rate of D2O consumption (i.e., the metabolic activity) of the cell.
A change in species, substrate intake, product profile, or simply the metabolic state can lead to a detectable alteration in SCRS. Therefore, a ramanome quantitatively profiles a wide variety of metabolic phenotypes for individual cells [24], such as quantifying the intake rate of hydrogen- [26] and carbon-containing [27] substrates, determining the diversity and content of various Raman-sensitive intracellular products (e.g., pigments [28], triglycerides [29], starch [30], and proteins [31]), characterizing the environmental stress responses of cells (e.g., antimicrobial susceptibility of pathogens [32], mechanisms of microbial drug response [33], and drug resistance and its mechanisms in tumor cells [34]), detecting intercellular metabolism interactions [35], reconstructing intracellular metabolite interconversion networks (i.e., Intra-Ramanome Correlation Analysis, or IRCA [25]), and distinguishing microbial species [36, 37]. The scope of ramanome applications is rapidly expanding.
By quantitatively converting the molecular spectrum of intracellular metabolites into major metabolic phenotypes, ramanome is an omics approach and data type that may be closer to functions than the transcriptome, proteome, or metabolome. Additionally, as a molecular spectroscopic approach, ramanome offers many advantages that are particularly appealing to deployment in a clinical microbiology laboratory. It is label free, which suggests simplicity in operation and general applicability to all cell types. Ramanome also provides rich information, which allows landscape-like phenotyping, that is, revealing multiple phenotypes simultaneously or serving multiple purposes with one SCRS (e.g., species classification and vitality measurement). It is also high speed, which greatly accelerates the turn-around time of diagnosis because the acquisition of each SCRS occurs in milliseconds for yeast and less than 1 s for most bacteria. Moreover, ramanome is of low cost since SCRS acquisition does not involve reagents or other expensive consumables, which greatly expands the scope of clinical applications.
Importantly, the non-destructiveness of ramanome allows coupling of metabolic phenome profiling to downstream sequencing, mass spectrometry, or cultivation of the cell of a targeted metabolic function via Raman-activated cell sorting (RACS) techniques [24]. In particular, by coupling RACS to genome sequencing, we have proven the feasibility of simultaneously obtaining metabolic phenome (and morphological phenome if necessary as morphological features are also readily revealed by the microscope in a Raman-activated cell sorting coupled to sequencing or RACS-seq instrument) and its corresponding high-coverage genomes at precisely one bacterial cell resolution and from various types of clinical and environmental microbiome samples [38-40]. The output is a new type of clinical data called single cell metabolic phenome and genome or (MP-G)n, which captures the metabolic functions and genomes from each of n cells sampled from a cellular population in a specific state, describing who (encoded by the genome sequence) is doing what (depicted by the SCRS) in the population at a particular spatiotemporal coordinate. These workflows, which exploit the complementary strength of molecular microspectroscopy (i.e., ramanome profiling) and genome sequencing, are collectively called the ramanome technology platform (Figure 1) [24].
4 RAMANOME-BASED DIAGNOSIS AND TREATMENT OF INFECTIONS VIA THE SCIVVS WORKFLOW
Using the ramanome technology platform, we developed a culture-free pathogen diagnosis and treatment strategy called single-cell identification, viability, and vitality test, and source tracking (SCIVVS; Figure 2). In this strategy, D2O-probed single cell Raman microspectroscopy of individual cells directly extracted from a specimen is employed for total live cell counting, organism identification, and species-resolved profiling of in situ viability, vitality, and susceptibility to a particular antimicrobial agent, whereas single-cell RACS-seq (scRACS-seq) is employed for full genome-based source tracking of only one cell.
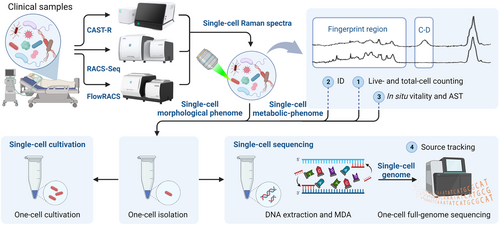
Single cell identification, viability and vitality tests and source tracking workflow for total live bacteria counting, rapid identification, species-resolved in situ vitality, and antimicrobial susceptibility test, and source tracking at precisely one cell resolution directly from an infected clinical specimen. Examples of instruments that support the workflow are also shown. Created with BioRender.com.
Specifically, because cells of different species carry a distinct SCRS, the fingerprint region in a D2O-probed SCRS can be employed to classify or even identify species from only one cell using a microspectrometer by searching the SCRS against a reference SCRS database of microorganisms. Moreover, because all live cells consume D2O and vitality-dependent assimilation of D2O into the cell causes the emergence of the C-D band in the other silent region of an SCRS [26], the viability and vitality of the cell can also be determined by the SCRS. Furthermore, this principle can be used for a culture-independent AST of microbes [32] and cancer cells [34] by D2O-probed single cell Raman microspectroscopy based on quantitative inhibition of metabolic vitality of individual cells using a particular antimicrobial drug that results in either a reduction in the C-D band for susceptible cells or no change in the C-D band versus the drug-free control for resistant cells in each SCRS. Correspondingly, the new concept of minimal inhibitory concentration (MIC) via metabolic activity (MIC-MA) was introduced as a single cell resolution, quantitative parameter to measure antimicrobial susceptibility, which has general applicability to fast and slow growing pathogens [32, 38, 41]. Compared with the conventional, population average-based parameter of MIC, which is defined as the lowest drug dose at which cellular growth stops, MIC-MA offers important advantages. (a) The single-cell resolution, which means a faster turnaround time of the AST report and revealing heterogeneity in resistance; (b) detection of metabolic inhibition, which is more accurate for AST than growth-based detection methods and able to distinguish persister cells [42]; (c) being label-free, which allows correlating the resistance phenotype and genotypes at single-cell resolution and direct isolation of individual cells with a target resistance phenotype for downstream culture; (d) general applicability to all cellular organisms because all live cells consume water, despite their vastly different rates of water consumption.
In terms of workflow, SCIVVS consists of three sequential steps, which start with microbial cells extracted from the clinical sample and proceeds in a culture-independent, integrated manner as follows (Figure 2). (a) Treatment of extracted microbial cells in liquid medium with 100% D2O for 3 h for D2O intake. (b) Automated SCRS acquisition for D2O-treated cells, which reports the total live cell count, ID, and species-resolved specifically in situ viability and vitality. Notably, antimicrobial susceptibility of each cell can be quantified by its C-D band under a particular type and duration of antibiotic administration. (c) Sorting of individual cells into a microdroplet for a precise one-cell reaction of multiple displacement amplification and whole-genome sequencing using scRACS-seq. Collectively, because culture-based strain isolation prior to ID or AST is not required, the total live bacteria count, ID, and organism-resolved in situ viability, vitality, and antimicrobial susceptibility can be reported within 5 h, whereas the time for source tracking depends on the sequencing platform.
5 A COMPREHENSIVE AND INTEGRATED INSTRUMENT SOLUTIONS TO SUPPORT CLINICAL DEPLOYMENT OF SCIVVS
To support the complete SCIVVS workflow and enable the plethora of circumstances in a clinical microbiology laboratory, a series of microbial single cell multiomics instruments have been developed and validated with clinical specimens (Figure 2). (a) The clinical antimicrobial susceptibility test ramanometry (CAST-R) instrument [38, 41], which includes hardware devices for automated clinical sample pretreatment and intelligent SCRS acquisition and analysis in a parallel, 60-well plate-based format, reports AST results for dozens of antimicrobials in each patient sample and processes dozens of patient samples in each run. (b) The flow-mode Raman-activated cell sorter (FlowRACS) instrument [43, 44], which is a microfluidics-based system, profiles the full spectrum spontaneous SCRS in a cell population with high speed (30 per min for Escherichia coli and 270 per min for budding yeasts) via its Raman flow cytometry mode, thereby providing a novel solution for automated, high-throughput SCRS-based ID and AST of pathogens [44]. (c) The RACS-seq instrument sorts individual microbial cells by their SCRS (i.e., based on identity, antimicrobial susceptibility, or other phenotypes as depicted by SCRS) and corresponding morphological phenome as depicted by brightfield microscopic images, and then exports target cells to a 96-well plate in an index-based, what-you-see-is-what-you-get and one-cell-one-well manner. The design of the RAGE (Raman-activated Gravity-driven cell Encapsulation) chip in the RACS-seq system allows low bias amplification of genomic DNA from precisely one post-RACS microbial cell. Thus, the RACS-seq instrument produces both a high-quality SCRS and its matching genome sequence of very high coverage (i.e., exceeds 95% and even reaches 99%) from just one bacterial cell [40]. This capability has been demonstrated using human specimens from gastric biopsies [38] and urine [40] as well as environmental samples (e.g., marine [45], waste-water [46], and soil [39]).
Notably, when pathogen isolation is required, the cell sorting module in both FlowRACS and RACS-seq systems can be coupled to a DNA/RNA sequencer or mass spectrometer for proteome or metabolome profiling and cultivation of the sorted cells with the target SCRS (Figure 2). In fact, designs of the positive dielectrophoresis induced deterministic lateral displacement-based Raman Flow Cytometry chip [44] in the Flow RACS instrument and the RAGE chip in the RACS-seq instrument [40] have carefully ensured the preservation of cellular vitality along the complete operation that includes sample preparation, SCRS acquisition, cell sorting, and cell exportation. This RACS-culture procedure, which has been demonstrated for both humans [40] and environmental microbiomes [39], selects a target cell directly from a clinical specimen and then deposits the cell into a well of a 96/384-well plate or into a microdroplet for liquid medium-based cultivation. By skipping the conventional step of agar plate-based colony formation and picking, RACS-culture can be more efficient in terms of time, human effort, and consumables, and more automated, and thus has the potential to greatly accelerate the strain isolation process in a clinical microbiology laboratory.
On the basis of ramanome and MIC-MA concepts, the (MP-G)n data type, and the instrument series of CAST-R, RACS-seq, and FlowRACS, a comprehensive SCIVVS workflow to diagnose and treat infectious diseases has been demonstrated by clinical applications. For example, we established the CAST-R-HP approach that accomplishes single-cell ID, AST, and high-coverage whole-genome sequencing of H. pylori directly from a gastric biopsy [38]. This approach consists of three steps: (a) treatment of clinical gastric biopsy specimens in medium with 50% D2O, 1% IsoVitale X™, Dent supplement, and a particular antibiotic, and then microaerobic incubation for 2–3 days for drug exposure without the need to isolate H. pylori; (b) acquisition of SCRS for D2O-treated, antibiotic-challenged cells directly from the biopsy sample for rapid ID and AST of H. pylori single cells; (c) sorting of individual cells whose SCRS identifies H. pylori or the AST phenotype into microdroplets in a RAGE chip for a precisely one cell reaction of multiple displacement amplification and then shotgun sequencing, which produces a precisely one cell assembled genome with high coverage (>95%) to support genotype-based validation of drug susceptibility, identification of new drug resistance mutations, and full genome-based tracking of pathogens at one cell resolution [38]. Because no isolation or pure culture of H. pylori is involved, the ID can be obtained within 20 min because no D2O treatment or antibiotic exposure is required, and the AST within 3 days upon biopsy sample arrival. Notably, single cell sorting and genome sequencing of H. pylori can start immediately after the ID step and proceed in parallel with the AST. Thus, the complete phenotyping-genotyping process, including ID, AST, and sequencing, can be completed within 3 days [38].
6 FUTURE DIRECTIONS
Bacterial and fungal infections have remained a major threat to human health. The rampant increase in antimicrobial drug-resistant bacteria and patient populations susceptible to infections pose additional challenges to healthcare. In SCIVVS, the integration of Raman microspectroscopy and single-cell sorting, lysis, and DNA amplification in a RAGE chip [40] allows direct coupling of SCRS-based, non-invasive metabolic phenome profiling to downstream single cell genome sequencing. This design takes full advantage of (a) the high speed and throughput of the former for rapid ID as well as vitality and viability tests, and (b) the high resolution of the latter in source tracking. Because speed and throughput are the priority when processing a large volume of clinical samples, while genome-based applications such as source tracking are only applied to a small number of selected samples, a metabolic phenome-guided single cell sequencing approach such as SCIVVS can be the most efficient in terms of both time and consumable costs for circumstances such as live pathogenic cell analysis in clinical settings. Moreover, the SCIVVS approach is highly complementary to recently introduced methods that sequence thousands of single microbial cells in parallel (e.g., Microbe-seq [47]). Instead of sequencing single cells randomly sampled from a microbiota in an indiscriminate, brute force manner, SCIVVS is a targeted, screen first and sequence second approach, in that it first rapidly identifies cells with targeted metabolic or morphological phenomes, and then employs phenome-based sorting coupled with sequencing to ensure that precise resources, such as sequencing and computational genome analysis, are focused only on those small numbers of cells with the targeted function. In this manner, despite its low throughput at present, the single-cell omics data produced by SCIVVS can be of higher efficiency because of (a) the inclusion of the genome data and matching phenome data; (b) the high quality of one cell genome assemblies (e.g., 90%–99% in genome coverage [38-40, 45, 46]) versus the ∼20% on average for brute force approaches [47] because of the higher sequence coverage for each cell; and (c) the low cost of DNA or RNA sequencing as only the very small, but important portion of the cell population, is sequenced.
Despite its strengths and value, further development of SCIVVS is required to test, extend, and validate its full potential in clinical applications. For example, reference SCRS databases should be expanded to include more species and physiological states, so that the scope and resolution of SCRS-based species identification can be fully and thoroughly tested. Additionally, in the AST step, the turnaround time can be reduced by employing stimulated Raman scattering (SRS) instead of the spontaneous Raman to detect the C-D band, although the much higher instrumentation cost of SRS and the challenge of simultaneously capturing the fingerprint region for ID with SRS can be a disadvantage that hinders its wider deployment in clinics [48].
By adopting the Raman flow cytometry mode in FlowRACS [43, 44], new clinical or scientific applications such as high-throughput, label-free metabolic profiling of live microbial cells will emerge, which are highly valuable to resolve the long-standing challenge of detecting and quantifying hetero-resistance [49]. At the one-cell RACS-seq or RACS-culture step, new designs of the RAGE chip that improve the precision of cell capture, such as optical tweezer-assisted pool screening [50] or the use of artificial intelligence for automated sorting of cells [50, 51], can greatly enhance the throughput, while the automation of SCRS acquisition, sorting, and one cell-one well disposition would ensure downstream single cell genome extraction, amplification and sequencing or cultivation can proceed without human intervention. Notably, in circumstances when cost-efficient sequencing of a larger number of RACS-sorted microbial cells are important, barcoded single cell genome sequencing that is directly coupled to indexed, low-throughput RACS-seq or unindexed, high-throughput FlowRACS should be established [39].
Summary The ability to diagnose and evaluate treatment effects at the resolution of one pathogenic cell has profound implications. For example, because one cell in the original sample corresponds to one colony (∼109 cells) on the agar plate, the one-cell phenome-genome profiling in SCIVVS directly skips time-consuming cell isolation and pure culture without sacrificing any resolution, at least theoretically. Instead, it can provide a more accurate overview of the diversity and structure of both genotypes and phenotypes of the original sample because the insertion of plate-based isolation and culture step can skew or even misrepresent such overview, owing to the variation in growth rate among species. Therefore, such single cell technologies that profile in situ morphological and metabolic phenomes, and high-coverage genomes at the ultimate resolution of one cell and in an integrated manner will provide new standards for pathogen diagnosis and AST. Such standards are particularly timely for clinical microbiology [3] because of the lack of fast, accurate, low cost, and generally applicable methods that range from strain identification and drug resistance phenotype profiling to source tracking [52, 53]. Therefore, we envision that the single cell metabolic phenome and genome profiling in SCIVVS will become an advantageous approach, and (MP-G)n will emerge as a universally applicable data type for pathogens and pathology to support a new generation of clinical practice, standard workflows, informatics systems, and big data for evidence-based diagnosis and treatment of infections.
AUTHOR CONTRIBUTIONS
Jian Xu and Yi-Wei Tang conceptualized and wrote the manuscript. All authors edited the manuscript and approved the final version.
ACKNOWLEDGMENTS
We thank Yang Liu for graphics support. This study was funded by the National Key R&D Program of China (2022YFA1304101), CAS (XDB29050400), the National Natural Science Foundation of China (32030003), and Shenzhen - Hong Kong Innovation Circle Plan (SGDX2019081623060946).
CONFLICT OF INTEREST STATEMENT
Jian Xu and Bo Ma are founders of Qingdao Single-Cell Biotech. Co., Ltd. Professor Jian Xu and Yi-Wei Tang are the members of the iLABMED Editorial Board. To minimize bias, they were excluded from all editorial decision-making related to the acceptance of this article for publication. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
Not applicable.
INFORMED CONSENT
Not applicable.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




