One-carbon metabolism biomarkers and upper gastrointestinal cancer in the Golestan Cohort Study
Abstract
Incidence of esophageal and gastric cancer has been linked to low B-vitamin status. We conducted matched nested case–control studies of incident esophageal squamous cell carcinoma (ESCC; 340 case–control pairs) and gastric cancer (GC; 352 case–control pairs) within the Golestan Cohort Study. The primary exposure was plasma biomarkers: riboflavin and flavin mononucleotide (FMN) (vitamin B2), pyridoxal phosphate (PLP) (B6), cobalamin (B12), para-aminobenzoylglutamate (pABG) (folate), and total homocysteine (tHcy); and indicators for deficiency: 3-hydroxykyurenine-ratio (HK-r for vitamin B6) and methylmalonic acid (MMA for B12). We estimated odds ratios (ORs) and 95% confidence intervals (CIs) using conditional logistic regression adjusting for matching factors and potential confounders. High proportions of participants had low B-vitamin and high tHcy levels. None of the measured vitamin B levels was associated with the risk of ESCC and GC, but elevated level of MMA was marginally associated with ESCC (OR = 1.42, 95% CI = 0.99–2.04) and associated with GC (OR = 1.53, 95% CI = 1.05–2.22). Risk of GC was higher for the highest versus lowest quartile of HK-r (OR = 1.95, 95%CI = 1.19–3.21) and for elevated versus non-elevated HK-r level (OR = 1.59, 95% CI = 1.13–2.25). Risk of ESCC (OR = 2.81, 95% CI = 1.54–5.13) and gastric cancer (OR = 2.09, 95%CI = 1.17–3.73) was higher for the highest versus lowest quartile of tHcy. In conclusion, insufficient vitamin B12 was associated with higher risk of ESCC and GC, and insufficient vitamin B6 status was associated with higher risk of GC in this population with prevalent low plasma B-vitamin status. Higher level of tHcy, a global indicator of OCM function, was associated with higher risk of ESCC and GC.
Graphical Abstract
What's new?
One-carbon metabolism (OCM) refers to a series of biochemical reactions essential for DNA methylation, synthesis, and repair. These reactions are often catalyzed by enzymes and cofactors derived from B vitamins, and B vitamin deficiency has been linked to cancer. Here, the authors investigated the relationship between B vitamin deficiency and risk of esophageal and gastric cancers in Iran, where the incidence of these cancers is high. Elevated levels of plasma biomarkers that indicate vitamin B6 and B12 deficiency were associated with higher risk of esophageal and gastric cancers, they found. Improving vitamin intake could reduce the incidence of these cancers in the populations with high prevalence of vitamin B deficiency.
1 INTRODUCTION
Esophageal and gastric cancers are major causes of morbidity and mortality worldwide. Incidence and mortality of these cancers vary by region and culture in the world.1, 2 While those cancers make up a small proportion of cancer diagnosed in many Western countries, esophageal cancer is much more common Asia and eastern and southern Africa.3 Within these high-incidence regions, esophageal squamous cell carcinoma (ESCC) makes up 90% of esophageal cancer cases as opposed to lower-incidence regions where esophageal adenocarcinoma is more common.4 Gastric cancer (GC) incidence and mortality are high particularly in eastern and central Asia (e.g., Japan, China).2, 5 Besides well-established risk factors, such as Helicobacter pylori (H. pylori) infection (for GC), tobacco smoking, and alcohol drinking (for ESCC), certain lifestyle factors including low fruit and vegetable intake, high intake of food rich in nitrogenous components, and opium use have been identified as risk factors for ESCC and GC.2, 3, 6, 7 Yet, those factors do not completely explain the regionally and culturally varied incidence and mortality of these cancers in the world.
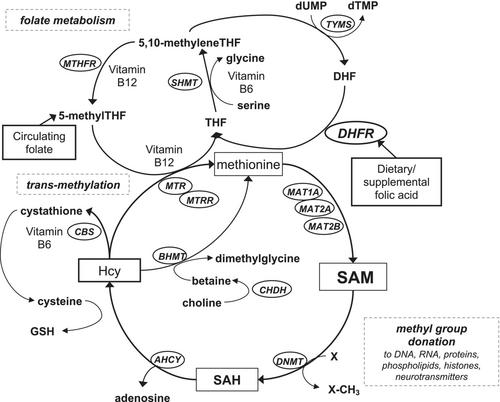
One-carbon metabolism (OCM) encompasses a series of biochemical reactions that are essential for DNA methylation, synthesis, and repair (Figure 1). Folate metabolism is a central part of OCM by transferring methyl groups, but many enzymes and co-factors which catalyze metabolic reactions in OCM are derived from other B-vitamins. Therefore, a deficiency or imbalance of B-vitamins and other OCM metabolites can interfere with DNA methylation, repair, and replication, leading to carcinogenesis. B-vitamins are essential water-soluble vitamins and need to come from dietary sources. Vitamin B deficiency is commonly observed in certain populations, where the sources of B-vitamins are mainly diets limited in food rich in those vitamins and not dietary supplements or food fortification. In the previous study in the rural area of China, where ESCC and GC incidences are high and vitamin B deficiency is commonly observed, lower risk of ESCC was observed among people with higher serum riboflavin and cobalamin.8 Circulating cobalamin was also inversely associated with risk of GC in previous studies in Europe.9, 10
Here, we evaluated plasma levels of B-vitamins and other biomarkers of OCM in relation to esophageal and gastric cancers in a large prospective cohort study in Iran, where incidence of these cancers is high and B-vitamin intake was not mainly from dietary supplements or fortified food.
2 MATERIALS AND METHODS
2.1 Study population
Participants of the current study were selected from the Golestan Cohort Study (GCS), a large prospective cohort study. The study design has been described in detail elsewhere.11 In short, 50,045 men and women aged 40–75 were recruited from January 2004 to June 2008 in the Golestan Province of Northeastern Iran. At the enrollment, trained interviewers administered a comprehensive questionnaire to obtain detailed information on demographics, lifestyle factors, family history, medical history, and diet. Study participants have been followed for incident cancer and deaths.
2.2 Case and control selection
Incident cancer and death were identified by annual telephone surveys or home visits for all study participants. Provincial cancer and death registry databases were also reviewed monthly to identify incident cancer cases and deaths. When an incident cancer was reported, trained staff visited the participant's home and the medical center where diagnostic or therapeutic procedures were performed. All clinical and pathology reports and hospital records were obtained. Incident cancer cases and deaths were independently verified by two physicians based on available documents, and the date of outcome occurrence and the International Classification of Diseases, Tenth Revision (ICD-10) were determined by each physician. When the diagnosis disagrees between physicians, a third physician independently reviewed the documents and made the final diagnosis.
In the esophageal cancer study, we included 340 incident ESCC cases confirmed through January 15, 2021, and had at least two straws of blood sample available in the repository. For the GC study, we included 352 incident cases (206 cardia, 98 non-cardia, and 48 unknown or overlapping subtype) with a confirmed diagnosis through January 15, 2021, and had at least two straws of blood sample available in the repository. We excluded one GC case diagnosed with neuroendocrine carcinoma and all others were adenocarcinomas. We randomly selected one-to-one matched controls for both cancer sites (n = 340 for ESCC and n = 352 for GC) by incidence density sampling without replacement, matching by age (± 5 years), sex, place of residence (urban or rural), enrollment period, duration of follow-up in the cohort, and tobacco use (for ESCC only; never, former, current cigarettes or hookah, current nass, current cigarettes, hookah, or nass).
2.3 Plasma biomarkers of OCM
Blood samples were collected by a trained technician at enrollment. In the urban areas, blood samples were immediately centrifuged and aliquoted in 500 μL straws in the central laboratory and stored at −80°C. In the rural area, blood samples were kept in refrigerators at 4°C and transferred in cooling boxes to the central laboratory for processing. The maximum duration from blood sample collection to final processing was 8 h. Otherwise, samples were handled identically. Half of the samples were subsequently shipped to the IARC repository in Lyon, France, at regular intervals, where they were stored in nitrogen vapor at approximately −135°C.
Plasma samples for the current study were shipped from the IARC repository to the laboratory of BEVITAL in Bergen, Norway, where the samples were aliquoted. Assays were performed for biomarkers including riboflavin and flavin mononucleotide (FMN) ((vitamin B2) vitamer), pyridoxal phosphate (PLP; vitamin B6), para-aminobenzoylglutamate equivalents (pABG; folate), 3-Hydroxykynurenine (HK), kynurenic acid (KA), anthranilic acid (AA), xanthurenic acid (XA), and 3-hydroxyanthranilic acid (HAA) by a liquid chromatography tandem mass spectrometry (LC–MS/MS), cobalamin (vitamin B12) by a microbiological assay, and methylmalonic acid (MMA) and total homocysteine (tHcy) by a gas chromatography tandem mass spectrometry (GC–MS/MS). Because flavin is light sensitive and subject to degradation, we measured FMN, one of the most biologically active forms of riboflavin, and computed a sum of riboflavin and FMN as an indicator of vitamin B2 status. In addition to cobalamin, we used MMA, as an indicator of vitamin B12 status, because MMA is stable and unlikely affected by preparation and stability of blood samples. Under vitamin B12 deficiency, MMA levels increase because MMA reacts with vitamin B12 to produce coenzyme A.12 tHcy levels can be high with both vitamin B12 and folate deficiency; therefore, using both tHcy and MMA may enable us to differentiate folate deficiency and vitamin B12 deficiency. Besides PLP, the prevailing vitamin B6 form in plasma and the most commonly used indicator of vitamin B6 status, we computed HK ratio (HK-r), a novel functional marker of vitamin B6, by computing a ratio of HK to a sum of four products of the PLP-dependent enzymes (KA, XA, AA, and HAA).13, 14 As a folate biomarker, we measured pABG, which can be used to assess folate status in biosamples stored for decades.15 The plasma samples were randomly placed on the plate and a matched case and control were placed next to each other. We placed three pooled quality control (QC) samples per plate to test within and across plate variabilities. Measured levels of nine biomarkers and functional indicators in QC samples showed good overall agreements with coefficients of variation (CVs) ranging from 2.9 to 10.6%. Besides the pooled QC samples created within the GCS, BEVITAL placed three in-house control plasma samples with known biomarker concentrations in each plate, and good validity was shown with CVs ranging from 2.6 to 10.3%. For 28 participants (4%; 17 cases and 11 controls) of the ESCC study and 43 participants (6%; 22 cases and 21 controls) of the GC study whose cobalamin values were under detectable limit (<30 pmol/L), we imputed their cobalamin values by the detectable limit value divided by square root of 2 (=21.213 pmol/L). We assessed correlations between biomarkers and indicators by Pearson correlation coefficients (r). Participants were categorized based on quartiles of each biomarker or indicator among controls for each cancer.
2.4 Covariates
For socioeconomic status, the composite wealth score was previously created using multiple correspondence analyses with data on ownership of household items.16 Data on oral health were obtained during the physical exam by dentist-trained health personnel at study baseline and parameterized into an age-specific metric.17, 18 Dietary intake was collected using the food frequency questionnaire by trained interviewers. Using these data, total fruit intake (g/day), total vegetable intake (g/day), and processed meat intake (g/day) were calculated. H. pylori antibody assays were performed in the previous GC case–control study using a multiplex serology method to assess 15 antibodies against H. pylori proteins.19 This information was available for 530 participants (265 matched case–control pairs) of the current GC study. H. pylori seropositivity was determined as the number of H. pylori positive proteins (<3 or > =3).19
2.5 Statistical analysis
We computed odds ratios (ORs) and their 95% confidence intervals (CIs) for each cancer outcome by quartiles of each biomarker or indicator using conditional logistic regression models adjusted for matching factors and potential cofounders determined by literature review and prior experience in this cohort, including age, sex, place of residence (urban or rural), tobacco use (for GC only; never, former, current cigarettes or hookah, current nass, current cigarettes, hookah, or nass), current alcohol intake (yes or no), current opium use (yes or no), preferred tea temperature (<60°C, 60–64°C, ≥ 65°C, or no tea drinking), number of teeth lost (expected or less; and more than expected, quartile 1 to 4), and dietary intake of total fruits (g/day), total vegetables (g/day), and processed meat (g/day). A missing category was created for a small number of participants who lacked information on tea temperature or dietary factors. Linear associations were assessed using a continuous scale of half an interquartile range (IQR) as a unit. For GC, we assessed the associations for all cases, and cardia and non-cardia cancer separately. We further adjusted the GC associations for H. pylori seropositivity as the number of H. pylori positive proteins (<3 or ≥3) among 530 participants who had H. pylori data.
We also assessed ORs by sufficient and deficient status (for riboflavin, FMN, sum of riboflavin and FMN, PLP, cobalamin, and pABG) or by non-elevated and elevated status (MMA, HK-r, and tHcy) of biomarkers referring to the BEVITAL's commonly reported levels using the same conditional logistic regression model: cut points for biomarkers or indicators were 5 nmol/L for riboflavin, 3 nmol/L for FMN, 8 nmol/L for a sum of riboflavin and FMN, 15 nmol/L for PLP, 0.4 for HK-r, 150 pmol/L for cobalamin, 0.26 μmol/L for MMA, 7.5 nmol/L for pABG, and 15 μmol/L for tHcy. These cutpoints were defined based on typical concentrations of these analytes in prior studies.20 All analyses were performed using SAS version 9.4. P-values <.05 were considered statistically significant. We did not adjust for multiple comparisons as each biomarker and each disease represent distinct hypotheses.
3 RESULTS
Among 680 adults in the ESCC study and 704 adults in the GC study, the average age was 58 to 59 years both in cases and controls (Table 1). ESCC cases were more likely to be Turkmen, have no or less formal education, and drink tea at higher temperature compared with ESCC controls. GC cases were more likely to have less education and never brush teeth or brush teeth non-daily compared with the controls.
| ESCC | GC | |||||
|---|---|---|---|---|---|---|
| Case | Control | p | Case | Control | p | |
| Number of subjects | 340 | 340 | 352 | 352 | ||
| Sexa | ||||||
| Male | 175 (51.5) | 175 (51.5) | 1.00 | 256 (72.7) | 256 (72.7) | 1.00 |
| Female | 165 (48.5) | 165 (48.5) | 96 (27.3) | 96 (27.3) | ||
| Age,a mean (SD) | 58.6 (8.9) | 58.3 (8.9) | .72 | 58.1 (8.9) | 57.8 (9.1) | .69 |
| Ethnicity | ||||||
| Turkmen | 293 (86.2) | 264 (77.7) | .004 | 280 (79.6) | 281 (79.8) | .93 |
| Otherb | 47 (13.8) | 76 (22.3) | 72 (20.4) | 71 (20.2) | ||
| Education | ||||||
| No formal education | 294 (86.5) | 277 (81.5) | .004 | 283 (80.4) | 252 (71.6) | .02 |
| ≤ 8 years | 46 (13.5) | 53 (15.6) | 47 (13.4) | 61 (17.3) | ||
| > 8 years | 0 (0.0) | 10 (2.9) | 22 (6.2) | 39 (11.1) | ||
| Residencya | ||||||
| Urban | 30 (8.8) | 30 (8.8) | 1.00 | 50 (14.2) | 50 (14.2) | 1.00 |
| Rural | 310 (91.2) | 310 (91.2) | 302 (85.8) | 302 (85.8) | ||
| Composite wealth scorec | ||||||
| Q1 (lowest SES) | 146 (42.9) | 123 (36.2) | .27 | 138 (39.2) | 124 (35.2) | .39 |
| Q2 | 86 (25.3) | 88 (25.9) | 75 (21.3) | 75 (21.3) | ||
| Q3 | 70 (20.6) | 85 (25.0) | 82 (23.3) | 79 (22.5) | ||
| Q4 (highest SES) | 38 (11.2) | 44 (12.9) | 57 (16.2) | 74 (21.0) | ||
| Tobacco used | ||||||
| Never | 235 (69.1) | 235 (69.1) | 1.00 | 221 (62.8) | 227 (64.5) | .46 |
| Former | 13 (3.8) | 13 (3.8) | 24 (6.8) | 29 (8.2) | ||
| Current cigarette or hookah | 51 (15.0) | 51 (15.0) | 44 (12.5) | 50 (14.2) | ||
| Current nass | 32 (9.4) | 32 (9.4) | 50 (14.2) | 37 (10.5) | ||
| Current cigarette, hookah, or nass | 9 (2.7) | 9 (2.7) | 13 (3.7) | 9 (2.6) | ||
| Current opium use | ||||||
| No | 256 (75.3) | 245 (72.1) | .34 | 261 (74.2) | 272 (77.3) | .33 |
| Yes | 84 (24.7) | 95 (27.9) | 91 (25.8) | 80 (22.7) | ||
| Current alcohol drinking | ||||||
| No | 337 (99.1) | 331 (97.4) | .08 | 332 (94.3) | 334 (94.9) | .74 |
| Yes | 3 (0.9) | 9 (2.6) | 20 (5.7) | 18 (5.1) | ||
| Tea temperature | ||||||
| < 60°C | 102 (30.0) | 130 (38.2) | .048 | 104 (29.5) | 119 (33.8) | .43 |
| 60–64°C | 136 (40.0) | 129 (37.9) | 132 (37.5) | 135 (38.3) | ||
| > 65°C | 95 (27.9) | 77 (22.7) | 113 (32.1) | 95 (27.0) | ||
| No tea intake | 3 (0.9) | 4 (1.2) | 2 (0.6) | 3 (0.9) | ||
| Missing | 4 (1.2) | 0 (0.0) | 1 (0.3) | 0 (0.0) | ||
| Tooth brushing | ||||||
| Never | 252 (74.1) | 244 (71.8) | .78 | 260 (73.8) | 220 (62.5) | .003 |
| Nondaily | 41 (12.1) | 44 (12.9) | 40 (11.4) | 47 (13.4) | ||
| Daily | 47 (13.8) | 52 (15.3) | 52 (14.8) | 85 (24.1) | ||
| Number of teeth lost | ||||||
| Expected or less | 158 (23.2) | 156 (45.9) | .99 | 180 (51.2) | 171 (48.6) | .55 |
| > Expected, Q1 | 46 (13.5) | 46 (13.5) | 39 (11.1) | 52 (14.8) | ||
| > Expected, Q2 | 46 (13.5) | 49 (14.4) | 43 (12.2) | 49 (13.9) | ||
| > Expected, Q3 | 58 (17.1) | 60 (17.7) | 54 (15.3) | 48 (13.6) | ||
| > Expected, Q4 | 32 (9.4) | 29 (8.5) | 36 (10.2) | 32 (9.1) | ||
| Dietary intake (g/d)e | ||||||
| Total fruits | 140.5 (145.5) | 127.2 (91.1) | .16 | 155.7 (154.2) | 161.3 (134.2) | .61 |
| Total vegetables | 109.5 (65.7) | 113.5 (57.1) | .40 | 117.6 (72.2) | 126.1 (69.9) | .12 |
| Processed meat | 1.6 (3.5) | 1.6 (3.6) | .77 | 2.0 (3.8) | 2.0 (5.1) | .89 |
| Number of H. Pylori positive proteins | ||||||
| < 3 | - | - | - | 43 (12.2) | 35 (10.0) | .59 |
| ≥ 3 | - | - | 227 (64.5) | 237 (67.3) | ||
| Missing | - | - | 82 (23.3) | 80 (22.7) | ||
| Biomarker/indicatore | ||||||
| Riboflavin, nmol/L | 8.3 (13.5) | 9.8 (16.1) | .18 | 9.5 (14.7) | 10.2 (15.6) | .57 |
| FMN, nmol/L | 7.9 (5.0) | 8.6 (6.6) | .12 | 8.3 (6.5) | 8.4 (7.0) | .87 |
| Total vitamin B2, nmol/Lf | 16.2 (17.1) | 18.5 (21.9) | .14 | 17.9 (20.4) | 18.6 (21.5) | .64 |
| Cobalamin, pmol/L | 193.4 (92.0) | 196.6 (137.5) | .72 | 158.2 (71.4) | 165.0 (73.1) | .21 |
| MMA, μmol/L | 0.6 (1.0) | 0.5 (0.4) | .07 | 0.48 (0.41) | 0.44 (0.36) | .14 |
| PLP, nmol/L | 26.7 (21.5) | 26.8 (16.2) | .94 | 25.4 (13.2) | 27.7 (22.6) | .10 |
| HK-r | 0.50 (0.23) | 0.50 (0.26) | .98 | 0.49 (0.19) | 0.45 (0.20) | <.01 |
| pABG equivalents, nmol/L | 19.7 (10.7) | 20.8 (11.4) | .21 | 20.0 (11.3) | 19.4 (12.5) | .50 |
| tHcy, μmol/L | 15.5 (14.0) | 13.3 (8.7) | .01 | 14.7 (10.7) | 13.4 (9.6) | .08 |
- Abbreviations: ESCC, esophageal squamous cell carcinoma; GC, gastric cancer; FMN, flavin mononucleotide; PLP, pyridoxal phosphate; MMA, methylmalonic acid; HK-r, 3-hydroxykynurenine ratio; pABG, para-aminobenzoylglutamate equivalent; tHcy, total homocysteine.
- a Matching factor (by frequency) for cases and controls in both esophageal squamous cell carcinoma and gastric cancer studies.
- b Fars, Turk, Sistani, Baluch, Kurd, and other.
- c Quartiles were in the entire Golestan Cohort Study cohort.
- d Matching factor (by frequency) for cases and controls in the gastric cancer study.
- e Mean (standard deviation) without missing values.
- f Sum of riboflavin + FMN.
Meaningful correlations were observed among the nine measured OCM biomarkers and indicators (Table 2). Riboflavin and its biologically active form, FMN, were highly correlated (r = .755 for ESCC and 0.789 for GC, p < .001 for both). MMA, an indicator of cobalamin deficiency, was negatively correlated with cobalamin level (r = −.1, p < .01 for ESCC and −.091, p = .02 for GC). Similarly, PLP and HK-r, an indicator of insufficient vitamin B6, were negatively correlated (r = −.204, p < .0001 for ESCC and −.211, p < .0001 for GC). tHcy level was negatively correlated with pABG (r = −.136, p < .01 for ESCC and −.098, p < .01 for GC) but more strongly correlated with MMA (r = .231, p < 00001 for ESCC and r = 0326, p < 00001 for GC), indicating that there was more prevalent vitamin B12 deficiency than folate deficiency in this population.
| A. Esophageal squamous cell carcinoma | ||||||||
|---|---|---|---|---|---|---|---|---|
| FMN | Ribo + FMN | Cobalamin | MMA | PLP | HK-r | pABG | tHcy | |
| Riboflavin | 0.755 | 0.981 | 0.056 | −0.001 | 0.037 | 0.026 | 0.014 | −0.043 |
| <0.0001 | <0.0001 | 0.14 | 0.97 | 0.34 | 0.50 | 0.71 | 0.26 | |
| FMN | 0.869 | 0.185 | −0.004 | 0.171 | −0.014 | −0.017 | −0.052 | |
| <0.0001 | <0.0001 | 0.92 | <0.0001 | 0.72 | 0.66 | 0.17 | ||
| Ribo + FMN | 0.098 | −0.002 | 0.079 | 0.015 | 0.006 | −0.048 | ||
| 0.01 | 0.96 | 0.04 | 0.69 | 0.88 | 0.21 | |||
| Cobalamin | −0.100 | 0.101 | 0.057 | −0.151 | −0.079 | |||
| <0.01 | 0.008 | 0.14 | <0.0001 | 0.04 | ||||
| MMA | −0.038 | −0.045 | 0.076 | 0.231 | ||||
| 0.32 | 0.24 | 0.047 | <0.0001 | |||||
| PLP | −0.204 | 0.0005 | −0.071 | |||||
| <0.0001 | 0.99 | 0.07 | ||||||
| HK-ratio | 0.092 | −0.005 | ||||||
| 0.017 | 0.89 | |||||||
| pABG | −0.136 | |||||||
| 0.0004 | ||||||||
| B. Gastric cancer | ||||||||
|---|---|---|---|---|---|---|---|---|
| FMN | Ribo + FMN | Cobalamin | MMA | PLP | HK-r | pABG | tHcy | |
| Riboflavin | 0.789 | 0.98 | 0.009 | 0.0001 | 0.065 | −0.003 | 0.013 | −0.086 |
| <0.0001 | <0.0001 | 0.82 | 0.997 | 0.08 | 0.93 | 0.73 | 0.02 | |
| FMN | 0.895 | 0.127 | 0.050 | 0.164 | −0.051 | −0.019 | −0.053 | |
| <0.0001 | 0.0007 | 0.19 | <0.0001 | 0.19 | 0.61 | 0.16 | ||
| Ribo + FMN | 0.047 | 0.016 | 0.100 | −0.019 | 0.003 | −0.079 | ||
| 0.21 | 0.67 | 0.008 | 0.62 | 0.93 | 0.04 | |||
| Cobalamin | −0.091 | 0.174 | −0.061 | −0.193 | −0.084 | |||
| 0.02 | <0.0001 | 0.11 | <0.0001 | 0.03 | ||||
| MMA | −0.023 | −0.025 | 0.030 | 0.326 | ||||
| 0.54 | 0.51 | 0.42 | <0.0001 | |||||
| PLP | −0.211 | 0.022 | −0.060 | |||||
| <0.0001 | 0.57 | 0.11 | ||||||
| HK-ratio | 0.045 | −0.061 | ||||||
| 0.24 | 0.11 | |||||||
| pABG | −0.098 | |||||||
| <0.01 | ||||||||
- Abbreviations: ESCC, esophageal squamous cell carcinoma; FMN, flavin mononucleotide; PLP, pyridoxal phosphate; MMA, methylmalonic acid; HK-r, 3-hydroxykynurenine ratio; pABG, para-aminobenzoylglutamate eauivalent; tHcy, total homocysteine.
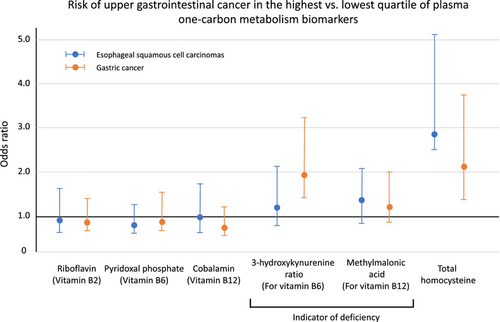
None of the measured B-vitamins, including cobalamin and riboflavin, were associated with ESCC (Table 3). However, there was marginally increased risk of ESCC per unit of MMA (OR = 1.06, 95% CI = 0.99–1.13) in the linear model. Higher level of tHcy indicates imbalanced biomarkers in the OCM, such as vitamin B deficiency, and was associated with higher risk of ESCC. The OR (95% CI) was 2.19 (1.28–3.76), 2.09 (1.18–3.67), and 2.81 (1.54–5.13) for 2nd to 4th quartiles of tHcy, respectively, compared with the lowest quartile (Figure 2). Alternatively, the risk increased by 9% with an increment of one-half of the IQR (OR = 1.09, 95% CI = 1.01–1.18).
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Continuousa | |
|---|---|---|---|---|---|
| Riboflavin | |||||
| Case/Control | 75/85 | 99/85 | 91/86 | 75/84 | |
| OR (95% CI) | 1.00 (ref.) | 1.20 (0.75–1.92) | 1.33 (0.81–2.19) | 0.95 (0.57–1.59) | 0.98 (0.95–1.01) |
| FMN | |||||
| Case/Control | 82/85 | 110/85 | 69/85 | 79/85 | |
| OR (95% CI) | 1.00 (ref.) | 1.35 (0.82–2.21) | 0.74 (0.44–1.26) | 0.93 (0.54–1.62) | 0.94 (0.88–1.01) |
| Riboflavin + FMN | |||||
| Case/Control | 77/85 | 105/85 | 87/85 | 71/85 | |
| OR (95% CI) | 1.00 (ref.) | 1.27 (0.78–2.08) | 1.07 (0.64–1.80) | 0.89 (0.52–1.53) | 0.97 (0.92–1.01) |
| Cobalamin | |||||
| Case/Control | 83/85 | 80/85 | 88/85 | 89/85 | |
| OR (95% CI)b | 1.00 (ref.) | 0.84 (0.50–1.39) | 0.87 (0.51–1.47) | 1.01 (0.58–1.77) | 0.96 (0.88–1.04) |
| PLP | |||||
| Case/Control | 99/85 | 93/85 | 77/85 | 71/85 | |
| OR (95% CI) | 1.00 (ref.) | 0.87 (0.55–1.37) | 0.94 (0.59–1.50) | 0.82 (0.50–1.32) | 1.01 (0.94–1.07) |
| pABG | |||||
| Case/Control | 89/83 | 103/86 | 74/85 | 74/86 | |
| OR (95% CI) | 1.00 (ref.) | 1.10 (0.69–1.75) | 0.84 (0.51–1.37) | 0.69 (0.39–1.20) | 0.92 (0.83–1.01) |
| MMA | |||||
| Case/Control | 68/85 | 74/85 | 91/85 | 107/85 | |
| OR (95% CI) | 1.00 (ref.) | 0.92 (0.54–1.55) | 1.20 (0.74–1.93) | 1.32 (0.80–2.07) | 1.06 (0.99–1.13) |
| HK-rc | |||||
| Case/Control | 71/82 | 96/83 | 69/83 | 95/83 | |
| OR (95% CI) | 1.00 (ref.) | 1.38 (0.84–2.27) | 0.76 (0.45–1.28) | 1.26 (0.76–2.10) | 0.99 (0.92–1.06) |
| tHcy | |||||
| Case/Control | 51/85 | 87/85 | 91/85 | 111/85 | |
| OR (95% CI) | 1.00 (ref.) | 2.19 (1.28–3.76) | 2.09 (1.18–3.67) | 2.81 (1.54–5.13) | 1.09 (1.01–1.18) |
- OR, odds ratio; CI, confidence interval; FMN, flavin mononucleotide; PLP, pyridoxal phosphate; pABG, para-aminobenzoylglutamate equivalent; MMA, methylmalonic acid; HK-r, 3-hydroxykynurenine ratio; tHcy, total homocysteine.
- a One half of interquartile range as a unit.
- b OR and 95% CI were adjusted for age (continuous), ethnicity (Turkmen, others), education (none, ≤8 years, >8 years), composite wealth score (quartiles), opium use (yes, no), alcohol intake (never, current), tea temperature (<60°C, 60–64°C, ≥ 65°C, no tea intake, missing), tooth brushing (never, nondaily, daily), tooth loss (≤expected, >expected quartiles), total fruit intake (g/day), total vegetable intake (g/day), and processed meat intake (g/day).
- c Analysis was limited to 662 participants excluding 18 participants who lacked HK-r data because of high hemolysis in their samples and their matched cases/controls.
Similarly, none of the measured B-vitamin levels was associated with the risk for GC (Table 4). However, higher level of HK-r, reflecting insufficient vitamin B6, was associated with higher risk of GC (OR for the highest vs. lowest quartiles = 2.19, 95% CI = 1.04–4.62), although the association in the continuous scale did not reach the statistically significant level (OR per one-half IQR = 1.08, 95% CI = 0.95–1.23) (Figure 2). Higher tHcy level was also associated with higher risk of GC. Relative to the lowest quartile, the OR (95% CI) was 1.52 (0.90–2.59), 2.08 (1.22–3.55), and 2.09 (1.17–3.73) for 2nd to 4th quartiles of tHcy, respectively. The observed associations remained in a subset of 530 subjects who had H. pylori data, but the HK-r association was attenuated to nonsignificant with further adjustment for H. pylori seropositivity. When we assessed the association for cardia and non-cardia cancers separately, the association for higher risk of ESCC was limited to non-cardia cancer (OR per one-half IQR = 1.37, 95% CI = 1.02–1.84), although the non-cardia GC estimates had limited statistical power.
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Continuousa | ||
|---|---|---|---|---|---|---|
| Riboflavin | ||||||
| Case/Control | 94/88 | 86/87 | 89/87 | 85/88 | ||
| Allb | 1.00 (ref.) | 0.91 (0.57–1.44) | 0.90 (0.58–1.40) | 0.88 (0.55–1.41) | 0.98 (0.95–1.02) | |
| Cardia (case n = 206) | 1.00 (ref.) | 1.10 (0.56–2.14) | 0.90 (0.48–1.71) | 1.03 (0.55–1.95) | 0.99 (0.94–1.04) | |
| Non-cardia (case n = 98) | 1.00 (ref.) | 0.44 (0.14–1.42) | 1.55 (0.51–4.78) | 1.24 (0.30–5.10) | 0.94 (0.84–1.05) | |
| Subsetc | 1.00 (ref.) | 0.98 (0.57–1.68) | 1.00 (0.59–1.72) | 1.07 (0.62–1.86) | 1.00 (0.96–1.04) | |
| Adjusted for H. Pylorid | 1.00 (ref.) | 0.97 (0.56–1.67) | 1.00 (0.58–1.71) | 1.09 (0.63–1.90) | 1.01 (0.96–1.05) | |
| FMN | ||||||
| Case/Control | 92/88 | 88/87 | 86/89 | 86/88 | ||
| All | 1.00 (ref.) | 1.00 (0.62–1.61) | 0.89 (0.55–1.42) | 0.95 (0.59–1.54) | 0.99 (0.93–1.05) | |
| Cardia | 1.00 (ref.) | 0.85 (0.43–1.68) | 0.71 (0.37–1.38) | 1.10 (0.56–2.13) | 1.00 (0.91–1.11) | |
| Non-cardia | 1.00 (ref.) | 0.55 (0.13–2.38) | 0.88 (0.26–2.96) | 0.61 (0.16–2.33) | 0.97 (0.81–1.17) | |
| Subset | 1.00 (ref.) | 1.07 (0.62–1.86) | 1.05 (0.60–1.86) | 1.05 (0.60–1.86) | 1.01 (0.94–1.08) | |
| Adjusted for H. Pylori | 1.00 (ref.) | 1.07 (0.61–1.86) | 1.06 (0.60–1.88) | 1.20 (0.67–2.12) | 1.01 (0.94–1.08) | |
| Riboflavin + FMN | ||||||
| Case/Control | 102/87 | 69/88 | 94/89 | 87/88 | ||
| All | 1.00 (ref.) | 0.53 (0.33–0.88) | 0.84 (0.54–1.31) | 0.74 (0.36–1.20) | 0.98 (0.94–1.03) | |
| Cardia | 1.00 (ref.) | 0.58 (0.27–1.24) | 0.72 (0.39–1.35) | 0.88 (0.45–1.71) | 0.99 (0.93–1.05) | |
| Non-cardia | 1.00 (ref.) | 0.49 (0.16–1.50) | 1.31 (0.44–3.86) | 1.02 (0.29–3.58) | 0.94 (0.81–1.08) | |
| Subset | 1.00 (ref.) | 0.69 (0.38–1.24) | 1.09 (0.64–1.85) | 1.09 (0.64–1.85) | 1.00 (0.96–1.05) | |
| Adjusted for H. Pylori | 1.00 (ref.) | 0.70 (0.39–1.27) | 1.11 (0.65–1.89) | 0.93 (0.53–1.63) | 1.01 (0.96–1.06) | |
| Cobalamin | ||||||
| Case/Control | 95/86 | 87/89 | 91/89 | 79/88 | ||
| All | 1.00 (ref.) | 0.76 (0.45–1.28) | 0.83 (0.49–1.38) | 0.70 (0.40–1.22) | 0.93 (0.84–1.03) | |
| Cardia | 1.00 (ref.) | 0.79 (0.37–1.68) | 1.01 (0.49–2.10) | 0.84 (0.36–1.97) | 0.92 (0.80–1.07) | |
| Non-cardia | 1.00 (ref.) | 0.52 (0.13–2.07) | 0.95 (0.25–3.56) | 0.28 (0.07–1.14) | 0.86 (0.66–1.12) | |
| Subset | 1.00 (ref.) | 0.63 (0.34–1.17) | 0.96 (0.53–1.74) | 0.67 (0.34–1.33) | 0.91 (0.80–1.03) | |
| Adjusted for H. Pylori | 1.00 (ref.) | 0.66 (0.35–1.24) | 1.02 (0.56–1.89) | 0.73 (0.37–1.45) | 0.92 (0.81–1.04) | |
| PLP | ||||||
| Case/Control | 90/88 | 121/87 | 66/89 | 75/88 | ||
| All | 1.00 (ref.) | 1.56 (0.97–2.50) | 0.65 (0.39–1.09) | 0.89 (0.52–1.52) | 0.95 (0.88–1.02) | |
| Cardia | 1.00 (ref.) | 1.51 (0.76–3.00) | 0.46 (0.20–1.04) | 0.47 (0.19–1.16) | 0.90 (0.80–1.01) | |
| Non-cardia | 1.00 (ref.) | 0.36 (0.09–1.52) | 0.31 (0.08–1.19) | 0.43 (0.09–2.09) | 0.86 (0.67–1.10) | |
| Subset | 1.00 (ref.) | 1.60 (0.89–2.86) | 0.53 (0.29–0.99) | 1.06 (0.55–2.05) | 0.97 (0.91–1.05) | |
| Adjusted for H. Pylori | 1.00 (ref.) | 1.59 (0.89–2.84) | 0.55 (0.29–1.02) | 1.08 (0.56–2.09) | 0.97 (0.91–1.04) | |
| pABGe | ||||||
| Case/Control | 75/89 | 66/85 | 110/89 | 88/91 | ||
| All | 1.00 (ref.) | 0.94 (0.57–1.56) | 1.64 1.01–2.68) | 1.27 (0.73–2.22) | 1.02 (0.92–1.12) | |
| Cardia | 1.00 (ref.) | 0.97 (0.49–1.95) | 1.98 (0.93–4.21) | 1.46 (0.64–3.34) | 1.09 (0.93–1.27) | |
| Non-cardia | 1.00 (ref.) | 0.76 (0.10–2.88) | 1.02 (0.33–3.19) | 1.83 (0.48–6.97) | 1.08 (0.88–1.34) | |
| Subset | 1.00 (ref.) | 0.93 (0.52–1.66) | 1.43 (0.82–2.49) | 1.28 (0.67–2.44) | 1.02 (0.91–1.15) | |
| Adjusted for H. Pylori | 1.00 (ref.) | 0.92 (0.51–1.66) | 1.43 (0.82–2.50) | 1.28 (0.67–2.46) | 1.02 (0.91–1.15) | |
| MMA | ||||||
| Case/Control | 60/87 | 91/88 | 119/89 | 82/88 | ||
| All | 1.00 (ref.) | 1.47 (0.88–2.45) | 1.80 (1.08–2.99) | 1.18 (0.69–2.00) | 1.03 (0.96–1.12) | |
| Cardia | 1.00 (ref.) | 2.11 (0.92–4.87) | 3.25 (1.46–7.23) | 1.60 (0.70–3.65) | 1.04 (0.94–1.16) | |
| Non-cardia | 1.00 (ref.) | 1.64 (0.51–5.30) | 2.36 (0.73–7.63) | 2.37 (0.63–8.89) | 1.20 (0.94–1.54) | |
| Subset | 1.00 (ref.) | 1.79 (0.94–3.41) | 2.07 (1.09–3.95) | 1.57 (0.82–3.03) | 1.05 (0.96–1.15) | |
| Adjusted for H. Pylori | 1.00 (ref.) | 1.82 (0.95–3.45) | 2.07 (1.08–3.97) | 1.55 (0.81–2.99) | 1.04 (0.95–1.14) | |
| HK-rf | ||||||
| Case/Control | 76/86 | 52/87 | 89/86 | 129/87 | ||
| All | 1.00 (ref.) | 0.59 (0.35–0.98) | 1.24 (0.76–2.04) | 1.95 (1.19–3.21) | 1.14 (1.04–1.26) | |
| Cardia | 1.00 (ref.) | 0.38 (0.18–0.81) | 1.06 (0.51–2.22) | 2.19 (1.04–4.62) | 1.08 (0.95–1.23) | |
| Non-cardia | 1.00 (ref.) | 0.26 (0.06–1.22) | 2.66 (0.67–10.61) | 2.08 (0.49–8.82) | 1.37 (1.02–1.84) | |
| Subset | 1.00 (ref.) | 0.68 (0.38–1.24) | 1.21 (0.69–2.13) | 2.03 (1.13–3.65) | 1.11 (1.00–1.24) | |
| Adjusted for H. Pylori | 1.00 (ref.) | 0.68 (0.38–1.24) | 1.25 (0.71–2.22) | 2.03 (1.12–3.66) | 1.11 (1.00–1.23) | |
| tHcy | ||||||
| Case/Control | 63/87 | 80/88 | 108/89 | 101/88 | ||
| All | 1.00 (ref.) | 1.52 (0.90–2.59) | 2.08 (1.22–3.55) | 2.09 (1.17–3.73) | 1.04 (0.98–1.10) | |
| Cardia | 1.00 (ref.) | 1.41 (0.63–3.13) | 2.25 (1.03–4.93) | 2.04 (0.86–4.87) | 1.03 (0.95–1.13) | |
| Non-cardia | 1.00 (ref.) | 2.19 (0.59–8.13) | 3.95 (0.95–16.33) | 5.11 (1.14–22.89) | 1.11 (0.97–1.45) | |
| Subset | 1.00 (ref.) | 1.63 (0.83–3.18) | 2.40 (1.26–4.59) | 2.17 (1.06–4.42) | 1.02 (0.96–1.09) | |
| Adjusted for H. Pylori | 1.00 (ref.) | 1.56 (0.80–3.07) | 2.31 (1.21–4.44) | 2.01 (0.97–4.18) | 1.02 (0.96–1.08) | |
- Abbreviations: FMN, flavin mononucleotide; PLP, pyridoxal phosphate; MMA, methylmalonic acid; HK-r, 3-hydroxykynurenine ratio; pABG, para-aminobenzoylglutamate equivalent; tHcy, total homocysteine.
- a One half of interquartile range as a unit.
- b Odds ratios (95% confidence intervals) were adjusted for age (continuous), ethnicity (Turkemen and others), education (none, ≤8 years, and >8 years), composite wealth score (quartiles), tobacco use (never, former, current cigarette or hookah, current nass, and current cigarette or hookah and nass), opium use (yes, no), alcohol intake (never, current), tea temperature (<60°C, 60–64°C, ≥ 65°C, no tea intake, missing), tooth brushing (never, nondaily, daily), tooth loss (≤ expected, >expected quartiles), total fruit intake (g/day), total vegetable intake (g/day), and processed meat intake (g/day).
- c Analysis was limited in a subset of 530 subjects excluding 174 subjects (162 subjects without number of sero-positive H. pylori protein data and their matched cases/controls).
- d Additionally adjusted for number of H. Pylori positive proteins (< or ≥3). The analysis was limited in 530 subjects excluding 174 subjects (162 subjects without this information and their matched cases/controls).
- e Analysis was limited to 702 participants (351 case–control pairs) excluding 2 participants (1 case whose sample volume was insufficient for the assay and their matched control). The analysis adjusting for H.Pylori positive proteins was limited to 528 participants (264 case–control pairs) additionally excluding 174 participants (162 participants who lacked information on number of H. Pylori positive proteins and their matched cases or controls).
- f Analysis was limited to 692 participants (346 case–control pairs) excluding 12 participants (6 subjects who lacked HK-r data because of high hemolysis in their samples and their matched cases or controls). The analysis adjusting for H.Pylori was limited to 518 participants (259 case–control pairs) additionally excluding 174 participants (162 participants who lacked information on number of sero-positive H. pylori proteins and their matched cases or controls).
Vitamin B deficiency was observed in high proportions of individuals in the two case–control studies (Table 5). In the ESCC study, high prevalence of deficient status was seen for riboflavin (51.6%), cobalamin (34.3%), a sum of riboflavin and FMN (22.2%), and PLP (15.6%). Similarly high prevalence of deficiency was observed for riboflavin (47.6%), cobalamin (48.4%), a sum of riboflavin and FMN (20.7%), and PLP (14.8%) in the GC study. The prevalence of elevated HK-r and MMA (indicators of low vitamin B6 and B12 status, respectively) and tHcy (a global indicator of low vitamin B status) were also high in the ESCC study (67.4%, 66.0%, and 28.2%, respectively) and in the GC study (66.9%, 62.7%, and 25.3%, respectively) indicating even higher prevalence of vitamin B deficiency, especially vitamin B6 and B12. For ESCC, the OR for elevated versus non-elevated status was borderline significantly increased for MMA (OR = 1.42, 95% CI = 0.99–2.04) and tHcy (OR = 1.52, 95% CI = 0.99–2.33). These associations are in concordance with the observed associations in categorical and linear models shown in Table 3. The OR was elevated for deficient status of cobalamin (OR = 1.32, 95% CI = 0.88–1.98) and PLP (OR = 1.19, 95% CI = 0.74–1.90) compared with sufficient status but was not statistically significant. In the GC study, higher OR was observed for elevated status of MMA (OR = 1.53, 95% CI = 1.05–2.22) and HK-r (OR = 1.59, 95% CI = 1.13–2.25) compared with non-elevated status, indicating potential association of deficient vitamin B6 and B12 with higher risk of GC. Higher ORs were observed for deficient status of FMN (OR = 1.52, 95% CI = 0.66–3.50), a sum of riboflavin and FMN (OR = 1.28, 95% CI = 0.85–1.93), and cobalamin (OR = 1.16, 95% CI = 0.81–1.66) compared with sufficient status; however, none of them reached a statistically significant level. The OR for elevated tHcy level was also increased relative to non-elevated status but not statistically significant (OR = 1.14, 95% CI = 0.77–1.70).
| ESCC | GC | |||
|---|---|---|---|---|
| Sufficient | Deficienta | Sufficient | Deficient | |
| Riboflavin | ||||
| N (%) | 329 (48.4) | 351 (51.6) | 369 (52.4) | 335 (47.6) |
| Case/Control | 161/168 | 179/172 | 183/186 | 169/166 |
| OR (95% CI)b | 1.00 (ref.) | 1.00 (0.70–1.43) | 1.00 (ref.) | 1.07 (0.77–1.50) |
| FMN | ||||
| N (%) | 642 (94.4) | 38 (5.6) | 671 (95.3) | 33 (4.7) |
| Case/Control | 325/317 | 15/23 | 334/337 | 18/15 |
| OR (95% CI) | 1.00 (ref.) | 0.48 (0.21–1.10) | 1.00 (ref.) | 1.52 (0.66–3.50) |
| Riboflavin + FMN | ||||
| N (%) | 529 (77.8) | 151 (22.2) | 558 (79.3) | 146 (20.7) |
| Case/Control | 269/260 | 71/80 | 273/285 | 79/67 |
| OR (95% CI) | 1.00 (ref.) | 0.86 (0.56–1.33) | 1.00 (ref.) | 1.28 (0.85–1.93) |
| Cobalamin | ||||
| N (%) | 447 (65.7) | 233 (34.3) | 363 (51.6) | 341 (48.4) |
| Case/Control | 221/226 | 119/114 | 176/187 | 176/165 |
| OR (95% CI) | 1.00 (ref.) | 1.32 (0.88–1.98) | 1.00 (ref.) | 1.16 (0.81–1.66) |
| PLP | ||||
| N (%) | 574 (84.4) | 106 (15.6) | 600 (85.2) | 104 (14.8) |
| Case/Control | 280/294 | 60/46 | 298/302 | 54/50 |
| OR (95% CI) | 1.00 (ref.) | 1.19 (0.74–1.90) | 1.00 (ref.) | 1.00 (0.61–1.62) |
| pABGc | ||||
| N (%) | 666 (97.9) | 14 (2.1) | 674 (96.0) | 28 (4.0) |
| Case/Control | 332/334 | 8/6 | 339/335 | 12/16 |
| OR (95% CI) | 1.00 (ref.) | 1.06 (0.33–3.37) | 1.00 (ref.) | 0.62 (0.27–1.42) |
| Not elevated | Elevateda | Not elevated | Elevated | |
|---|---|---|---|---|
| MMA | ||||
| N (%) | 222 (32.6) | 458 (67.4) | 233 (33.1) | 471 (66.9) |
| Case/Control | 97/125 | 243/215 | 99/134 | 253/218 |
| OR (95% CI) | 1.00 (ref.) | 1.42 (0.99–2.04) | 1.00 (ref.) | 1.53 (1.05–2.22) |
| HK-rd | ||||
| N (%) | 225 (34.0) | 437 (66.0) | 258 (37.3) | 434 (62.7) |
| Case/Control | 109/116 | 222/215 | 113/145 | 233/201 |
| OR (95% CI) | 1.00 (ref.) | 0.97 (0.67–1.40) | 1.00 (ref.) | 1.59 (1.13–2.25) |
| tHcy | ||||
| N (%) | 488 (71.8) | 192 (28.2) | 526 (74.7) | 178 (25.3) |
| Case/Control | 231/257 | 109/83 | 258/268 | 94/84 |
| OR (95% CI) | 1.00 (ref.) | 1.52 (0.99–2.33) | 1.00 (ref.) | 1.14 (0.77–1.70) |
- Abbreviations: ESCC, esophageal squamous cell carcinoma; GC, gastric cancer; FMN, flavin mononucleotide; PLP, pyridoxal phosphate; MMA, methylmalonic acid; HK-r, 3-hydroxykynurenine ratio; pABG, para-aminobenzoylglutamate equivalent; tHcy, total homocysteine.
- a Cut points for sufficient/deficient or not elevated/elevated status were 5 nmol/L for riboflavin, 3 nmol/L for FMN, 8 nmol/L for a sum of riboflavin and FMN, 15 nmol/L for PLP, 0.4 for HK-r, 150 pmol/L for cobalamin, 0.26 μmol/L for MMA, 7.5 nmol/L for pABG, and 15 μmol/L for tHcy.
- b Adjusted for age (continuous), sex, ethnicity (Turkmen and others), education (none, <=8 years, and >8 years), composite wealth score (quartiles), tobacco use (never, former, current cigarette or hookah, current nass, and current cigarette or hookah and nass), opium use (yes, no), alcohol intake (never, current), tea temperature (<60°C, 60–64°C, ≥ 65°C, no tea intake, missing), tooth brushing (never, nondaily, daily), tooth loss (≤ expected, >expected quartiles), total fruit intake (g/day), total vegetable intake (g/day), and processed meat intake (g/day).
- c Analysis for GC was limited to 702 participants (351 case–control pairs) excluding one case who lacked pABG data because of insufficient sample volume and their matched control.
- d Analysis was limited to 662 participants for ESCC and 692 participants for gastric cancer, excluding 18 and 12 participants, respectively, who lacked HK-r data because of high hemolysis in their samples and their matched cases or controls.
4 DISCUSSION
In the nested case–control studies within the large prospective cohort study in Golestan, Iran where ESCC and GC incidence are high and vitamin B intake is low, high prevalence of deficient plasma vitamin B were observed. Although plasma levels of riboflavin and cobalamin were not associated with the risks of ESCC and GC, elevated level of MMA, an indicator of deficient B12 status, was associated with higher risk of ESCC and GC. Higher level of HK-r, indicating deficient vitamin B6 status, was associated with higher risk of GC but not ESCC. Higher plasma level of tHcy, a global indicator of low vitamin B status, was associated with higher risk of ESCC and GC.
B-vitamin deficiencies are endemic in populations which rely on diets lacking food items rich in each B-vitamin. In contrast, cereals and grain products are often fortified by B-vitamins and can be main sources of B-vitamins in Western countries. Dietary supplements are also main sources of B-vitamins in populations where they are commonly used. Indeed, lower blood levels of B-vitamins and other OCM biomarkers have been shown in populations where food fortification and dietary supplement use are not commonly practiced. In 20 large prospective cohort studies participating in the Lung Cancer Cohort Consortium (LC3), blood levels of riboflavin, PLP, folate, and vitamin B12 were the highest in the U.S. cohorts and were low in Asian cohorts.21, 22 Conversely, circulating levels of OCM metabolites, which are inversely correlated with B-vitamins, were high in Asian cohorts. These differences are likely explained by variations in dietary intake of these vitamins and particularly a high prevalence of dietary supplement use in the United States, because blood levels of B-vitamin biomarkers were considerably lower among nonusers of multivitamins than among users in the U.S. cohorts.22
Studies assessing circulating levels of B-vitamins and other OCM biomarkers and upper gastrointestinal cancer have been limited. In the nested case–control study within the General Population Nutrition Intervention Trial (NIT) cohort in Linxian, China, where ESCC and GC incidences are high and B-vitamin deficiency is commonly observed, participants in the highest quartile of serum riboflavin levels had 44% lower risk of ESCC than those in the lowest quartile (HR = 0.56, 95% CI = 0.41–0.75).8 Higher cobalamin level was also marginally associated with lower risk of ESCC (HR = 0.95, 95% CI = 0.89–1.01 by one-half of IQR). In the 25-year follow-up of the NIT participants after the completion of the trial, study participants who had received the intervention of daily riboflavin and niacin supplementation had 13% lower risk of esophageal cancer death compared with the risk among those who had received a placebo.23 In the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) study, lower serum level of cobalamin was associated with a 5.8-fold increased risk of non-cardia gastric adenocarcinoma (95% CI = 2.65–12.56 for the lowest vs. highest quartile, P-trend <0.001).9 The inverse association between circulating levels of cobalamin and a risk of GC was also observed in the European Prospective Investigation into Cancer and Nutrition study (OR = 0.79 per log scaled standard deviation [SD], 95% CI = 0.66–0.95), but the association was confined to cancer cases with low pepsinogen A levels, a marker of severe atrophic gastritis (OR = 0.38 per log scaled SD, 95% CI = 0.21–0.68).10
Null associations between circulating riboflavin or cobalamin level and ESCC or GC in the current study are not consistent with the previously reported associations.8-10, 23 One possible reason is that the distributions of circulating levels of these vitamins vary by populations and regions. For example, in the current study in Golestan, Iran, the mean (SD) of plasma cobalamin level among GC controls was 165 (73.1) pmol/L, which is considerably lower than the mean in the EPIC study controls (303 [170] pmol/L).10 Most plasma B-vitamin levels measured in the current study were considerably lower than the levels reported in the U.S. and even Asian cohorts included in the aforementioned study in the LC3.22 High proportions of individuals had low or deficient levels of B-vitamins with vitamin B12 as the most prominent deficiency. The low vitamin B status was reflected in the indices used in the current study. About 67% of individuals in both ESCC and GC studies had elevated MMA, indicating insufficient vitamin B12. Indeed, 4% and 6% of the ESCC and GC study, respectively, had undetectable levels of plasma cobalamin. Although deficient PLP was observed in 15%–16% of individuals, a majority of study participants had elevated HK-r, indicating insufficient vitamin B6 status. Despite a lack of associations for cobalamin and PLP levels, we observed the higher risk of ESCC and GC for elevated MMA and the higher risk of GC for elevated HK-r. Further, relatively low B-vitamin status is in consistent with elevated tHcy observed in about 27% of individuals. The observed high risks of ESCC and GC in relation to higher tHcy may reflect the association for overall low vitamin B in the current study population.
Our study benefited from relatively large numbers of ESCC and GC cases because the Golestan region in Iran is one of the highest-risk areas of esophageal and gastric cancers in the world.24 Typical diets in the GCS population heavily relied on refined grains, especially rice, which were not fortified by B-vitamins. Dietary supplement use was not a common practice in the Golestan region. Thus, we had wide distributions of plasma B-vitamins particularly at lower levels. The GCS was originally designed to examine risk factors for upper gastrointestinal cancers, and therefore most of the known key risk factors for ESCC and GC had been assessed and studied. Using these data, we were able to minimize potential confounding in the analyses. Incident cancer cases were ascertained with high accuracy using multiple methods (annual telephone surveys or home visits, clinical, pathology, and hospital records, and linkage to cancer registry). However, several limitations should also be noted. Blood samples were collected only at recruitment and had been stored for years at the time of the assays. However, using functional indicators of B-vitamin status allowed us to better assess the associations of circulating B-vitamins with ESCC and GC, compensating potential effects of long-term storage or sample processing on B-vitamins in stored plasma samples and any decrement in measured values will be nondifferential by case status. For example, we identified potential vitamin B6 deficiency more by using HK-r, a novel functional indicator of vitamin B6, than using PLP. Although we had relatively large numbers of ESCC and GC cases, we lacked statistical power for certain analyses, such as the stratified analysis by cardia and non-cardia GC. H. pylori seropositivity information was available only for the subgroup of the study participants. However, the proportion of H. pylori positivity in this population was very high and the results among all participants remained after adjusting for H. pylori seropositivity in the subgroup analysis. Finally, the fortification of folate and iron was implemented in Iran for a limited period from 2007 to 2009, but only for flour used for baked products in bakeries. Although this time frame overlapped the recruitment period of the GCS (2004–2008), the consumption amount of baked goods purchased from bakeries was not assessed in the questionnaire. However, a diet in this cohort heavily relied on rice, and thus folate intake from purchased fortified baked products was likely low. Indeed, plasma folic acid, the form of folate used for food fortification and dietary supplements, was undetectable among virtually all participants of the prior pilot study within a subset of the GCS participants (data not shown).
In this study in Golestan, Iran where ESCC and GC incidence are high and B-vitamin intake is low, high proportions of individuals had deficient plasma B-vitamin status, particularly vitamin B2, B6, and B12. Previously reported associations between circulating riboflavin or cobalamin and ESCC or GC were not observed in this population with low vitamin B status. However, individuals with deficient status of B12, indicated by elevated MMA, had higher risk of ESCC and GC, and those with deficient B6 status, reflected in elevated HK-r, had higher risk of GC. Low plasma tHcy was associated with higher risk of ESCC and GC. Increasing intake of vitamin B6 and B12 may be beneficial to prevent ESCC and GC in populations where vitamin B deficiency is prevalent.
AUTHOR CONTRIBUTIONS
Maki Inoue-Choi: Conceptualization; data curation; formal analysis; methodology; project administration; writing—original draft; and writing—review & editing. Neal D. Freedman: Conceptualization; methodology; supervision; funding acquisition; and writing—review & editing. Arash Etemadi: Resources; data curation; and writing—review & editing. Maryam Hashemian: Resources; data curation; and writing—review & editing. Paul Brennan: Resources and writing—review and editing. Gholamreza Roshandel: Resources and writing—review & editing. Hossein Poustchi: Resources and writing—review & editing. Paolo Boffetta: Resources and writing—review & editing. Farin Kamangar: Resources and writing—review & editing. Taghi Amiriani: Resources and writing—review & editing. Alireza Norouzi: Resources and writing—review & editing. Sandy Dawsey: Funding acquisition; resources; writing—review & editing. Reza Malekzadeh: Funding acquisition; resources; and writing—review & editing. Christian C. Abnet: Conceptualization; resources; supervision; funding acquisition; methodology; and writing—review & editing. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
ETHICS STATEMENT
This study was approved by the Institutional Review Boards of the Digestive Disease Research Institute of Tehran University of Medical Sciences, the International Agency for Research on Cancer (IARC), and the United States (U.S.) National Cancer Institute. Written informed consent was obtained from all study participants at enrollment.
Open Research
DATA AVAILABILITY STATEMENT
The data are managed and owned by the Digestive Diseases Research Institute (DDRI) of Tehran University, and de-identified data can be shared upon approval of a proposal by the GPCR Secretariat ([email protected]) and the Golestan Research Center of Gastroenterology and Hepatology (GRCGH), affiliated with the Golestan University of Medical Sciences (GOUMS), with a data transfer agreement. Additional limitations on sharable data may apply due to local regulations. Further information is available from the corresponding author upon request.