Representation of women in clinical trials supporting FDA-approval of contemporary cancer therapies
This work was presented in part under an American Society of Clinical Oncology (ASCO) Merit award at the 2022 ASCO annual scientific sessions.
The manuscript's content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Sujay Kalathoor, Sanam Ghazi, Beryl Otieno, and Melissa A. Babcook contributed equally to this study.
Abstract
Contemporary anticancer therapies frequently have different efficacy and side effects in men and women. Yet, whether women are well-represented in pivotal trials supporting contemporary anticancer drugs is unknown. Leveraging the Drugs@FDA database, clinicaltrials.gov, MEDLINE, and publicly available FDA-drug-reviews, we identified all pivotal (phase II and III) non-sex specific trials supporting FDA-approval of anticancer drugs (1998–2018). Observed-enrollment-rates were compared to expected-population-rates derived from concurrent US-National-Cancer-Institute's Surveillance-Epidemiology-and-End-Results (SEER) reported rates and US-Census databases. Primary outcome was the proportional representation of women across trials, evaluated by a participation-to-prevalence ratio (PPR), according to cancer type. Secondary outcome was the report of any sex-specific analysis of efficacy and/or safety, irrespective of treatment-arm. Overall, there were 148 trials, enrolling 60,216 participants (60.5 ± 4.0 years, 40.7% female, 79.1% biologic, targeted, or immune-based therapies) evaluating 99 drugs. Sex was reported in 146 (98.6%) trials, wherein 40.7% (24,538) were women, compared to 59.3% (35,678) men (p < .01). Altogether, women were under-represented in 66.9% trials compared to the proportional incidence of cancers by respective disease type; weight-average PPR of 0.91 (relative difference: -9.1%, p < .01). Women were most under-represented in gastric (PPR = 0.63), liver (PPR = 0.71), and lung (PPR = .81) cancer trials. Sex-based safety data was reported in 4.0% trials. There was no association between adequate female enrollment and drug efficacy (HR: 0.616 vs. 0.613, p = .96). Over time, there was no difference in the percentage of women recruited into clinical trials. Among pivotal clinical trials supporting contemporary FDA-approved cancer drugs, women were frequently under-represented and sex-specific-efficacy and safety-outcomes were commonly not reported.
Graphical Abstract
What's new?
Contemporary anticancer therapies frequently have different efficacy and side effects in men and women. Yet, whether women are well represented in pivotal trials is unknown. Here, the authors offer a comprehensive assessment of the representation of women and the reporting of sex-based analyses among pivotal clinical trials supporting FDA-approved anticancer therapies. The results show that among the more than 60,000 phase II and III clinical trial participants for the testing of nearly 100 anticancer therapies approved between 1998 and 2018, women were frequently under-represented, with sex-specific efficacy and safety outcomes commonly unreported.
1 INTRODUCTION
Novel cancer therapies are an increasingly common, but important component of effective cancer treatment.1 Over the last two decades there has been a dramatic rise in the number of novel cancer therapies, with >140 new approvals since 2000 alone.1 Targeting specific molecular, or immune-based alterations, many of these drugs have been associated with dramatic improvements in survival.2-5 However, emerging reports have suggested potential limitations in the wide applicability of trial findings supporting several therapies, particularly given differences in genetic, hormonal, and clinical utilization in broader populations.
Concurrently, national policies have encouraged increased focus on the representation of women in studies.6-8 Historically, women were underrepresented in clinical trials due to misconceptions that trial observations among men were uniformly applicable to women, and fears of the impact of anticancer therapy on reproductive potential.9 However, sex-specific differences have increasingly been linked with pathophysiologic and clinical efficacy differences with many common therapies.9-11 In addition, growing data support potential sex-based differences in clinical safety profiles of many novel anticancer therapies.12
In the US, novel anticancer therapies are reviewed by the Food and Drug Administration (FDA) for adequate representation and efficacy, with specific emphasis on supporting latter-phase clinical trials, prior to drug approval and use.12 Incumbent on these clinical trials is the adequate representation and evaluation of target populations to allow informed assessments and decisions by providers and patients. Yet, whether women are well represented within these key anticancer clinical trials is unknown.
2 METHODS
2.1 Study population
Leveraging the Drugs@FDA database, we manually searched all anticancer treatments approved for new drug applications (NDAs) from 1998 to 2018.13 Drugs or biologics given approval for patients 18 years and older were considered eligible for final inclusion (Supplementary Table 2). Non-cancer therapies were excluded; and sex-specific trials (breast, ovarian/cervical, and prostate) were not included in primary analysis. To allow uniform definitions, we primarily focused on sex rather than gender, given the heterogeneity in gender definitions. We accessed publicly available FDA drug labeling and reporting sites, as well as medical and statistical reviews available at Drugs@FDA to comprehensively capture all drugs approved for anticancer treatment. Later-phase (II and III) clinical trials related to drug approval, such as those identified from MEDLINE, published abstracts, trial supplements, clinicaltrial.gov registration, and any other publicly available FDA drug reviews were identified as well (Supplementary Figure 1).13-15 In cases of reporting ambiguity, corresponding authors were contacted. Prior to data entry, two independent reviewers obtained and examined all drug trial data. Following confirmation, all drug trial data was extracted, and pertinent cancer and sex variables in the trials were collected, including cancer type, funding source, study length, therapy/class type (ex. biologic or immunotherapy), number of participants, and number and percentage of women enrolled. Biologic sex was defined as sex reported within the original primary trial data, collected at the time of trial initiation.14, 15 To identify which clinical trials reported disaggregated outcomes by sex, the two independent reviewers comprehensively evaluated published manuscript text, tables, and figures. The corresponding publications were identified by using the following information combined with the Boolean operator “OR”: official title, trial registration number (ClinicalTrials.gov identifier), drug name, brand name, responsible parties (sponsor and principal investigator), and indexed publications titles. Additionally, if the Drugs@FDA entries contained links to associated publications, they were searched manually. A study was determined to report disaggregated outcomes by sex if any portion of the identified manuscript included primary outcome data described separately for male and female participants.
2.2 Outcomes
The primary outcome was the proportional representation of women within clinical trials for anticancer drugs compared to the expected representation of that cancer in women (i.e., the participation to prevalence ratio [PPR]), across major cancer types.16 We chose to report PPR for uniformity, but our calculation of PPR also considers incidence. This analysis primarily relied on cancer incidence per year by disease type.7, 17, 18 The secondary outcomes included the report of specific sex-based anticancer efficacy and safety, respectively.
2.3 Statistical analysis
Descriptive statistics were used to summarize patient characteristics, using mean ± standard deviation (SD) or median (interquartile range) for continuous variables, and frequency counts with percentages for categorical variables. Depending on the expected cell counts of the corresponding contingency tables, Chi-square or Fisher's exact test was used to explore the association between groups and other categorical variables. To understand non-reporting, multivariable stepwise logistic regression was used to assess for trial characteristics associated with sex non-reporting. Specifically, all variables were initially considered for the multivariable model.
To describe the proportional participation of women by cancer type, actual proportional cancer incidence rates in women were derived from published age-adjusted reported population rates, as listed within the NCI Surveillance, Epidemiology, and End Results (SEER) program annual cancer statistics.16 Specifically, incidence and distribution in men and women for each cancer type for comparison were derived from age-adjusted SEER Database cancer statistics. This program, collecting data from over a dozen cancer registries capturing nearly 30% of the US population, reflects the contemporaneous incidence of various cancers across the US. These rates were obtained for each individual malignancy. These rates were then summed across all cancer types for national estimates of cancer incidence rates, as reported in the annual summary SEER statistics. For comparison, reported annual incidence rates from SEER were utilized to perform representative summary analyses for the time period considered. The PPR was estimated by dividing the percentage of women enrolled in the clinical trials for a specific cancer type by the total incidence of those cancers, among SEER participants from 1998 to 2018. A calculated ratio of 1 indicates that the trial enrollment rates approximate that of the epidemiology data. A ratio <0.85 or >1.15 indicates that women were unrepresented or overrepresented, respectively, relative to the control population. For example, consider a trial where the observed number of women was 81 women, and the expected number of women was calculated to be 100 women. The observed-to-expected ratio would be: 81 (observed enrollees)/100 (expected enrollees) = 0.81, suggestive of under-enrollment.
To assess temporal change, we compared the proportion of women enrolled in each clinical trial by year, with SEER-derived incidence rates. To visually display change across time, three-year periods of reporting were serially divided by the index timepoint considered (i.e., 1998–2000). To assess the effect of adequate versus inadequate recruitment of women on clinical trial outcomes, PPR for each clinical trial was also estimated, with PPR <0.85 being inadequate recruitment of women, PPR 0.85–1.15 being adequate recruitment of women, and PPR >1.15 being over-recruitment of women.
To describe the differences in the magnitude of drug efficacy among trials that optimally enrolled or did not optimally enroll female participants, we used pooled binary endpoint hazard ratios (HRs). More specifically, to correlate the relationship between the enrollment of women and trial therapy efficacy, we used reported HRs and 95% confidence intervals for trials that reported binary endpoints (progression-free survival, disease response, or mortality). These values accounted for individual trial size within subgroups. The results from these trials were expressed by stratification according to trial enrollment of women in a single plot, for visual assessment. Drug approvals by primary cancer type (e.g., lung cancer) and therapeutic class (e.g., immunotherapy) were considered. These drugs were primarily approved on the basis of efficacy data from a single trial. Due to the significant heterogeneity in continuous endpoints among trials and the lack of consistent controls, we focused on binary endpoints to describe efficacy. Percentages and mean +/− SD values were reported to describe this distribution of categorical and continuous variables, respectively. All analyses were performed with SAS software version 9.4 (SAS Institute, Cary, NC), and the statistical tests were 2-sided with statistical significance evaluated at α = .05 significance level.
3 RESULTS
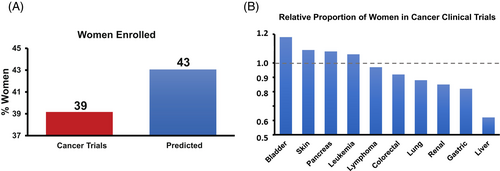
Overall, there were 234 trials supporting 189 anticancer therapies, enrolling a total of 97,566 patients including 148 trials evaluating 60,216 patients (testing 99 discrete therapies) for non-sex specific disease(s); Supplementary Figure 1. Sex was reported in 187 (98.9%) clinical trials, of which 42,229 (43.4%) were women compared to 55,267 (55.6%) men; p < .01. Among non-sex-specific trials, 40.7% of participants were women, and the median age of study participants was 60.5 years (interquartile range: 55.0 to 63.0 years), with a median trial size of 300–400 participants. Biologic, targeted, or immune-based therapies were evaluated in the majority (79.1%) of trials. For analysis uniformity, sex was considered as the conventional binary gender assigned at enrollment. In total, women were under-enrolled in 66.9% (99 trials) of trials, including 47 trials (31.8%) with under 33% female participation, and two trials with <5% female participation. Women were underrepresented in 84% of immune checkpoint inhibitor trials. Women were consistently under-represented in gastrointestinal (gastric, liver, and pancreas; PPR = 0.76) and lung (PPR = 0.81) cancer trials; Figure 1. Outside of lymphomas, there was no over-representation of women in trial enrollment by disease type, trial phase, or cancer therapeutic class. In multivariable analysis, outside of cancer type, no specific trial characteristics were associated with the presence or absence of lower female enrollment (Table 1). There was no association between higher relative proportional cancer incidence in women and the percentage of women enrolled (odds ratio: 6.6, 95% CI 0.19 to 225.81).
| Number of trials, No. (%) | No. of patients | ≤33.3% Female Enrollment, No. (%)a | p-Valueb | ||
|---|---|---|---|---|---|
| Overall | 148 | 60,216 | 47 (31.8) | Univariate | Multivariable |
| Cancer type | |||||
| Colorectal/GI | 24 (16.2) | 13,441 | 6 (25.0) | <.001 |
<.001 |
| Genitourinary | 14 (9.5) | 6647 | 13 (92.3) | ||
| Leukemia | 25 (16.9) | 8895 | 7 (29.2) | ||
| Lung | 19 (12.8) | 9666 | 5 (26.3) | ||
| Lymphoma | 24 (16.2) | 3976 | 6 (24.0) | ||
| Skin | 11 (7.4) | 7048 | 0 (0.0) | ||
| Other | 31 (20.9) | 10,543 | 10 (32.3) | ||
| Therapeutic-class | |||||
| Chemotherapy | 23 (15.5) | 9620 | 9 (39.1) | .32 |
- |
| Biologic or Immunotherapy | 56 (37.8) | 24,173 | 22 (39.3) | ||
| Hormonal | 2 (1.4) | 1852 | 0 (0.0) | ||
| Targeted | 61 (41.2) | 23,576 | 15 (24.6) | ||
| Other therapies | 6 (4.1) | 995 | 1 (16.7) | ||
| Trial size | |||||
| <100 | 21 (14.2) | 1257 | 6 (28.6) | .27 |
- |
| 100–499 | 84 (56.8) | 22,419 | 26 (31.0) | ||
| 500–999 | 33 (22.3) | 22,639 | 14 (42.4) | ||
| >1000 | 10 (6.8) | 13,901 | 1 (10.0) | ||
| Trial Phasec | |||||
| Phase 2 | 49 (33.1) | 6880 | 18 (36.7) | .45 |
- |
| Phase 3 | 99 (66.9) | 53,336 | 29 (29.3) | ||
| Funding Sourced | |||||
| Industry | 122 (82.4) | 51,525 | 38 (31.2) | .49 |
- |
| Government/Nonprofit | 3 (2.0) | 873 | 0 (0.0) | ||
| Both | 23 (15.5) | 7818 | 9 (39.2) | ||
| Start of enrollment | |||||
| Before 1998 | 8 (5.4) | 1943 | 4 (50.0) | .09 |
.11 |
| 1998–2002 | 25 (16.9) | 12,079 | 9 (36.0) | ||
| 2003–2007 | 30 (20.3) | 12,553 | 11 (36.7) | ||
| 2008–2012 | 57 (38.5) | 24,236 | 11 (19.3) | ||
| 2013–2018 | 28 (18.9) | 9405 | 12 (42.9) | ||
| Year of approval | |||||
| 1998–2002 | 19 (12.8) | 6118 | 5 (26.3) | .83 |
- |
| 2003–2007 | 22 (14.9) | 12,157 | 8 (36.4) | ||
| 2008–2012 | 39 (26.4) | 15,960 | 14 (35.9) | ||
| 2013–2018 | 68 (45.9) | 25,981 | 20 (29.4) | ||
| Trial duration (months) | |||||
| ≤24 | 59 (39.9) | 19,826 | 16 (27.1) | .33 |
- |
| 25–36 | 42 (28.4) | 17,496 | 16 (38.1) | ||
| 37–48 | 14 (9.5) | 8373 | 2 (14.3) | ||
| >48 | 26 (17.6) | 12,570 | 11 (42.3) | ||
| NA | 7 (4.7) | 1951 | 2 (28.6) | ||
| Trial setting | |||||
| Internationale | 140 (94.6) | 58,535 | 44 (31.4) | .71 | - |
| US only | 8 (5.4) | 1681 | 3 (37.5) | ||
| Number of women enrolledf | |||||
| ≤50 | 40 (27.0) | 5280 | 21 (52.5) | - |
- |
| 51–100 | 24 (16.2) | 5535 | 9 (37.5) | ||
| 101–499 | 78 (52.7) | 39,998 | 17 (21.8) | ||
| >500 | 6 (4.1) | 9403 | 0 (0.0) | ||
| Proportion of women enrolledf | |||||
| ≤20% | 15 (10.1) | 5387 | 15 (100) | - |
- |
| 21%–40% | 56 (37.8) | 23,420 | 32 (57.1) | ||
| 41%–59% | 65 (43.9) | 65 (0) | |||
| ≥60% | 12 (8.1) | 12 (0) | |||
- Abbreviations: CVD, cardiovascular disease; GI, gastrointestinal.
- a The percentages reported reflect a denominator of the number of trials within the subgroup considered.
- b The univariate p values presented describe associations estimated using Fisher's exact tests. All variables were considered and those with p values <.30 (i.e., cancer type, trial size, and year of enrollment start) were included in the multivariable stepwise regression model, where a p = .20 significance level was used to determine variables that remained in the final multivariable model (cancer type, year of enrollment start).
- c Several therapies were approved on the basis of breakthrough phase II data, with ongoing or as yet to be initiated phase III trials.
- d Not included in multivariable model due to low (government/nonprofit) cell count.
- e Most international trials, included sites in the US as well.
- f Hypothesis tests not done due to direct relationship with the outcome.
3.1 Reporting of sex-based efficacy
Among those trials evaluating non-sex-specific cancers (148), 45.3% (67) trials did not report sex-based efficacy. In multivariable analysis, only the trial phase (OR, 1.97, p < .001) and year of drug approval (OR 1.3–1.8, p = .04) were associated with the presence or absence of sex-based efficacy reporting (Table 2). There was no association between therapeutic mechanism class (including targeted or immune-based treatment) or trial size and the reporting of sex-based efficacy analysis.
| Number of trials, No. (%) | No. of Patients | Sex efficacy/ safety not reported, No. (%)a | p-Valueb | ||
|---|---|---|---|---|---|
| Overall | 148 | 60,216 | 67 (45.3) | Univariate | Multivariable |
| Cancer type | |||||
| Colorectal/GI | 24 (16.2) | 13,441 | 6 (25.0) | .05 |
- |
| Genitourinary | 14 (9.5) | 6647 | 6 (42.9) | ||
| Leukemia | 25 (16.9) | 8895 | 14 (58.3) | ||
| Lung | 19 (12.8) | 9666 | 6 (31.6) | ||
| Lymphoma | 24 (16.2) | 3976 | 16 (64.0) | ||
| Skin | 11 (7.4) | 7048 | 3 (27.3) | ||
| Other | 31 (20.9) | 10,543 | 16 (51.6) | ||
| Therapeutic-class | |||||
| Chemotherapy | 23 (15.5) | 9620 | 14 (60.9) | .53 |
- |
| Biologic or immunotherapy | 56 (37.8) | 24,173 | 25 (44.6) | ||
| Hormonal | 2 (1.4) | 1852 | 1 (50.0) | ||
| Targeted | 61 (41.2) | 23,576 | 25 (41.0) | ||
| Other therapies | 6 (4.1) | 995 | 2 (33.3) | ||
| Trial size | |||||
| <100 | 21 (14.2) | 1257 | 16 (76.2) | .01 |
- |
| 100–499 | 84 (56.8) | 22,419 | 35 (41.7) | ||
| 500–999 | 33 (22.3) | 22,639 | 11 (33.3) | ||
| >1000 | 10 (6.8) | 13,901 | 5 (50.0) | ||
| Trial phasec | |||||
| Phase 2 | 49 (33.1) | 6880 | 33 (67.4) | <.001 |
<.001 |
| Phase 3 | 99 (66.9) | 53,336 | 34 (34.3) | ||
| Funding source | |||||
| Industry | 122 (82.4) | 51,525 | 56 (45.9) | 1.0 |
- |
| Government/nonprofit | 3 (2.0) | 873 | 1 (33.3) | ||
| Both | 23 (15.5) | 7818 | 10 (43.5) | ||
| Start of enrollment | |||||
| Before 1998 | 8 (5.4) | 1943 | 6 (75.0) | .06 |
- |
| 1998–2002 | 25 (16.9) | 12,079 | 16 (64.0) | ||
| 2003–2007 | 30 (20.3) | 12,553 | 12 (40.0) | ||
| 2008–2012 | 57 (38.5) | 24,236 | 20 (35.1) | ||
| 2013–2018 | 28 (18.9) | 9405 | 13 (46.4) | ||
| Year of approval | |||||
| 1998–2002 | 19 (12.8) | 6118 | 12 (63.2) | .09 |
.04 |
| 2003–2007 | 22 (14.9) | 12,157 | 10 (45.5) | ||
| 2008–2012 | 39 (26.4) | 15,960 | 21 (53.9) | ||
| 2013–2018 | 68 (45.9) | 25,981 | 24 (35.3) | ||
| Trial duration (months) | |||||
| ≤24 | 59 (39.9) | 19,826 | 30 (50.9) | .41 |
- |
| 25–36 | 42 (28.4) | 17,496 | 16 (38.1) | ||
| 37–48 | 14 (9.5) | 8373 | 4 (29.6) | ||
| >48 | 26 (17.6) | 12,570 | 14 (53.9) | ||
| NA | 7 (4.7) | 1951 | 3 (42.9) | ||
| Trial setting | |||||
| Internationald | 140 (94.6) | 58,535 | 62 (44.3) | .47 |
- |
| US only | 8 (5.4) | 1681 | 5 (62.5) | ||
| Number of women enrolled | |||||
| ≤50 | 40 (27.0) | 5280 | 28 (70.0) | <.001 |
- |
| 51–100 | 24 (16.2) | 5535 | 11 (45.8) | ||
| 101–499 | 78 (52.7) | 39,998 | 25 (32.1) | ||
| >500 | 6 (4.1) | 9403 | 3 (50.0) | ||
| Proportion of women enrolled | |||||
| ≤20% | 15 (10.1) | 5387 | 9 (60.0) | .14 |
.20 |
| 21%–40% | 56 (37.8) | 23,420 | 25 (44.6) | ||
| 41%–59% | 65 (43.9) | 31 (47.7) | |||
| ≥60% | 12 (8.1) | 2 (16.7) | |||
- Abbreviations: CVD, cardiovascular disease; GI, gastrointestinal.
- a The percentages reported reflect a denominator of the number of trials within the subgroup considered.
- b The univariate p values presented describe associations estimated using Fisher's exact tests. All variables were considered and those with p values ≤.30 (i.e., Cancer type, trial size, trial phase, year of enrollment start, year of approval, number of women enrolled, and proportion of women enrolled) were included in the multivariable stepwise regression model, where a p ≤ .20 significance level was used to determine variables that remained in the final multivariable model (trial phase, year of approval, and proportion of women enrolled).
- c Several therapies were approved on the basis of breakthrough phase II data, with ongoing or as yet to be initiated phase III trials.
- d Most international trials, included sites in the US as well.
There were 66 drugs, supported by 89 trials, approved at least in part on the basis of a binary endpoint. Among these trials, there was no difference in efficacy on the basis of higher female representation (HR: 0.616 vs. 0.613, p = .96; Supplementary Figure 2). Additionally, whether sex-based efficacy was reported or not, did not affect efficacy (HR: 0.6 vs. 0.62, p = .6).
3.2 Reporting of sex-based adverse event risk
In follow-up, 96.0% of trials did not report adverse event or safety data by sex. Outside of cancer disease type, there were no trial characteristics, including year of trial initiation or the use of immune or targeted (precision) therapies, associated with the reporting of sex-specific assessments; Supplementary Table 1. There was no difference in safety risk based on higher or lower female representation (HR: 0.52; 95% CI [0.1, 0.9] vs. 0.4; 95% CI [−2.4, 3.2], p = .61).
3.3 Observed to expected female participation
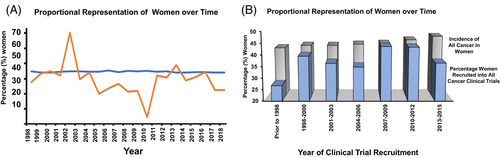
Moreover, among the 148 trials where patients with sex-specific cancers were excluded, 2 (1.4%) did not report any female participation. There were 23,749 (39.4%) women enrolled from a total of 60,216 participants. Compared with the published incidence of 43.1%, this translated into an overall observed versus expected participation ratio of 0.91 (or 9.2% lower relative participation; p < .001; Figure 1). There was no difference in female representation by time of trial initiation; with a non-significant trend toward lower enrollment in the latter trials; Figure 2.
4 DISCUSSION
In this evaluation of pivotal clinical trials supporting the FDA approval of contemporary anticancer therapies, nearly 50% of trials did not report efficacy or safety by sex. In those trials reporting outcomes by sex, the noted rates of safety reporting were markedly low. This pattern remained, even after accounting for the presence or absence of potentially excess cancer subtype risk in women, and the use of purportedly targeted or immune-based therapies. Furthermore, among all trials, women were consistently underrepresented by nearly 10%, a pattern most amplified in gastrointestinal and lung cancer trials. There was no difference in drug efficacy or adverse event rates, by the presence or absence of higher female enrollment. This is concerning, particularly given the rapidly increasing number of perceived precision therapies and the lack of consistent tools to guide the interpretation of long-term efficacy and toxicity risk.
The observation of underreporting of sex-based efficacy in key cancer trials adds to an increasing body of evidence. In an analysis of hematologic malignancy trials, the enrollment rates of women consistently trailed males in acute leukemia and myeloma-based trials.19 Similarly, in a preliminary analysis of federally registered trials, women were underrepresented in several solid-tumor-based studies.20 A similar relative absence of published sex-based efficacy analyses was observed in non-cancer populations.18, 21, 22 As noted by Murthy et al., the inclusion of women within pivotal cancer trials is still substandard.7 Although there have been at least some improvement in enrollment patterns over the last two decades, our data suggest continued efforts are needed. A comparable pattern amongst ophthalmic disease trials was also noted.21 Further, a similar trend was noted amongst cardiovascular disease trials wherein female participation was found to be substantially low or did not meet the targets.18 This suggests to us that even beyond cancer, other disease states may similarly see relative underrepresentation of women within pivotal clinical trials. Further, within the current analysis, we demonstrate for the first time that a similar sub-signal (relative absence of sex-based efficacy analyses) may exist in pivotal oncologic trials. Historically, clinical trials incorporate populations where disease prevalence and available subjects are prominent.23 Our results provide significant insights into the nature of female inclusion and biologic investigation within pivotal cancer trials. Prior, more isolated, or disease-specific studies have suggested potential discrepancies in the reporting patterns of sex-based evaluation within cancer-focused trials. This is exemplified by a trial of pazopanib for renal cell carcinoma where the incidence in women is estimated to approach 37%, but where enrollment trailed expected by 22%.16 Similarly, initial reports suggested similar adverse event risk with nivolumab, an immune checkpoint inhibitor. However, although the exact risk is unclear, treatment appears to be associated with a nearly 40% relative increase for high-grade adverse event risk in women, depending on the population studied.24 Despite our observation of some updates in the reporting of sex-based analyses among more recent studies, solutions for these discrepancies will require more systematic and prospective investigation. Yet, consideration for the implementation of a more diverse trial leadership structure, sex-specific patient navigators, planned assessment of sex-specific biologic effects, and the incorporation of sex-specific patient-reported outcomes may improve the capture of potentially relevant effects.
Clinicians and patients faced with difficult decisions on the initiation of potentially life-saving anticancer therapies rely on the rigor of reporting within supporting clinical trials. Often within this analysis, significant heterogeneity was observed in reporting sex-specific effectiveness. This was exemplified by the absence of sex-based safety reports within any “targeted” therapy trial, and by low rates of efficacy assessments in early immune checkpoint trials, treatment commonly marketed as precision therapies. Although some uptake was seen in the enrollment of women among more recent studies, well after the early to mid-2000s, a time when additional federal calls for gender-focused evaluations were widely circulated, many of the more recent trials evaluating therapies with apparent signals for sex differences did not report any investigation of potential efficacy or safety considerations.7, 25, 26 Similarly, drug companies may not be incentivized to publish potentially limiting or concerning sex-specific safety and/or efficacy data, particularly when signals are derived from underpowered (exploratory) studies.
Although the exact reasons for these observations are not clear, several plausible barriers to female recruitment and retention have been identified. Women have been underrepresented in clinical studies in part, anecdotally, due to underlying policies excluding women for reasons of reproductive health.20 Whereas the intention was to protect women of childbearing age from potential adverse outcomes, the unintended exclusion of females, contributed to a gap in literature regarding drug effects in this population.23 Even with adjustments in these policies, following recommendations by both NIH as well as FDA, data on whether outcomes are different between the sexes continues to be limited.25, 27 In a self-administered questionnaire study of non-cancer patients, underemployment and distrust in pharmaceutical institutions were more common among female participants.26 Lymphoma, a disease wherein enhanced awareness and robust social support groups are increasingly available, saw higher female proportional enrollment, suggesting these strategies may be advantageous.28 Childcare commitments and time away from work have been cited as barriers to participating in clinical trials in younger women.29, 30 Similarly, women of childbearing age may be excluded from participation due to concerns of potential harm. The fear of adverse effects on women's health was also cited as a reason for refusal to participate in trials.31 Moreover, in several countries, women may exhibit less self-autonomy in decisions to enroll in research, as frequently women are required to seek permission from a husband or parent.32 These barriers have been recognized to drive disproportionate enrollment of women in other populations. However, with cancer trials, elucidation of these barriers may require additional community-based and prospective studies.
While the reasons for the underreporting of sex-based efficacy and safety are not well understood, there are some potential theories to explain why. In a 2010 Society for Women's Health Research evaluation of 11 major journals, only 18.2% (two) had a requirement for sex-based data reporting. Similarly, editorial discomfort may have contributed to the relative deemphasis of sex-based differences, particularly where patterns were not clear.33 Additionally, some investigators may have elected not to publish underpowered sex-based safety or efficacy data if the data were more difficult to interpret. Accordingly, it may be reasonable to suggest that trial outcomes be disaggregated by sex when larger cohort sizes are achieved (e.g., >300 participants). Further, it may be reasonable to mandate the publication of sex-based efficacy for latter-phase clinical trials by medical journals. Beyond measures that have already been initiated since the landmark publication by Murthy et al.,7 it is imperative to consider policy benchmarks wherein the publication of any larger, phase II, III, or IV clinical trial (e.g., >200 enrolled subjects), or trials linked to the FDA's decision on oncologic drug approval, should be mandated to publish or make available online their sex-based efficacy data, even if exploratory. Similar to NIH's policy for all studies to include consideration of sex as a biologic variable, we also suggest a relative mandate by the journal for the publishing (or online availability) of sex-based efficacy and safety outcomes data supported by NIH funding (to further support broader implementation of these goals and standards). Yet, given the growing appreciation of the consequences of differences in safety profile with many novel targeted and immune therapies by sex, added priority to stratified data presentation is warranted.34 Despite efforts like the NIH Revitalization Act, which encourages adequate inclusion of women in clinical studies, challenges persist regarding the effectiveness of this reform.35, 36 Equitable enrollment of women is critical to elucidate potential biological differences, as has already been suggested in several fields of medicine. The lack of sex-based analysis and reporting in clinical studies undermines the ability to adequately deliver more precision-based care. This specifically hampers the development and implementation of targeted precision medicine approaches,37 while also potentially masking important sex differences in treatment response, toxicity, and adverse effects. Considering the potential broad public health implications (e.g., potentially lower efficacy or safety in women, data transparency, incomplete knowledge base), improved strategies are needed.
4.1 Limitations
Several limitations should be acknowledged. This investigation focused on latter-phase pivotal clinical trials supporting FDA approval and did not include data from early-stage or non-efficacious drug-related trials. However, given the desire to best reflect drugs commonly employed in contemporary clinical practice, we focused on these high-profile and more well-controlled studies. We could not account for potential variations in cancer incidence and burden across all non-US countries. Gender identity exists on a spectrum and is distinct from the sex assigned at birth. To allow more definitive analyses from available data, this study focused on sex assigned at birth using conventional binary classifications (i.e., female, male). We could not fully determine the sex of all trial leads. Participant age based on biologic sex was not uniformly available across trials. We were not powered to definitively determine the effect of higher relative cancer incidence in women on enrollment. Further, qualitative data collection may aid in the exploration of the reasons for underrepresentation in future cancer trials. Trials performed after the FDA's approval decision were not included. Screening data were not uniformly available. Information on clinical trial site locations within countries was not uniformly available. Further, as this evaluation focused on publicly available data, reported trial reviews, and investigator-provided information, some non-publicly available data may not have been captured, despite extensive search.
5 CONCLUSION
In summary, among clinical trials supporting contemporary cancer therapies, women were frequently underrepresented, and sex-based analyses were commonly not performed. This pattern remained even after accounting for trial characteristics, including the time of trial initiation. Given the potentially serious impacts of inadequate clinical and safety information, increased focus on the consistency and systematic nature of female enrollment and sex-based reporting is needed.
AUTHOR CONTRIBUTIONS
All of the authors had full access to all of the data in the study, reviewed drafts of the manuscript, and approved the final version. Concept and design: MAB, JMB, and DA. Acquisition, analysis, or interpretation of data: SuK, SG, BeO, MAB, JMB, and DA. Drafting of the manuscript: all authors. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: SuK, EM, MAB. Administrative, technical, or material support: DA. Supervision: DA. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
ACKNOWLEDGMENTS
The authors acknowledge and thank the patients, providers, and their families who supported the completion of these trials.
FUNDING INFORMATION
This work was supported in part by National Cancer Institute (NCI) Grant (P30 CA016058); as well as by National Institutes of Health (NIH) (K01HL142848 [KB], R01HL159216 [KB], R56HL159216 [KB], and L30HL148881 [KB], KL2-TR002733 [JB], K12-CA133250 [DA], K23-HL155890 [DA], R01HL170038 [DA], R01HL168045 [DA]). Dr. Breathett also receives funding from the Health Resources and Services Administration (HRSA). Dr. Carter was supported in part by charitable contributions from the Mary H. and J. Churchill Hodges Clinical Prevention Program Fund at the Ohio State University Wexner Medical Center. Mr. Kola-Kehinde was supported by Ohio State University Comprehensive Cancer Center's Pelotonia grant funds. Dr. Paskett has received research funding from the Breast Cancer Research Foundation, and The American Cancer Society. Dr. Addison was also supported by a Robert Wood Johnson Foundation (Harold Amos)- American Heart Association grant. Support also received from a 2022 American Society of Clinical Oncology (ASCO) Merit award (Dr. Babcook). All other authors have reported no relationships relevant to the contents of this paper to disclose. The funder did not play a role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
CONFLICT OF INTEREST STATEMENT
Dr. Paskett received research funding from NCI, Merck Foundation, Pfizer, Genentech, Guardant Health and Astra Zeneca and is an Advisory Board member for GSK and Merck. The other authors have no conflict to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data that support the results of this research are available upon request to the corresponding author, Dr. Addison. Patient-level data that support the results of this research are available at the following websites: https://www.fda.gov/drugs, https://clinicaltrials.gov/, https://pubmed.ncbi.nlm.nih.gov, https://seer.cancer.gov/, https://www.census.gov/.