Bottlenecks and recent advancements in detecting Mycobacterium tuberculosis in patients with HIV
Zixun Lin and Liqin Sun contributed equally to this work and shared the first authorship.
Abstract
Tuberculosis (TB) remains a leading cause of deaths among patients with acquired immunodeficiency syndrome patients. Early diagnosis of TB is essential for administering timely anti-TB therapy and improving health outcomes, particularly in the people living with HIV. However, conventional techniques used to detect Mycobacterium tuberculosis have significant drawbacks: for example, sputum smear microscopy has low sensitivity, and liquid culture is time-consuming in patients with HIV-TB co-infection due to low sputum production. In addition, while immunological-based methods involving tuberculin skin testing and interferon gamma release assays are commonly used for auxiliary TB diagnosis, they are often inaccurate in immunodeficient patients. Molecular techniques such as line probe assays, Xpert MTB, and lipoarabinomannan assay are recommended for early diagnosis by World Health Organization. However, no single technique is sufficicent for diagnosing HIV/TB co-infection, suggesting that multiple diagnostic tests should be used to detect TB. Here, we summarize the drawbacks and advantages of existing TB-diagnostic methods, as well as their applications to diagnosing HIV/TB co-infection. We describe newly emerging technologies such as whole genome sequencing and mass spectrometry, with the aim of providing updated guidelines and alternative strategies for TB diagnosis, and particularly HIV/TB diagnosis.
Abbreviations
-
- AFB
-
- acid-fast bacilli
-
- AIDS
-
- acquired immunodeficiency syndrome
-
- ATB
-
- active tuberculosis
-
- Cas
-
- CRISPR-associated protein
-
- CCR5
-
- C-C chemokine receptor type 5
-
- CFP-10
-
- cultured filtrate protein-10
-
- CRISPR
-
- Clustered regularly inter-spaced short palindromic repeat
-
- CSF
-
- cerebrospinal fluid
-
- ddPCR
-
- droplet digital polymerase chain reaction
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- ELSPOT
-
- enzyme-linked immunospot
-
- EPTB
-
- extra-pulmonary tuberculosis
-
- ESAT-6
-
- early secreted antigenic target 6
-
- FujiLAM
-
- Fujifilm SILVAMP TB LAM
-
- HBV
-
- hepatitis B virus
-
- HCV
-
- hepatitis C virus
-
- IFN-γ
-
- interferon gamma
-
- IGRAs
-
- interferon gamma release assays
-
- INH
-
- isoniazid
-
- LAM
-
- lipoarabinomannan
-
- LAMP
-
- loop-mediated isothermal amplification
-
- LF-LAM
-
- lateral flow urine lipoarabinomannan assay
-
- LPAs
-
- line probe assays
-
- LTBI
-
- latent tuberculosis infection
-
- MALDI-TOF MS
-
- matrix-assisted laser desorption ionization-time of flight mass spectrometry
-
- MDR
-
- multidrug-resistant
-
- MS
-
- mass spectrometry
-
- Mtb
-
- Mycobacterium tuberculosis
-
- NAAT
-
- nucleic acid amplification tests
-
- NTM
-
- nontuberculous mycobacteria
-
- PCR
-
- polymerase chain reaction
-
- PLWH
-
- people living with HIV
-
- POCT
-
- point-of-care testing
-
- PPD
-
- purified protein derivative
-
- QFT-GIT
-
- QuantiFERON-TB Gold Tube
-
- QFT-Plus
-
- QuantiFERON-TB Gold Plus
-
- qPCR
-
- real-time fluorescent quantitative PCR
-
- real-time PCR
-
- real-time polymerase chain reaction assay
-
- RIF
-
- rifampicin
-
- RS
-
- Raman spectroscopy
-
- TB
-
- tuberculosis
-
- TBM
-
- tuberculous meningitis
-
- TBST
-
- mycobacterium tuberculosis antigen-based skin test
-
- T-SPOT
-
- Oxford Immunotec T-SPOT®.TB
-
- TST
-
- tuberculin skin testing
-
- WANTAI
-
- Beijing Wantai's TB-IGRA
-
- WAS
-
- whole genome sequencing
-
- WGS
-
- whole-genome sequencing
-
- WHO
-
- World Health Organization
-
- Xpert Ultra
-
- Xpert MTB/RIF Ultra
1 INTRODUCTION
The causative agent (Mycobacterium tuberculosis [Mtb]) of tuberculosis induces pathological changes in both the lungs and extra-pulmonary tissues (i.e., blood lymphatic system, bones and joints), resulting in pulmonary tuberculosis (TB) and extra-pulmonary tuberculosis (EPTB), respectively [1, 2]. TB is a serious global health problem: in 2021, an estimated 10.6 million people were infected with TB, and 187 000 people living with HIV (PLWH) died of tuberculosis [3]. The main reasons why these two pathogens increase disease susceptibility and accelerate health deterioration are that: (a) the progressive immunodeficiency that occurs in HIV-infected individuals suppresses the T cell response to Mtb [4]. (b) Mtb promotes HIV replication and infection by increasing the expression of the viral coreceptor C-C chemokine receptor type 5 (CCR5) [5]. (c) and finally, but most importantly, diagnosis of Mtb infection in individuals co-infected with HIV has more technological bottlenecks than diagnosis of Mtb infection alone.
An autopsy study performed in sub-Saharan Africa showed that undiagnosed TB cases at death accounts for nearly 45.8% of HIV-infected patients had undiagnosed TB at death, because of the drawbacks of current diagnostic techniques [6]. TB diagnosis remains particularly challenging in HIV-positive individuals for a variety of reasons. Firstly, patients with HIV/TB co-infection exhibit atypical clinical manifestations of TB, such as less coughing and hemoptysis, resulting in a high frequency of smear-negative disease and limiting the use of routine tests (i.e., sputum smear microscopy and sputum culture). Second, EPTB accounts for 50% of cases of HIV/TB co-infection, which means that many cases are missed by conventional approaches to diagnosis of pulmonary disease [7]. Thirdly, immunological approaches, such as tuberculin skin testing (TST) that relies on the cellular immune response to mycobacterial antigens, are not indicated for immunodeficient patients.
Herein, we provided an overview of all available traditional techniques for diagnosing TB, particularly in patients co-infected with HIV. We also present principles and future directions regarding the development of more accurate diagnostic techniques based on newly emerging technologies in TB diagnosis for patients with HIV/TB co-infection.
2 CHALLENGES OF TRADITIONAL TECHNIQUES FOR DIAGNOSING TB IN PATIENTS WITH HIV
2.1 Microbiological diagnostic assays
2.1.1 Sputum smear microscopy
Sputum smear microscopy is the most common laboratory test used to diagnose TB, particularly in low-income countries (Figure 1). Although it is widely used, the sensitivity of the assay is low and variable (range 20%–60%) in some settings. In addition, the positive detection rate of the assay is even lower (20%–35%) in areas with high HIV prevalence areas owing to their poor laboratory conditions [8, 9]. Furthermore, the sensitivity of sputum smear microscopy has been reported to be lower (26%) in patients with HIV/TB co-infection than in patients without HIV because the low bacterial counts in the sputum are below the range of detection (>10 000 cfu/mL) [10, 11]. Therefore, while sputum smear microscopy is an affordable, readily available diagnostic method, it is not suitable for TB diagnosis in PLWH.
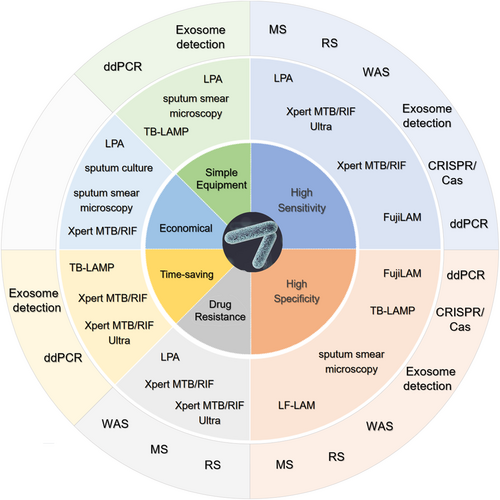
The feature of the diagnosis of each diagnostic method for Mtb. The inner-circle represents the advantages of these technologies, including high sensitivity, high specificity, application for drug resistance, time-saving, economical or easy-to-handle. The middle circle indicates the existing technology used in clinical. The outside circle displays the emerging techniques for TB diagnosis. ddPCR, droplet digital polymerase chain reaction; FujiLAM, Fujifilm SILVAMP TB LAM; LF-LAM, lateral flow lipoarabinomannan; LPA, line probe assay; MS, mass spectrometry; RS, Raman spectroscopy; TB-LAMP, tuberculosis loop-mediated isothermal amplification; WAS, whole genome sequencing.
2.1.2 Mycobacterial sputum culture
For sputum smear-negative patients suspected of having TB, mycobacterial sputum culture is a reliable alternative diagnostic method (Figure 2). Mycobacterial sputum culture can be used not only to detect infection but also to assess drug susceptibility, with high sensitivity (93%) [11, 12]. However, mycobacterial sputum culture is a time-consuming method. Generally, mycobacterial strains or subspecies can be identified after approximately 14 days and isolated within 21 days from smear-positive sputum [12]. However, in patients with HIV/TB co-infection, it may take longer to collect an adequate number of liquid specimens, especially in asymptomatic patients who produce less sputum, which is not conductive to rapid diagnosis and treatment. In addition, mycobacterial culture requires laboratories with a high level of biosafety (biosafety level 3) and personnel with specialized skills, which is another bottleneck in areas with limited medical resources.
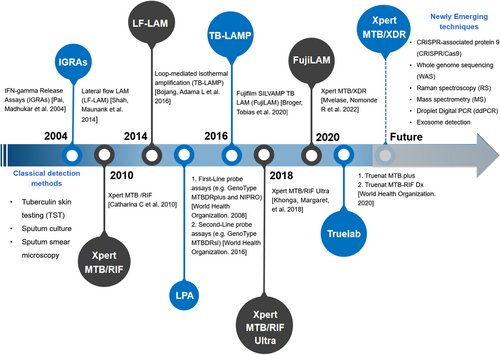
The timeline for the development of tuberculosis (TB) diagnosis.
2.2 Immunological methods for auxiliary diagnosis of tuberculosis
Tuberculin skin testing (TST), which detects memory cell-mediated immune responses to the Mtb complex, is commonly used in children, but this approach cannot distinguish between a latent or active TB infection. Despite being inexpensive, TST is not recommended for Mtb in patients with HIV/TB co-infection, because host immunosuppression state can yield false-negative results (Table 1). Immmunocompromised people such as patients with AIDS are at a higher risk of TST anergy due to decreased CD4+T cell count and impaired T-cell function [4]. Another alternative immunological test used for auxiliary TB diagnosis, the Interferon gamma (IFN-γ) release assays (IGRAs), shows higher specificity than TST in BCG unvaccinated individuals (88% vs. 70%) [13]. This assay detects interferon-γ released by antigen-specific T cells isolated from whole blood or peripheral blood mononuclear cell (PBMC) samples and stimulated with Mtb-specific antigens, including early secreted antigenic target 6 (ESAT-6) and/or cultured filtrate protein-10 (CFP-10), which is encoded by the Mtb gene RD1 [14, 15].
| Test | Patients | Sensitivity (%)a | Specificity (%)a | Time required | Potential recommended usage | Limitation | Refs |
|---|---|---|---|---|---|---|---|
| Sputum smear microscopy | TB with HIV-negative | 52–94 (CM) | 96–100 (CM) | Within a day | Cavitarypulmonary disease | Low sensitivity; unable to identify drug resistance | [10, 24] |
| 63–97 (FM) | 96–99 (FM) | ||||||
| TB with HIV-positive | 36 (CM) | 100 (CM) | |||||
| 73 (FM) | 100 (FM) | ||||||
| Mycobacterial sputum culture | TB with HIV-negative | 40 | 85 | 14–21 days | Drug susceptibility testing | Time-consuming; high requirement for laboratories (biosafety level 3) | [58, 59] |
| TB with HIV-positive | − | − | |||||
| TBST | TB with HIV-negative | 30.67 | 62.0 | 48–72 hours | Use to test for Mtb infection and require additional tests to confirm TB | Low specificity; low sensitivity in PLHIV | [16] |
| TB with HIV-positive | − | − | |||||
| IGRAs | TB with HIV-negative | 76.7 (QFT-GIT) | 76.1 (QFT-GIT) | Within 2 days | Used to test for LTBI in low- and middle-income countries; not used for PLHIV with suspected active tuberculosis in low-income countries | Low sensitivity in PLHIV | [16, 61, 62] |
| 77.4 (T-SPOT.TB) | 63.1 (T-SPOT.TB) | ||||||
| TB with HIV-positive | 61 (QFT-GIT) | 72 (QFT-GIT) | |||||
| 65 (T-SPOT.TB) | 70 (T-SPOT.TB) | ||||||
| Xpert MTB/RIF | TB with HIV-negative | 85 (pooled) | 98 (pooled) | Below 2 h | As an initial test for suspected of TB and EPTB; RIF- resistance detection | High needs for infrastructure and maintenance | [19, 28, 60] |
| 96 (RIF resistance) | 98 (RIF resistance) | ||||||
| TB with HIV-positive | 81 | 98 | |||||
| Xpert MTB/RIF ultra | TB with HIV-negative | 90 (pooled) | 96 (pooled) | Below 2 h | As an initial tests in suspected of TB and EPTB; RIF- resistance detection | Expensive instrument; high needs for infrastructure and maintenance | [19, 60] |
| 94 (RIF resistance) | 99 (RIF resistance) | ||||||
| TB with HIV-positive | 88 | 95 | |||||
| TB-LAMP | TB with HIV-negative | 78 | 98 | Within 2 h | As a replacement test for sputum smear microscopy in adults with TB signs; as a follow-on test to smear microscopy in adults with EPTB signs | Expensive instrument; unable to identify drug resistance | [19, 36] |
| TB with HIV-positive | 64 | 99 | |||||
| LPA | TB with HIV-negative | 88.4 (pooled) | 94.6 (pooled) | 1–2 days | First-line LPAs: As an initial test to detect resistance to RIF and INH | Unable to completely replace culture; require for specialized skills and high-level biosafety laboratories | [19, 24, 63] |
| 96.7 (RIF resistance) | 98.8 (RIF resistance) | ||||||
| 90.2 (INH resistance) | 99.2 (INH resistance) | Second-line LPAs: As an initial test to detect resistance to FQs, SLID. | |||||
| TB with HIV-positive | − | − | |||||
| LF-LAM | TB with HIV-negative | − | − | Within 15–20 min | As an additional point-of-care test for TB in PLHIV; an auxiliary test in PLHIV combined with other detection techniques | The sensitivity increases with a decrease in CD4+T cell | [19, 50] |
| TB with HIV-positive | 42 (pooled) | 92 (pooled) | |||||
| 16 (CD4+T cell count >200 cells/μL) | 94 (CD4+T cell count >200 cells/μL) | ||||||
| 45 (CD4+T cell count ≤200 cells/μL) | 89 (CD4+T cell count ≤200 cells/μL) | ||||||
| FujiLAM | TB with HIV-negative | − | − | Within a day | Use in children with suspected TB or PLHIV with signs and symptoms of TB; immunosuppressed people; as a rapid test to confirm TB diagnosis in PLHIV | Clinical trails insufficient | [50] |
| TB with HIV-positive | 60 (pooled) | 87 (pooled) | |||||
| 47 (CD4+T cell count ≥200 cells/μL) | 88 (CD4+T cell count ≥200 cells/μL) | ||||||
| 69 (CD4+T cell count <200 cells/μL) | 84 (CD4+T cell count <200 cells/μL) |
- Abbreviations: CM, conventional microscopy; EPTB, extrapulmonary tuberculosis; FM, fluorescence microscopy; FujiLAM, Fujifilm SILVAMP TB LAM; Fqs, fluoquinolones; HIV, human immunodeficiency virus; IGRAs, interferon gamma release assay; INH, isoniazid; LF-LAM, lateral flow lipoarabinomannan; LPA, line probe assay; LTBI, Latent tuberculosis; Mtb, Mycobacterium tuberculosis; PLHIV, people living with HIV; QFT-GIT, QuantiFERON-TB Gold In-Tube; RIF, rifampicin; SLID, second-line injectable drugs; TB, pulmonary tuberculosis; TB-LAMP, tuberculosis loop-mediated isothermal amplification; TBST, mycobacterium tuberculosis antigen-based skin test; WHO, World Health Organization.
- a Performance estimates have been retrieved from different studies and are not the result of head-to-head comparisons. Therefore, comparing performances between tests must be made with caution.
The first-generation commercial IGRAs for TB diagnosis, T-SPOT. TB, is widely used in countries with a high TB burden. However, this assay, which is based on the same principle as enzyme-linked immunospot (ELSPOT), faces the same problem as the TST method, in that it cannot distinguish between ATB and LTBI [13]. Studies have shown that ELISPOT has low sensitivity (77.4%) for diagnosing active tuberculosis (ATB) in patients with HIV/TB co-infection [16]. Another commonly used method called QuantiFERON-TB Gold Tube (QFT-GIT) is based on the lymphocyte response to Mtb-specific antigens (ESAT-6 and CFP-10) [17] and is more sensitive than TST at diagnosing latent tuberculosis infection (LTBI) with higher sensitivity [18]. WHO recommended QFT-GIT as an auxiliary diagnosis methods in detecting Mtb, especially in LTBI populations [19]. However, QFT-GIT has limited sensitivity for detecting ATB in patients with HIV/TB co-infection patients. Thus, the updated product QuantiFERON-TB Gold Plus (QFT-Plus) was developed to provide improved sensitivity in immunocompromised subjects and young children. As of 2021, the new ELISA-based IGRA assays recommended by the WHO for TB infection diagnosis are QFT-Plus and WANTAI TB-IGRA (Figure 3) [19]. Compared with the last generation product (QFT-GIT), QFT-Plus contains an additional tube that allows for the assessment of antigen-specific responses by both CD4+ and CD8+ T cells [20]. Petruccioli, Elisa et al. detected both CD4+ and CD8+T-cells responses using the QFT-Plus stimulation system. They showed that HIV-infected and HIV-uninfected individuals exhibited similar CD8+ T cell responses, indicating similar sensitivity in patients with HIV/TB co-infection and patients with TB only (90.9% vs. 91.9%) [21]. Thus, QFT-Plus might be useful for screening HIV-infected patients in low-income areas for TB [21]; however, larger, multi-center studies are needed to confirm these results.
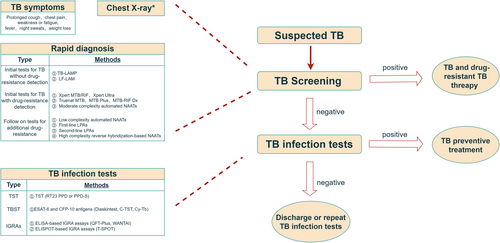
WHO-recommended TB diagnosis flow diagram. CFP-10, culture filtrate protein 10; ELISA, enzyme-linked immunosorbent assay; ELISPOT, enzyme-linked immunospot; ESAT-6, early secretory antigenic target 6 kDa protein; IGRA, interferon-gamma release assay; LAMP, loop-mediated isothermal amplification; LF-LAM, lateral flow urine lipoarabinomannan assay; LPA, line-probe assay; NAAT, nucleic acid amplification tests; PPD, purified protein derivative; QFT-Plus, QuantiFERON-TB Gold Plus; TB, tuberculosis; TBST, Mycobacterium tuberculosis antigen-based skin test; T-SPOT, Oxford Immunotec T-SPOT®.TB; TST, tuberculin skin test; WANTAI, Beijing Wantai's TB-IGRA. * Radiographic features depend on the type of infection and are discussed separately.
3 RECENT ADVANCES IN MOLECULAR DIAGNOSTIC METHODS FOR TUBERCULOSIS
3.1 Line probe assays
Line probe assays (LPAs), which are based on polymerase chain reaction (PCR), are mainly used to detect drug-resistant tuberculosis, especially rifampicin (RIF)-resistant tuberculosis, isoniazid (INH)-resistant tuberculosis and multidrug-resistant (MDR) tuberculosis. However, the utility of this method in patients with HIV/TB co-infection is limited for the following reasons: (a) LPAs requires a sufficient amount of bacillary DNA load, but the acid-fast bacilli (AFB) load in patients with HIV/TB co-infection is low [22]; (b) nearly 24%–61% [23] of patients with HIV/TB co-infection have AFB smear-negative sputum, and the sensitivity of LPAs for detecting AFB in smear-negative samples is lower (44.1%) than it is in AFB smear-positive samples (94.6%) [24]. Therefore, LPAs was recommended only for detecting drug-resistant TB, and not AFB smear-negative TB, by the WHO in 2008 (Figure 3) [14]. Thus, the LPAs needs to be updated for more accurate Mtb testing in AFB smear-negative samples (e.g., patients with HIV/TB co-infection).
3.2 Xpert MTB
3.2.1 Xpert MTB/RIF
A milestone in TB diagnosis was reached when nucleic acid amplification (NAAT) methods were developed. An automated real-time polymerase chain reaction assay (real-time PCR) assay called Xpert MTB/RIF (Cepheid, CA, USA) was designed for a rapid detection of Mtb and RIF resistance. The Xpert MTB/RIF test shows high sensitivity and specificity for the detection of drug-resistant TB, including 94.4% sensitivity and 98.3% specificity for RIF-resistant TB [25-27]. In addition, the Xpert MTB/RIF test is superior to smear microscopy with high accuracy specificity and sensitivity in pooled samples (89% and 99%, respectively), whereas the sensitivity in HIV-positive cases and HIV-negative cases is 79% and 86%, respectively [28].
3.2.2 Xpert MTB/RIF Ultra
To overcome the limitations of the Xpert MTB/RIF assay, the next-generation Xpert MTB/RIF Ultra (Xpert Ultra) assay was developed. While the Xpert Ultraassay uses the same GeneXpert platform as Xpert MTB/RIF, it has two different amplification targets (IS6110 and IS1081) and a larger DNA reaction chamber (50 μL vs. 25 μL)than Xpert MTB/RIF [27, 29]. Additionally, Xpert Ultra incorporates a melt curve analysis system to improve the accuracy of rifampicin-resistance detection. In contrast, Xpert relies on cycle threshold (Ct) values to detect rifampicin resistance, which may generate false-positive results, especially in samples that lack a sufficient amount of DNA [26, 34]. Xpert Ultra has a lower detection threshold for Mtb than Xpert (9 cfu/mL vs. 184 cfu/mL) [33]. More importantly, the WHO recommended Xpert Ultra as the initial test for TB and rifampicin resistance in both adults and children presenting with signs and symptoms of pulmonary TB in its most recent guidelines (2021) (Figure 3) [19]. As known, Tuberculous meningitis (TBM) is the most lethal and disabling type of TB, especially in patients who are also infected with HIV, in whom TB co-infection is associated with a high risk of death (50%) [29]. Although Xpert can be available for detecting Mtb in non-sputum specimens, such as pleural fluid and lymph node tissue, it has only 55% sensitivity for diagnosing TBM by using cerebrospinal fluid (CSF) [30]. In contrast, Xpert Ultra is more sensitive than Xpert for diagnosing TBM using CSF or liquid specimens culture (95.5% vs. 45.5%) [31]. In HIV-positive people with suspected meningitis, Xpert Ultra exhibits greater sensitivity than Xpert (70% vs. 43%) [27]. In recent studies, Xpert Ultra showed greater sensitivity than Xpert MTB/RIF in diagnosing TB in the general population (87.5% vs. 81.0%) [32, 33] in general populations, and TB among HIV-positive patients (87.6% vs. 74.9%) [34, 35], respectively. However, Xpert Ultra has slightly lower specificity and is slightly more expensive than Xpert MTB/RIF [32, 34]. In countries where individuals are high risk of HIV/TB co-infection, the increased sensitivity of Xpert Ultra could help significantly reduce mortality. However, in areas with low TB prevalence, the lower specificity may lead to overtreatment of false-positive cases [34]. Thus, Xpert Ultra or Xpert should be chosen on the basis of clinical demands and cost-effectiveness.
3.3 Loop-mediated isothermal amplification
In contrast to the high-cost Xpert MTB/RIF assay, the rapid (within 2 h) [36] and less expensive molecular test called TB-LAMP can be used to identify Mtb using the method of loop-mediated isothermal amplification (LAMP). TB-LAMP is easier to perform and yields clearer results than Xpert MTB/RIF, which needs a continuous supply or electricity and a temperature-controlled environment. A multi-center study reported that TB-LAMP has high sensitivity of 80% and high specificity of 96% [36]. However, TB-LAMP alone has low sensitivity (only 52.3%) for diagnosing TB in HIV-positive individuals [37]. Thus, the WHO only recommended TB-LAMP as an alternative method to microscopy in patients with typical indications and specific clinical symptoms of TB in 2016 (Figure 3) [38]. TB-LAMP combined with liquid culture and drug susceptibility testing can diagnose TB more accurately in HIV-positive patients than TB-LAMP alone [39].
3.4 Lipoarabinomannan (LAM)
3.4.1 LF-LAM
A more useful diagnostic technique for tuberculosis in patients with HIV/TB co-infection patients who fails to produce sputum (or produce scarce sputum) is the detection of urinary lipoarabinomannan (LAM) levels. LAM is an Mtb cell wall lipopolysaccharide. As WHO stated in 2015, the lateral flow LAM (LF-LAM) assay is a sensitive, easy-to-handle, and rapid technique for detecting various forms of TB in a variety of patient populations, including smear-negative TB patients, children with TB, and TB patients with HIV/TB co-infection. Peter et al. reported that the urinary LAM testing used as a rapid adjunct can reduce the 8-week mortality (4%) of patients with HIV/TB co-infection [40]. Furthermore, TB co-infection can be detected and diagnosed in approximately 35%–42% of patients with AIDS using LF-LAM [41-43]. Positive LF-LAM assay results, therefore, are considered an independent risk factor for death in patients with HIV/TB co-infection [43]. However, the sensitivity of the LF-LAM assay depends on CD4+ T-cell count: as one study reported, the sensitivity is 56% in subjects with a CD4+ T cell count ≤100 cells/μL but only 16% in subjects with a CD4+ T-cell count >200 cells/μL [44]. Therefore, combined Xpert/LF-LAM testing for tuberculosis diagnosis is considered to be more cost-effective than sputum smear microscopy assay or Xpert assay alone, especially in HIV-positive patients with a CD4+ T cell count <100 cells/μL [45]. Furthermore, combining Xpert MTB/RIF with urine LF-LAM testing can increase the overall TB diagnostic sensitivity to 75% and specificity to 93% [46]. Thus, the commercially available LF-LAM assay is a potentially useful for rapid point-of-care testing (POCT), but its sensitivity is suboptimal in patients with AIDS. More advanced methods are needed to diagnose TB in this patient population.
3.4.2 FL-FAM
Recently, the new generation FL-FAM—Fujifilm SILVAMP TB LAM (FujiLAM) assay was shown to have a higher sensitivity than LF-LAM assay (68.1% vs. 44.7%) for detecting EPTB in AIDS with patients [47, 48]. This technique combines two high-affinity monoclonal antibodies against the Mtb-specific antigen MTX-LAM with a silver-based amplification step [49, 50]. A meta-analysis by Broger et al. founded that FujiLAM has a significantly increased the positive detection rate for TB compared with LF-LAM assay (70.7% vs. 34.9%) in patients with HIV [48]. The FujiLAM assay is therefore highly valuable for diagnosing TB in patients with HIV. In addition, the FujiLAM assay is easy to perform, and can be performed more rapidly than the LF-LAM assay. Thus, it has the potential to become a new POCT diagnostic tool for HIV-positive individuals, although larger prospective studies are needed to verify these results.
4 FUTURE OPPORTUNITIES FOR AND POTENTIAL CHALLENGES OF TUBERCULOSIS DIAGNOSIS
Although numerous studies have attempted to identify a highly sensitive, highly specific, rapid, and cost-effective method for diagnosing TB, no single test meets all of these requirements (Figure 1). Therefore, recent advances in molecular techniques have led to the proposal of the next-generation diagnostic platforms for TB that could dramatically improve the rapidity and accuracy of TB diagnosis.
4.1 CRISPR/Cas
Clustered regularly inter-spaced short palindromic repeat (CRISPR)/CRISPR-associated protein (Cas) can be used to detect Mtb with high sensitivity and specificity. CRISPR/Cas systems recognize specific nucleic acid sequences and initiate a nucleic acid sequence cleavage. The Cas family proteins, with include Cas12, Cas13 and Cas14, mediate non-specific cleavage of other nucleic acid sequences in their activated state and can recognize and cleave specific target sequences under gRNA guidance [51]. For example, the CRISPR-Cas12b-based platform can detect as few as 0.06 copies/μL of Mtb-cfDNA (Mtb-IS6110 gene) in blood samples within 1 h [52], and could therefore be used to diagnose cases with few TB bacilli, such as children with HIV/TB co-infection [51]. Interestingly, Sam et al. combined LAMP with CRISPR/Cas12b technique to develop a novel MTB genetic detection platform, called TB-QUICK, that exhibited higher sensitivity than Xpert (80.5% vs. 57.1%) and culturing (80.5% vs. 46.2%) in AFB-negative samples [51]. CRISPR-based diagnostics have been developed only recently, however (within the past 5 years), so a substantial amount of work is still needed to translate this technique from bench to bedside.
4.2 Whole-genome sequencing
Recently, whole genome sequencing (WGS) has been used for Mtb molecular epidemiology and laboratory diagnostics. WGS not only detects tuberculosis with extreme precision, it can also detect drug-resistance mutations, which can predict drug susceptibility and the epidemiology of different TB strains. One systematic review showed that WGS has high sensitivity and specificity for detecting drug-resistant Mtb, at 98% and 97%, respectively, for rifampicin and 97% and 96%, respectively, for isoniazid (Figure 1) [53, 54]. In 2018, the WHO recommended that WGS be used to diagnose TB and detect drug-resistant mutations. Although it has high sensitivity for Mtb and can accurately detect Mtb drug resistance, this method still faces many challenges regarding the diagnosis of patients with HIV/TB co-infection. First, it is difficult to obtain enough bacterial DNA for sequencing and further analysis. Second, even in areas with high TB prevalence, it is a high-cost approach that is not suitable for low-income areas.
4.3 Droplet digital polymerase chain reaction
Droplet digital polymerase chain reaction (ddPCR), as a new generation of precision nucleic acid quantification technology, is commonly used to detect rare mutations in various pathogenic microorganisms, such as hepatitis B virus (HBV), hepatitis C virus (HCV), and enterovirus. DdPCR has a lower limit of detection for circulating virulent M. tb-specific CFP10 compared with real-time fluorescent quantitative PCR (qPCR) (1.2 copies/μL vs. 15.8 copies/μL) [55]. The sensitivity and specificity of ddPCR using exoDNA as a template are 76.9% and 98.0%, respectively [56]. Conceivably, ddPCR could be used to quantify Mtb in patient specimens, and could therefore be broadly applied for rapid and accurate diagnosis of TB.
4.4 Matrix-assisted laser desorption ionization-time of flight mass spectrometry
Very recently, matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) has been shown to be very efficacy at identifying bacteria. Cao et al. founded that this method has a high rate of accuracy in identifying nontuberculous mycobacteria (NTM) (e.g., M. haemophilum) and Mycobacterium tuberculosis at 93% and 92%, respectively [57]. However, the technique has difficultly to distinguishing among different mycobacterial species owing to their high degree of genetic similarity. In addition, the high cost of MALDI-TOF MS equipment means that the assay is unavailable in resource-limited countries.
4.5 Raman spectroscopy
Raman spectroscopy (RS) detects the vibrational energy levels of molecular bonds within a sample [64, 65]. When a laser light source is directed at a sample, a unique pattern of light is scattered back, known as a Raman spectrum [66]. In the context of TB diagnosis, RS can detect the unique chemical composition of Mtb bacteria. The cell walls of TB bacteria contain characteristic molecules, such as mycolic acids and lipids, that can be detected by RS [64]. By analyzing the Raman spectra of a sample, it is possible to identify the presence of Mtb bacteria (with or without the presence of other microorganisms), as well as the strain and the drug resistance profile. However, there are limitations to the application of RS to TB detection, specifically: (a) complex sample preparation: RS requires samples to be prepared in a suitable form, such as a thin film or powder, so specimens such as sputum or blood may result in a poorer Raman signal [67]; (b) cost-effectiveness: RS equipment is too expensive to be used for TB detection particularly in countries with limited healthcare resources; (c) time-consuming: RS also requires complex data analysis and interpretation, which can be time-consuming and may not be feasible in some clinical settings where rapid diagnosis is critical. Therefore, more basic research is needed to determine whether RS-based TB diagnosis is suitable for use in a clinical setting (Figure 1).
4.6 Exosome detection
Recent studies have shown that exosomes are a potential biomarker for TB (Figure 1). These small, membrane-bound vesicles that contain specific proteins and nucleic acids associated with TB infection are released by cells into the extracellular space, and can be isolated from biological samples such as blood, urine, and sputum. Urine-based TB-exosome testing is a useful and promising diagnostic tool for TB, as TB-exosomes levels are correlated with disease severity [68]. Another potential application of TB-exosomes is to distinguish between ATB and LTBI by detecting exosomal miRNA expression in blood samples with high sensitivity and specificity [69-71]. However, further research is needed to validate the diagnostic utility of TB-exosomes.
5 DISCUSSION
In the past 2 decades, numerous techniques have been developed for diagnosing Mtb, including microbiological-based assays, immunological-based assays, and molecular techniques. With continuing advances in molecular biology, more effective and sensitive diagnostic methods have been developed and implemented to enhance tuberculosis control (Figure 2). However, no single technical approach is fully capable of diagnosing all cases of TB, especially for HIV-associated TB (Table 1). Normally, TB diagnosis is based on screening tests, assessment of clinical symptoms (prolonged cough, chest pain, weakness or fatigue, fever, night sweats, or weight loss), and chest X-ray (Figure 3). Clinical symptoms and chest X-ray signs are key factors in routine TB diagnosis. However, one third of cases of HIV-associated TB are asymptomatic and present with non-specific chest X-ray signs, such as lower lobe infiltrates, hilar or mediastinal lymphadenopathy instead of lung fibrosis, cavitation, or upper-lobe illness, which makes it more challenging to diagnose TB in HIV-infected individuals than in otherwise healthy persons.
The gold standard for TB diagnosis is smear microscopy, which is rapid and cost-effective but has low sensitivity in patients with HIV/TB co-infection, a high proportion of whom produce very little sputum. Sputum culture, another common method, is time-consuming and requires access to a laboratory with a high biosafety level, so it is not practical for rapid diagnosis of TB (Figure 3). Immunodiagnostic assays (including TST and IGRAs) are highly dependent on CD4+ T cells count and function. Thus, the low CD4+ T-cell counts seen in immunocompromised patients, such as those with AIDS, may lead to false-negative results in immunological assays, rendering the tests invalid. Another way to diagnose tuberculosis is to amplify Mtb-specific nucleic acids from patient specimens using PCR. However, Mtb DNA levels are low in the scarce sputum samples that can be obtained from patients with HIV/TB co-infection, limiting the utility of this approach. Finally, molecular diagnostic methods, including TB-LAMP and Xpert, are significantly less sensitive in diagnosing patients with smear-negative TB that those with smear-positive TB, particularly in the setting of HIV co-infection [28].
According to WHO guidelines, an ideal method for TB diagnosis is accurate, affordable, rapid, and easy to perform. The diagnosis of TB in patients with HIV continues to be more challenging than the diagnosis of TB alone. Promising future directions for developing new approaches for TB diagnosis include molecular techniques, such as WGS, and CRISPR/Cas. However, various bottlenecks still limit the translation of these techniques from bench to bedside. First, it remains unclear whether tests based on these methods can be implemented in a cost-effective manner. Second, because these technologies have only recently been developed (mostly within the past 5 years), they need to be validated in well-designed and strictly controlled clinical trials. Overall, the techniques mentioned above are not suitable for replacing the culture-based methods that are currently used in clinical microbiology laboratories. Thus, combining multiple diagnostic tools is likely to provide the most clinical benefit and practical value.
AUTHOR CONTRIBUTIONS
Conceptualization: Hongzhou Lu; Project administration: Zixun Lin, Hongzhou Lu, Qian Li; Supervision: Hongzhou Lu, Qian Li; Writing of the original draft: Zixun Lin, Qian Li; Revision of the paper: Qian Li, Liqin Sun, Hongzhou Lu, Cheng Wang, Fuxiang Wang. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
The authors wish to acknowledge and thank all participants for their contributions.
CONFLICT OF INTEREST STATEMENT
Professor Hongzhou Lu is the member of the iLABMED Editorial Board. To minimize bias, he was excluded from all editorial decision-making related to the acceptance of this article for publication. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
No human subjects were involved in this study; therefore, consent was waived.
INFORMED CONSENT
All participants provided written informed consent prior to inclusion in this study.
Open Research
DATA AVAILABILITY STATEMENT
There is no experiment data in this review.




