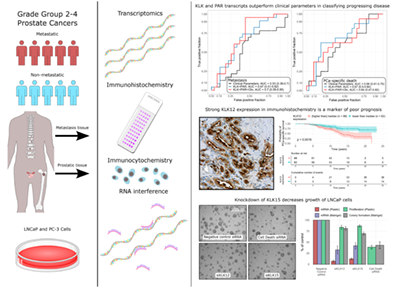
Prognostic impact of kallikrein-related peptidase transcript levels in prostate cancer
Abstract
We aimed to study mRNA levels and prognostic impact of all 15 human kallikrein-related peptidases (KLKs) and their targets, proteinase-activated receptors (PARs), in surgically treated prostate cancer (PCa). Seventy-nine patients with localized grade group 2-4 PCas represented aggressive cases, based on metastatic progression during median follow-up of 11 years. Eighty-six patients with similar baseline characteristics, but no metastasis during follow-up, were assigned as controls. Transcript counts were detected with nCounter technology. KLK12 protein expression was investigated with immunohistochemistry. The effects of KLK12 and KLK15 were studied in LNCaP cells using RNA interference. KLK3, -2, -4, -11, -15, -10 and -12 mRNA, in decreasing order, were expressed over limit of detection (LOD). The expression of KLK2, -3, -4 and -15 was decreased and KLK12 increased in aggressive cancers, compared to controls (P < .05). Low KLK2, -3 and -15 expression was associated with short metastasis-free survival (P < .05) in Kaplan-Meier analysis. PAR1 and -2 were expressed over LOD, and PAR1 expression was higher, and PAR2 lower, in aggressive cases than controls. Together, KLKs and PARs improved classification of metastatic and lethal disease over grade, pathological stage and prostate-specific antigen combined, in random forest analyses. Strong KLK12 immunohistochemical staining was associated with short metastasis-free and PCa-specific survival in Kaplan-Meier analysis (P < .05). Knock-down of KLK15 reduced colony formation of LNCaP cells grown on Matrigel basement membrane preparation. These results support the involvement of several KLKs in PCa progression, highlighting, that they may serve as prognostic PCa biomarkers.
Graphical Abstract
What's new?
Several kallikrein-related peptidases (KLKs) are associated with prostate cancer, most notably KLK3, also called prostate-specific antigen. The relationship between most human KLKs and prostate cancer, however, remains unclear. In our study, mRNA levels and prognostic utility of KLKs and protease-associated receptors (PARs), which influence tumor growth, were investigated in advanced prostate cancer. Analyses revealed associations between poor prognosis and decreased KLK15 mRNA expression and increased KLK12 immunostaining. Consideration of KLK and PAR expression improved prediction of metastasis and cancer-specific death. Moreover, knockdown of KLK15 reduced LNCaP cell growth. The findings identify promising new biomarkers for prostate cancer.
Abbreviations
-
- AR
-
- androgen receptor
-
- AUC
-
- area under the curve
-
- CRPC
-
- castration-resistant prostate cancer
-
- FFPE
-
- formalin-fixed paraffin-embedded
-
- GG
-
- grade group
-
- IHC
-
- immunohistochemistry
-
- KLK
-
- kallikrein-related peptidase
-
- LOD
-
- limit of detection
-
- NE
-
- neuroendocrine prostate cancer
-
- PAR
-
- proteinase-activated receptor
-
- PCa
-
- prostate cancer
-
- PSA
-
- prostate-specific antigen
-
- pT stage
-
- pathological tumor stage
-
- RNAi
-
- RNA interference
-
- RP
-
- radical prostatectomy
-
- RT-qPCR
-
- quantitative reverse transcription-polymerase chain reaction
-
- TMA
-
- tissue microarray
1 INTRODUCTION
Prostate cancer (PCa) is the second most frequent cancer and the fifth leading cause of cancer deaths in men globally.1 The prognosis of individual PCa patients is highly variable.2 Histological classification, using Gleason grading and the Grade Group (GG) system, is one of the strongest predictors of PCa outcome.3 Most new PCa cases cluster in GG2-4,4 in which the highest or predominant Gleason grade pattern is 4. For this group, there are no good prognostic markers and, perhaps excluding GG4, tumor grading alone cannot adequately distinguish indolent cases from those who will develop aggressive PCa.5 Thus, novel prognostic and predictive markers are needed.
Prostate-specific antigen (PSA, also known as kallikrein-related peptidase [KLK]-3) is a widely used PCa marker.6 However, the accuracy of blood-based PSA tests to identify clinically significant PCa is poor.7 The accuracy can be increased by analyzing, in addition to total PSA, different PSA forms, such as free PSA, PSA-density, and markers, such as KLK2.8, 9 PSA (referred hereinafter as KLK3) is a member of a human KLK family, consisting of 15 proteases.10 Several KLKs are expressed in the prostate and are regulated by androgens, the most abundant being KLK3 and -2.10, 11 Although the blood concentrations of KLK2 and -3 are increased in PCa, often positively correlating with disease burden, the opposite has been found in PCa tissue, where low KLK2 and -3 expression is associated with dedifferentiation and poor prognosis.12-15 In addition to KLK2 and -3, other KLKs are potential markers in various cancers,16, 17 including PCa where they are differentially expressed compared to benign prostate, and may have prognostic significance.16, 18
The physiological function of the major prostatic KLKs has been proposed to relate to promotion of sperm motility by dissolving seminal clot formed after ejaculation.10 KLKs may drive PCa progression, although the opposite has also been suggested for some of them.10, 19-21 Perhaps the most studied, in this respect, is KLK3, which has been proposed to have activities that both promote and suppress the PCa growth and progression.20-23 KLKs are involved in proteolytic cascades, which may facilitate PCa growth and metastasis.20, 24 Several KLKs are able to activate growth-factors and protease-associated receptors (PARs), which are involved in tumor growth and metastatic dissemination.10, 25-29 Especially, PAR1, also known as thrombin receptor, has been strongly implicated in promotion of cancer cell invasion.30, 31 Overexpression of PAR1 has also been shown to predict biochemical recurrence in PCa and is a marker of late-stage disease.32, 33 Among the four human PARs (PAR1-4, encoded by genes F2R, F2RL1, F2RL2 and F2RL3, respectively), especially the activation of PAR1 and -2 have been found to be regulated by several KLKs.27, 28 PARs can also be activated by other proteases, including thrombin and matrix metalloproteinases, present in the prostate tumor microenvironment.28 Notably, PAR-activation by different proteases may lead to different responses.28
Most of the studies addressing either the protein or mRNA expression of KLKs and PARs in PCa have included a relatively small number of nonaggressive to highly aggressive cases,16, 18 not reflecting the clinical need for prognostic biomarkers in GG2-4 PCa. Previous studies have also addressed only a single or a few KLKs at a time. Thus, we aimed to study tissue mRNA levels of all human KLKs and PARs and their prognostic value in the clinically challenging GG2-4 PCa. KLK12 was also studied by immunohistochemistry and, together with KLK15, using RNA interference (RNAi).
2 MATERIALS AND METHODS
2.1 Patient summary and study design
The primary case-control study for mRNA analyses consisted of 165 GG2-4 patients treated by radical prostatectomy in Helsinki University Hospital in 1992-2015. The selection process of patients has been previously described.34 Seventy-nine patients with metastatic progression or PCa-specific death, during the median follow-up of 11 years, were selected to present cases. Patients with similar clinical baseline characteristics, but no metastasis during the follow-up (n = 86), were assigned as controls, representing a clinically indolent phenotype of GG2-4 PCa. Additionally, 12 samples from distant PCa metastases, seven samples of castration-resistant PCa (CRPC) and six neuroendocrine PCa (NE) were included. Nineteen benign prostate samples from men without detected PCa, but with benign prostatic hyperplasia, were also studied. One of the metastases was from a para-aortic lymph node, from the level of renal vein and was extracted 10 years after RP. The other metastases were from bone (vertebra, humerus, femur, n = 5), bone marrow (iliac crest, n = 1), spinal cord (n = 1), liver (n = 1), appendix (n = 1), testis (n = 1) and epididymis (n = 1). All patients in the CRPC group, 10 in the metastasis group and three in the NE had received hormonal treatment before the samples were extracted. Patients in other groups were hormone-naïve at the time of sample extraction. During quality control, eight aggressive cases, four controls and three metastasis samples were excluded from the final analyzes. The histological slides of all the patients were rereviewed by an expert uropathologist (TM) and the index cancer lesions were annotated for RNA extraction and tissue microarray (TMA) construction using the formalin-fixed paraffin-embedded (FFPE) blocks. Detailed demographics of analyzed samples are available in Table 1.
| Variable | Case | Control | Metastases | CRPC | NE | Benign |
|---|---|---|---|---|---|---|
| Sample type, n (%) | ||||||
| RP | 71 (100) | 82 (100) | 0 | 0 | 1 (16.7) | 2 (10.5) |
| TURP | 0 | 0 | 0 | 7 (100) | 5 (83.3) | 17 (89.5) |
| Metastasis biopsy | 0 | 0 | 9 (100) | 0 | 0 | 0 |
| Age at RP/TURP/met biopsy (y) | ||||||
| Mean (SD) | 61.0 (6.4) | 63.8 (5.9) | 72.2 (7.5) | 79.5 (9.6) | 73.2 (11.0) | 65.1 (8.2) |
| RP/TURP grade group, n (%)a | NAb | |||||
| 1 | 0 | 0 | 0 | |||
| 2 | 20 (28.1) | 39 (47.6) | 0 | |||
| 3 | 35 (49.3) | 27 (32.9) | 0 | |||
| 4 | 16 (22.5) | 16 (19.5) | 0 | |||
| 5 | 0 | 0 | 7 (100) | |||
| pT stage, n (%)c | NA | NA | NA | |||
| T2 | 22 (31.0) | 32 (39.0) | ||||
| T3a | 22 (31.0) | 31 (37.8) | ||||
| T3b | 27 (38.0) | 19 (23.2) | ||||
| Presurgery PSA (ng/mL) | ||||||
| Median (IQR)d | 9.4 (6.1) | 8.8 (7.4) | 53.0 (491) | 12.1 (23.1) | 2.0 (4.1) | 12.2 (7.8) |
| Follow-up time (y) | 10.0 | 11.9 | ||||
| Metastasis, n | 71 | 0 | 9 | 5 | 4 | 0 |
| PCa-specific death, n | 47 | 0 | 7 | 4 | 3 | 0 |
| Death from other reasons, n | 3 | 6 | 0 | 2 | 2 | 0 |
- Abbreviations: CRPC, castration-resistant PCa; IQR, interquartile range; NE, neuroendocrine PCa; PCa, prostate cancer; PSA, prostate-specific antigen; pT stage, pathological tumor stage according to American Joint Committee on Cancer (AJCC) TNM 8th edition; RP, radical prostatectomy; T2-3, tumor stage; TURP, transurethral resection of the prostate.
- a P = .038 between case and control groups (permutation test of independence).
- b P = .11 between case and control groups (permutation test of independence).
- c P = .97 between case and control groups (Mann-Whitney U test).
- d Neuroendocrine prostate cancer is not graded with Gleason score or grade group.
2.2 RNA extraction and transcript analysis
RNA extraction was performed using previously published methodology.34 In brief, one to two 1 mm diameter punches were sampled from FFPE blocks. The punches were deparaffinized, homogenized and proteinase K digested. Then RNA was extracted using QIASymphony RNA kit (QIAGEN, Venlo, Netherlands). RNA concentration was analyzed using RiboGreen kit (Invitrogen, Waltham, MA) and the RNA integrity (range = 1.0-7.6, mean = 2.24, SD = 0.82) was measured with Agilent Bioanalyzer kit (Agilent Technologies, Santa Clara, CA). Transcript counts were measured using the NanoString nCounter platform (NanoString Technologies, Seattle, WA). The transcripts were hybridized with probes of a custom 93-gene NanoString Reporter and Capture CodeSet. Among others, probes included those for KLK1-15 and PAR genes, androgen receptor gene (AR), and 12 housekeeping genes (Table S1). The housekeeping genes were ACTB, ALAS1, B2M, CLTC, G6PD, GAPDH, GUSB, HPRT1, POLR2A, SDHA, TBP and TUBB.
2.3 Tissue microarray composition and immunohistochemical staining
The samples punched into TMA for an immunohistochemistry (IHC) study overlapped largely with the samples punched for mRNA extraction. The IHC study additionally contained 11 samples of matched primary PCa for the metastasis samples. For case, control, CRPC and NE specimens, two punches of 1 mm in diameter of cancer tissue were sampled. Benign tissue adjacent to cancer tissue, was also sampled from case and control blocks. For metastasis samples, two punches were extracted from FFPE blocks from metastases, and two samples from the index tumor in the prostate. For patients without any detected PCa, two punches of benign tissue were extracted. Punches were sampled from a location as close as possible from those used for RNA extraction, so the TMA cores can be considered representative of the mRNA punches.
TMA sections of 3.5 μm were first hematoxylin and eosin stained in a clinical laboratory (Huslab, Helsinki, Finland). The TMA slides were scanned using Pannoramic 250 Flash III scanner (3DHISTECH, Budapest, Hungary) with 20X magnification (NA 0.8). Digitized slides were then consensus-assessed by an expert uropathologist (TM) and a nonpathologist researcher (TPKL) for percentage area of total epithelium and cancerous epithelium in relation to total epithelium. TMA cores in the case group had mean total epithelial area of 67% (SD = 18.7) of which 93% (SD = 16.1) was cancerous. In the control group, the mean total epithelial area was 67% (SD = 18.4) of which 89% (SD = 23.7) was cancerous.
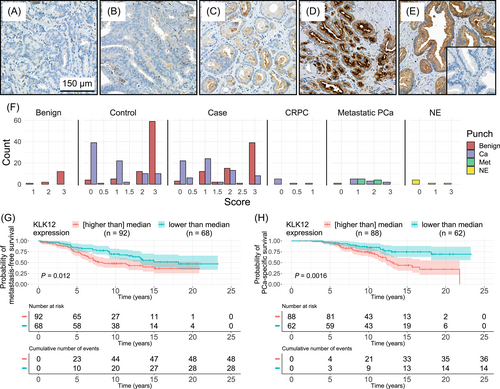
TMA sections of 3.5 μm were immunostained using rabbit-anti-KLK12 antibody, previously validated for IHC of prostatic tissue.35 The slides were incubated at +37°C overnight and at +56°C for 1 hour before deparaffinization in xylene and rehydration in a decreasing ethanol series. Antigen retrieval was performed with Dako PT Link Pretreatment module (Agilent Technologies) using Dako Envision TM Flex Target Retrieval solution, low pH (Agilent Technologies). The staining was carried out in an Autostainer 480 (LabVision Corporation, Fremont, CA) using Dako REAL Envision Detection system Peroxidase/DAB+, Rabbit/Mouse (Agilent Technologies). The KLK12 antibody, diluted at 1:500, was incubated for 1 hour at room temperature and the secondary antibody (of the Dako REAL Envision Detection system) for 20 minutes at room temperature. In control sections the primary antibody was replaced by Rabbit IgG control antibody (Vector Laboratories, Newark, CA). Finally, tissues were counterstained with hematoxylin (Histolab, Espoo, Finland), washed with water and mounted using Aquatex (Merck Millipore, Burlington, MA). The slides were again scanned with a Pannoramic 250 Flash III slide scanner, with 20X magnification (NA 0.8). Staining intensities of the tissue samples were evaluated by three observers (RK, HK and TM). Only the cancerous areas were evaluated in cancerous TMA spots, and benign areas in cancer-adjacent benign spots. Apart from this classification, staining evaluation was blinded regarding the clinical data. Staining intensity in the epithelial cells was assessed in an ordinal scale as negative (0), weak (1), moderate (2) or strong (3) and their combinations, for example, moderate-strong (2.5). Only the areas with the strongest staining for the spot were assessed, given that those represented >5% of the total cancerous epithelial area in the spot. One aggressive case, two metastasis samples, one sample of matched primary PCa and four samples of benign prostate tissue were excluded due to not being scorable.
2.4 Cell culture
LNCaP cells (ATCC, CRL-1740, RRID: CVCL_0395), originally isolated from lymph node metastasis of human prostate adenocarcinoma, were maintained in RPMI-1640 media (Lonza, Basel Switzerland) supplemented with 10% fetal bovine serum (Biowest, Nuaillé, France), 100 units/mL penicillin, 0.1 mg/mL streptomycin, 1 M glucose, 1 M HEPES, 100 mM sodium pyruvate, L-glutamine and nonessential amino acid cell culture supplement (Lonza) at +37°C, 5% CO2. AR-negative bone metastasis-derived PC-3 cells (ATCC, CRL-1435, RRID: CVCL_0035) were cultured in Ham's F12 Nutrient mixture (F-12) (Gibco) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 0.1 mg/mL streptomycin, and 7.5% sodium bicarbonate (Gibco). Culture media was changed every 2 to 3 days. Upon reaching 80% confluence, cells were detached from flasks using trypsin-EDTA solution (Lonza). Trypsin was inactivated with complete growth media and cells were centrifuged. Cell pellet was resuspended in fresh media and cells were counted using Countess II automated cell counter (Invitrogen). All cell lines were authenticated using short tandem repeat (STR) profiling within the last 3 years. All experiments were performed with mycoplasma-free cells.
2.5 RNA interference
For RNAi, cells were seeded 24 h prior to transfection with siRNAs (all from QIAGEN). Four different siRNAs targeting KLK12 (KLK12_1, SI00124201, TGGGAACTTCTTGGAACTTTA; KLK12_2, SI00124208, TCCCTGGAGTCTACACCTATA; KLK12_3, SI00124215, TCCCGGGAGAATCACGAGCAA; KLK12_8, SI05119912, ATAGTCTGGAATAAATATAAA) and KLK15 (KLK15_1, SI00134974, CCCGCCTGACATGGAACAGAA; KLK15_3, SI00134988, CACCTGTTTAATGCCAAGATA; KLK15_5, SI02642647, CCCGTGTGATCTTGAACAAGA; KLK15_6, SI03033485, AAGCGCGATGGCCCAGAGCAA) were used in two to three independent experiments for each siRNA. AllStars Negative Control and AllStars Hs Cell Death Control siRNAs were used as controls. siRNAs were transfected, at a final concentration of 10 nM, using Lipofectamine RNAiMAX transfection reagent (Invitrogen) in serum-free OptiMEM-1 medium (Invitrogen) according to the manufacturer's instructions.
The efficiency of siRNAs was determined using quantitative reverse transcription-polymerase chain reaction (RT-qPCR). One milliliter of cold Matrigel (Corning, Corning, NY) was pipetted into wells of 6-well plates on ice and allowed to solidify at +37°C for an hour. LNCaP cells (2 × 104 cells/well) were seeded and, after 24 hours incubation, transfected as above. Cells were washed three times with ice-cold PBS on ice 72 hours after transfection, and 2 mL of Cell Recovery solution (Corning) was added into each well. The cells and Matrigel were detached from the bottom of the well using cell scraper and the plate was incubated on ice for 30 minutes. The contents of the wells were collected into 50 mL tube, and the wells rinsed with 2 mL of Cell Recovery solution. The tubes were incubated on ice for 30 minutes followed by the centrifugation at 1000g for 5 minutes at +4°C. LNCaP cells were also seeded on a 6-well plate (250 000 cells per well) and incubated at +37°C for 24 hours prior to transfection as above. Seventy-two hours after transfection, the cells were washed once with PBS and detached using trypsin-EDTA solution (Lonza). Trypsin was inactivated with complete growth media and cells were centrifuged. For both on Matrigel and on plastic grown cells, the supernatants were discarded, and cell pellets were resuspended in 1 mL of ice-cold PBS, transferred into the microcentrifuge tubes and centrifuged at 10 000g for 5 minutes at +4°C. The washing step was repeated, and the cell pellets were stored at −80°C until RNA extraction. Total RNA was isolated using RNeasy Mini kit (QIAGEN) and quantified using DS-11+ spectrophotometer (DeNovix, Inc, Wilmington, DE).
cDNA was obtained using SensiFAST cDNA Synthesis Kit (Meridian Bioscience, Cincinnati, OH) according to the supplier's protocol. RT-qPCR was conducted using TaqMan gene expression assays (Applied Biosystems, Waltham, MA) on Applied Biosystems 7500 Fast Real-time PCR system according to the manufacturer's instructions. TaqMan assays used for KLK12, KLK15 and GAPDH were Hs00377603_m1, Hs00930396_g1 and Hs02786624_g1, respectively. Relative mRNA levels were analyzed as the ratio of KLK12 or KLK15 to GAPDH using 2-ΔΔCt method.
2.6 Overexpression of KLK12 and KLK15 in PC-3 cells
KLK12 (UniProt: Q9UKR0) and KLK15 (Q9H2R5) encoding cDNAs were cloned as synthetic genes in pcDNA3.1(+) plasmid using EcoRI/NotI sites (GenScript Biotech, Rijswik, Netherlands) (see Supplementary Material). The codons were optimized for mammalian expression (GenScript) and the propeptides, following the signal peptides of KLK12 and KLK15, were replaced by a furin-activable propeptide (APLRLRR), which has previously been successfully used for activation of KLK3 in PC-3 cells.36 For controls, codons for Ser200 in KLK12 and Ser209 in KLK15 in active sites were replaced by a codon encoding Ala.
The plasmids were transfected in PC-3 cells using FugeneHD transfection reagent (Promega, Madison, WI). For selection of KLK12/KLK15 expressing cells, the cells were grown, at least for 28 days, in Ham's F12 media (Gibco) with supplements as above, containing also 200 μg/mL of geneticin (Gibco). For the detection of the expression of KLK12, KLK15 and their mutant forms, RNA was isolated and reverse-transcribed to cDNA as described above, and subjected to RT-qPCR and SybrGreen detection (Meridian bioscience, Cincinnati, OH). For primers for codon optimized KLK12 and KLK15 see Supplementary Material. The expression was assessed using ΔΔCt method (i.e., ΔCt of KLK transfected cells − ΔCt of wild-type cells, where ΔCt = threshold cycle [Ct] of the gene of interest − Ct of GAPDH). If the Ct was not detectable it was set to 40 (as seen sometimes for KLK12 and KLK15 in wild-type PC-3 cells). Secreted proteins were also detected in conditioned cell culture supernatants by immunoassays.11 For this, 40 000 cells were grown on wells of 12-well plate for 7 days in 1.2 mL medium. KLK12 was also detected by immunocytochemical staining (ICC). For ICC the cells grown on glass surface were fixed for 5 min with acetone. After washing twice with PBS, essentially the same protocol, including the antibody, as used for IHC staining of KLK12 in tissue sections was followed, but without antigen retrieval and steps before that.
2.7 Cell growth assays
To assess the effect of siRNAs on cell viability and growth on plastic surface, 6000 LNCaP cells/well were seeded on a 96-well plate and left to grow for 24 hours prior to siRNA transfection as above. To study the effect of overexpression of KLK12 and KLK15 on cell proliferation, PC-3 cells (3000 cells/well) were seeded on a 96-well plate. After 3-4 days, 10 μL of Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan) reagent was added into each well and the plate was incubated at +37°C for 1-2 hours. The absorbance at 450 nm was measured using Multiskan EX plate reader (Labsystems, Vantaa, Finland). Three independent experiments were conducted in triplicate.
For growth of cells on top of Matrigel, 150 μL of cold Matrigel was pipetted into wells of a 48-well plate on ice and allowed to solidify at +37°C for an hour. LNCaP cells were seeded and transfected as above for RNA isolation. Half of the media was replaced with fresh media every 3 days. The effect of KLK12 and KLK15 knockdown on the number and area of LNCaP cell colonies grown on Matrigel, was quantitated from images acquired using EVOS FL cell imaging system (Advanced Microscopy Group, Bothell, WA) with 2X objective. Images were analyzed using Fiji ImageJ.37 The background subtraction was performed using the Gaussian blur method and image thresholding using Otsu method. The Analyze particles-command was used to analyze the cell clusters in the segmented images. The minimum particle size was set to 200 pixels to remove the background debris. Cell clusters touching the border were excluded from the analysis. Median size of cell clusters from each siRNA treated sample was multiplied by the number of the cell clusters to obtain a colony formation score.
2.8 Invasion and migration
The invasion assays were performed using Nunc Polycarbonate cell culture inserts (Thermo Scientific) and migration assays using Millicell cell culture inserts (Millipore, Burlington, MA). For invasion assays, the 24-well inserts were coated with 50 μL of Matrigel (0.5 mg/mL) and incubated at room temperature for 2.5 hours followed by the incubation with serum-free culture media for an hour. Transfected and wild-type PC-3 cells (300 000) were seeded in serum-free culture media on top of Matrigel in the upper chamber of the Nunc inserts. For migration assays, 300 000 cells were seeded in serum-free culture media in the upper chamber of the Millicell inserts. For both assays, F-12 culture media with 10% FBS was used as a chemoattractant in the lower compartments. After 2-4 days incubation, the cells from the upper chamber were removed with a cotton swab soaked in ultrapure water. Migrated cells were stained with 0.1% crystal violet for 10 minutes and rinsed with ultrapure water. Finally, the inserts were incubated in 200 μL of extraction solution (Cell Biolabs, Inc, San Diego, CA) for 10 minutes at room temperature. One hundred microliters of the extracts were then transferred to a 96-well plate and the absorbance was measured at 560 nm using Multiskan EX plate reader.
2.9 Statistical analyses
Statistical analyses were performed using nSolver Analysis Software (NanoString Technologies), v. 4.0.70 and R, v.4.0.2 (R Development Core Team, Vienna, Austria). Detailed statistical methodology can be found in the Supplementary statistical methods.
Transcript data quality control was performed in nSolver software in compliance with NanoString's guidelines. Raw counts were exported into R. Limit of detection (LOD) was determined as mean + SD of negative control probes. The eight best performing housekeeping genes, including ACTB, B2M, CLTC, GAPDH, GUSB, POLR2A, SDHA and TUBB were used to analyze variation caused by technical sources. Next, differential gene expression analysis was performed. Differentially expressed genes were determined based on multiple hypothesis testing adjusted P < .05.
Transcript counts were variance-stabilizing transformed for further analyses. Metastasis-free and PCa-specific survival of patients in the case and control study groups was analyzed using Kaplan-Meier estimator and Mantel-Haenszel log-rank test. Random forests were used to classify metastatic disease and PCa-specific death. The data was randomly split to training and validation sets with stratification, which consisted of 67% and 33% of case and control samples respectively. Class imbalance of training set was overcome by synthetic minority oversampling technique. The model performance was assessed using receiver operating characteristic areas under the curves (AUCs). AUCs were compared using DeLong test. Goodness of models was also assessed by stratifying the validation set based on the random forest model classifications in Kaplan-Meier survival analysis.
When multiple spots representing same patients and tissue type were available, the ones showing strongest staining were used for analyses. The IHC scores of cancer spots were compared to adjacent benign tissue using Mann-Whitney U-test. The IHC scores of cancer adjacent benign spots of case and control samples were also compared to benign tissue samples from patients with no detected PCa. Spearman's rank order correlation of KLK12 IHC scores and transcript counts was calculated. Statistical analysis of cell growth assays was conducted using two-tailed one-sample t-test after assessing normality with Shapiro-Wilk test.
3 RESULTS
3.1 Low KLK15 expression is associated with poor prognosis
We set out to study the expression of all human KLKs and PARs in PCa on mRNA level. KLK3, -2, -4, -11, -15, -10 and -12, in decreasing order of mean mRNA expression, were expressed in PCa and benign prostate samples over LOD (Figure 1A). Expression of these KLKs over LOD was observed also in metastases and CRPC. The mean expression levels of other KLKs were close to background. Very high expression of KLK2, -3 and -4 was observed in PCa and benign prostate, however in NE samples the expression of KLK2-4 was low. On the contrary, KLK12 expression was highest in NE. Among the four human PARs, PAR1 and PAR2 were highly expressed in benign tissues and all subgroups of PCa. Low expression of PAR3 and PAR4 was also observed in PCa groups. AR was highly expressed in all samples aside from NE.
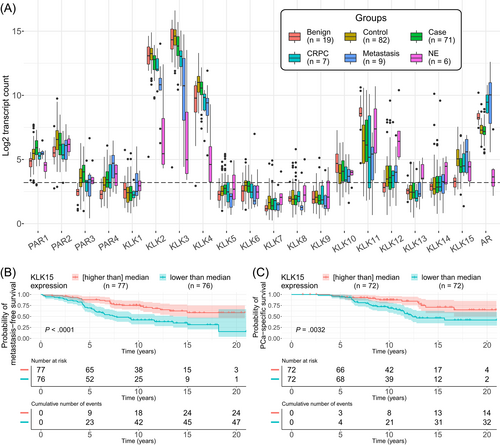
Among the significantly expressed KLKs, the expression of KLK4, -12 and -15 was increased, and that of KLK10 and -11 decreased, in cancer (cases and controls combined), compared to benign prostate (P < .05 for all) (Figure 1A and Table 2). However, when aggressive cases were compared to controls (Figure 1A and Table 2), the expression of KLK2, -3, -4 and -15 was found to be decreased, and that of KLK12 increased, in cases (P < .05 for all). Higher expression of all PARs was observed in cancer (cases and controls combined) compared to benign prostate (P < .05). The expression of PAR1 and PAR4 was increased in cases compared to controls, while PAR2 was decreased (P < .05). AR expression was similar in case and control groups (P = .73). AR appeared to be up-regulated in metastatic and CRPC samples compared to localized cancer and down-regulated in neuroendocrine cancers as shown in Figure 1A, although no statistical tests were performed due to low number of samples in these groups.
| Cancer vs benigna | Case vs Controla | Survival (Kaplan-Meier)b | |||
|---|---|---|---|---|---|
| Log2 fold-change (P-value) | Log2 fold-change (P-value) | Metastasis free | PCSD | Prognostic impactc | |
| KLK2 | −0.0001 (1.0) | −0.55 (.005) | 0.00017 | 0.1 | Positive |
| KLK3 | 0.13 (.73) | −0.54 (.012) | 0.00029 | 0.07 | Positive |
| KLK4 | 1.2 (.00003) | −0.45 (.014) | 0.057 | 0.9 | |
| KLK10 | −0.84 (.001) | 0.29 (.11) | 0.43 | 0.76 | |
| KLK11 | −1.6 (.0006) | 0.15 (.67) | 0.8 | 0.7 | |
| KLK12 | 1.9 (.00003) | 0.77 (.012) | 0.84 | 0.32 | |
| KLK15 | 2.2 (<.00001) | −0.63 (.0012) | <0.0001 | 0.0032 | Positive |
| PAR1 | 1.1 (<.00001) | 0.38 (.012) | 0.024 | 0.009 | Negative |
| PAR2 | 1.1 (.0002) | −0.39 (.046) | 0.25 | 0.7 | |
| PAR3 | 1.9 (<.00001) | 0.30 (.13) | 0.063 | 0.038 | Negative |
| PAR4 | 1.2 (<.00001) | 0.47 (.012) | 0.05 | 0.14 | Negative |
| AR | −0.92 (.00001) | −0.05 (.73) | 0.69 | 0.74 | |
- Note: Only the genes showing mean expression over LOD (Figure 1A) are shown.
- a Change in expression levels in cancer (both cases, as defined by metastasis or death during the follow-up time, and controls with no clinical events during follow-up) compared to completely benign prostate tissues and between cases and controls. Expressed as log2 of fold-change. Values <−0.58 or >0.58 (i.e., those showing >1.5-fold difference in either direction) are bolded. P-values computed using Wald test in parentheses. Significant P-values (<.05) are bolded.
- b Metastasis free and prostate cancer specific death (PCSD) Kaplan-Meier survival estimates with Mantel-Haenszel log-rank tests (P-values) of cancer patients (both cases and controls) as divided in groups based on median expression levels of the studied genes.
- c Positive and negative prognostic impact indicates that low or high expression, respectively, is associated with poor prognosis.
Low KLK2, -3 and -15, and high PAR1 and PAR4 expression was associated with poor metastasis-free survival (Table 2). Further, low KLK15 and high PAR1 and PAR3 were associated with poor PCa-specific survival (Table 2). Survival plots depicting association of KLK15 with metastasis-free and PCa-specific survival are shown in Figure 1B,C. High GG was associated with adverse metastasis-free survival in the binary comparison of GG2 vs GG3-4 (P = .0074), but not with PCa-specific survival (P = .071). However, only PAR2 showed significant differences in mRNA expression across GG2 and 3, and none of the analyzed transcripts were differentially expressed across pathological stages. Differential expression analysis results across GGs and pathological stages are shown in Table S2.
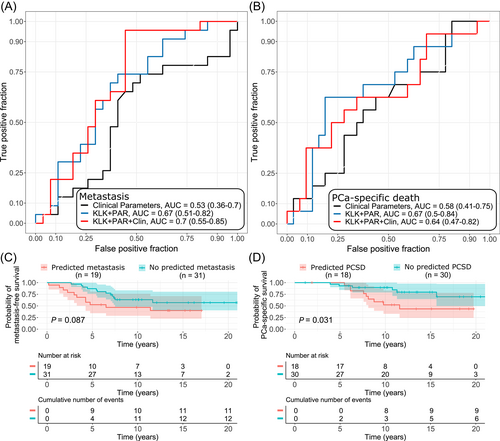
3.2 KLKs and PARs improve classification of progressing PCa over clinical parameters
When all KLKs and PARs, that showed expression levels over LOD, were combined into a random forest model, higher classification performance for metastasis and PCa-specific death was observed compared to that of clinical parameters GG, stage and PSA combined (AUC = 0.67 vs 0.53 and AUC = 0.67 vs 0.58, respectively) (Figure 2A,B). Combining the clinical parameters with KLKs and PARs improved classification of metastatic disease, but not PCa-specific death over the combination of KLKs and PARs alone (AUC = 0.70 and AUC = 0.64, respectively). The observed improvements were not statistically significant in DeLong test due to lack of statistical power in the validation set. In Kaplan-Meier analysis, those with panel-predicted metastasis in the combined model had shorter PCa-specific survival (P = .031), however, no statistically significant differences were observed in metastasis-free survival (P = .087) (Figure 2C,D).
The expression of KLK2 and -3 correlated with AR both in case and control groups, while for KLK4 and -15 such correlation with AR was observed only in the control group (ρ > 0.4, P < .001 for all). Correlations of AR with KLKs and PARs showing mean expression over LOD can be found in Table S3.
3.3 High KLK12 protein expression is associated with poor survival
We also studied KLK12 protein expression using IHC (Figure 3). The intensity of KLK12 expression was stronger in cancer-adjacent benign spots (mean score = 2.46) compared to cancerous spots (mean = 0.96, P < .0001), and similar to that in benign spots from patients without detectable cancer (mean = 2.73, P = .21). In case and control samples, high KLK12 scores were associated with poor metastasis-free and PCa-specific survival (Figure 3G,H). KLK12 IHC scores and transcript levels showed low or no correlation for cases (ρ = 0.25, P = .04), controls (ρ = −0.16, P = .15), benigns (ρ = 0.29, P = .29), CRPCs (ρ = 0.76, P = .049), distant metastases (ρ = 0.27, P = .51) and NEs (ρ = 0.34, P = .51).
3.4 Knockdown of KLK15 reduces the formation of LNCaP colonies on Matrigel
To investigate the functional effects of KLK12 and KLK15 expression on prostate adenocarcinoma, KLK12 and KLK15 were down-regulated in LNCaP cells using four different siRNAs for each gene (Figure 4). When the cells were grown on plastic, all siRNAs targeting either KLK12 or KLK15 reduced KLK12 and KLK15 expression, respectively, below 20% of that in control transfected cells, as determined by RT-qPCR. In the cells grown on Matrigel basement membrane preparation the knock-down efficiency of siRNAs was, in most cases, less pronounced, but still clearly distinguishable from the control (Figure 4). Two siRNAs targeting KLK12 and two siRNAs targeting KLK15 had a statistically significant effect on proliferation of the cells grown on plastic surface (for all P < .05, n = 3), while one of the siRNAs targeting KLK12 and three targeting KLK15 reduced the colony formation score of cells grown on Matrigel (for all P < .05, n = 4).
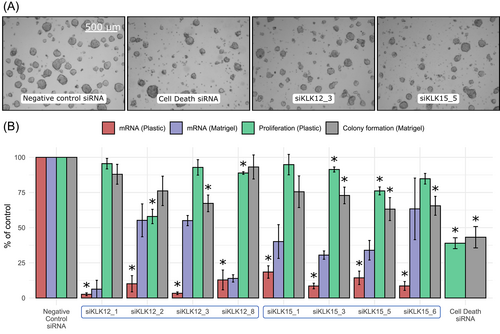
3.5 Overexpression of KLK12 and KLK15 does not affect growth, migration or invasion of PC-3 cells
Since LNCaP cells are poorly invasive we used for invasion studies PC-3 cells that were transfected with KLK12 and KLK15 expression constructs and, as controls, constructs directing expression of active site mutant forms of these KLKs. Unlike the wild-type PC-3 cells, the transfected cells exhibited significant mRNA and protein expression of KLK12 and KLK15 (Table S4 and Figure S1). The overexpression of KLK12 and KLK15 did not significantly change the cell proliferation on plastic surface (Table S4). Also chemotactic migration and invasion were unaffected by overexpression.
4 DISCUSSION
We studied mRNA levels of all 15 human KLKs and their targets, PARs, in patients having GG2-4 PCa. The expression of several KLKs was found to be increased in cancer, but, contrary to KLK12, the expression of KLK2-4 and -15 was decreased in aggressive PCa. Furthermore, we showed, that high KLK12 protein expression is associated with poor survival, and that knockdown of KLK15 reduces colony formation of LNCaP cells on Matrigel basement membrane preparation. One of the major advantages in our study was the analysis of all KLK and PAR transcripts simultaneously in the same samples, allowing comparison of their relative expression and prognostic impact.
The observed mRNA expression profiles of KLKs and PARs in benign and malignant prostate are in line with previous studies regarding the major KLKs, KLK2-4.10, 11, 16, 32 However, KLK1 and -14 showed weak expression in our study, but have been reported to have higher expression in some studies. While the differences in the expression of KLKs in benign prostate and prostatic tumors, and their prognostic significance in PCa have been widely reported,16, 18 this is the first study concentrating on GG2-4 PCas. Among the KLKs expressing over LOD, the expression levels of KLK4, -12 and -15 were found to be higher, and those of KLK10 and -11 lower in cancerous than in benign prostate, agreeing with many, but not all studies.16, 38, 39 Observed variation between studies may arise from differences in sample sets and methodology, including the probes used for mRNA detection. Notably, in our study, benign samples were from men who were not diagnosed with cancer. Most studies have used benign tissues adjacent to cancer as “benign” controls. Such tissues may differ from truly benign ones, perhaps due to paracrine regulation.40, 41
KLK splicing variants appear to be common in PCa.42 Thus, the custom mRNA detection probes for our study were designed for maximum coverage across all transcript variants. Given the observed variation in expression levels among individual samples in our study, the studies including only a very small number of samples may not be representative. All these factors, excluding the potential splicing variants associated with advanced cancer, should be identical across our study groups and, therefore, not compromise the major goal of our study, that is, elucidation whether changes in KLK mRNA levels have prognostic significance.
When mRNA expression levels of KLKs in aggressive cases were compared to those with indolently behaving control group, the expression levels of KLK2, -3, -4 and -15 were found to be decreased, and that of KLK12 increased. Low expression of KLK2, -3 and -15 was associated with poor metastasis-free survival and, in case of KLK15, also with poor PCa-specific survival. Association of low KLK2 and KLK3 protein levels and poor prognosis has been reported.12-14, 43 Others have also reported higher KLK15 expression in cancer than in benign prostate, but, contrary to our results, found that high protein or mRNA expression was associated with advanced PCa and shorter progression-free survival.44-46 An integrated analysis of KLK expression data found in Oncomine and OncoLnc databases has suggested that dysregulation of especially KLK1-KLK5, KLK11 and KLK12 are associated with metastatic progression of PCa.17 A study with prostate and breast cancer cell lines suggests that KLK12, like many other KLKs, is up-regulated by androgen and other steroid hormones.10, 47 However, apparently there are other mechanisms involved as well, as neuroendocrine tumors that had very low AR expression showed prominent expression of KLK12. Indeed, also hypoxia and HIF1α may regulate KLK12 expression.48 As hypoxia is linked to prostate cancer progression,49 this may explain why we found higher KLK12 expression in aggressive cases than in less aggressive ones. Importantly some patients with neuroendocrine tumors had received hormonal treatment before sample extraction.
PARs showed higher expression in cancerous than benign tissue. While PAR1 and -4 expression was higher in aggressive cases compared to controls, the opposite was observed with PAR2. High PAR1 and -4 expression was associated with poor metastasis-free survival and high PAR1 was also associated with poor PCa-specific survival. In accordance with our results, a small IHC study identified PAR1 protein overexpression as a predictor of biochemical recurrence after radical prostatectomy.32 Interestingly, this was observed in periglandular stromal cells, but not in epithelial cells, and PAR2 and PAR4 were not found to predict the outcome.32 Another study found PAR1 to be overexpressed in metastatic PCa compared to localized disease, on both transcript and protein levels.33 Notably, in the study by Kaushal and coworkers, the metastatic PCa more resembles our CRPC samples, than the aggressive cases in our case-control study, since the metastasis patients were not baseline-matched and were undergoing androgen ablation therapy.33 When KLKs and PARs were used as a panel with or without clinical parameters (GG, pathological stage and PSA), they improved classification of metastatic PCa and lethal disease. The demonstrated classification performance was similar to that of publicly available genes included in commercially available PCa risk stratification panels in our previous study.34
KLK2, -3 and -4 are up-regulated by androgens and their levels have been reported to correlate with AR status.10 We also found such correlation with expression levels of these KLKs and AR, while KLK15 correlated with AR in nonaggressive control group, but not in the aggressive PCas. The hormonal regulation of KLK15 is controversial, some studies suggesting regulation by steroid hormones, while the others have not found such an effect.10, 50, 51 Since we did not find any difference in AR expression between the aggressive cancer and control groups it is unlikely that the observed differences in expression of androgen-regulated KLKs are explained by AR expression.
IHC staining of KLK12 revealed that while the benign glands showed significantly higher staining intensities than the cancerous tissue, high KLK12 protein expression was associated with poor metastasis-free and PCa-specific survival. Similar observation of differential KLK12 staining of the benign and malignant prostatic tissue has been previously reported.35 We found only moderate correlation between the IHC staining intensities and the mRNA expression levels of the samples from the same patients. This may be explained by the different quantitation methods: in IHC the KLK12 protein staining represents the areas of the strongest staining. However, mRNA analysis corresponds to the whole tissue samples used for RNA extraction that may contain different ratios of stromal and epithelial cells. These cell types might have differential expression across the analyzed genes, including housekeeping genes. To note, TMA cores, considered representative of the punches used for RNA extraction, had almost identical content of total epithelial and cancerous areas in the case and control groups.
Association of low expression of KLK2, -3 and -15 mRNAs, and strong KLK12 immunostaining with adverse postoperative metastasis-free survival, may reflect functions of these KLKs. However, it is speculative to draw conclusions of their potential roles solely based on the changes in expression levels as those may not correlate with the activity of KLKs, which are expressed as zymogens. Furthermore, the activity of proteases is regulated by several other mechanisms, like endogenous inhibitors,52 which were not addressed in the present study. Such inhibitors are present also in prostate52, 53 and, for example, in ovarian cancer ascites fluid only a small portion of the immunodetectable KLK proteins have enzymatic activity, which is at least partially explained by the presence of proteinase inhibitors.54 Thus, based only on expression levels, it is challenging to know the concentration of active proteases in a tumor microenvironment. Still, the low expression of KLK3 associated with adverse prognosis agrees with the proposed antiangiogenic activity of KLK3.55, 56 However, several studies have suggested that KLK3 may also promote PCa growth.21, 23 KLK2 has also been proposed to promote tumor growth, for example, by activating PARs.20, 27 It is also possible that KLKs have different roles in different stages of cancer development and metastatic dissemination, as we have previously suggested for KLK3.19 This agrees with our findings showing that while KLK4 and KLK15 were expressed at higher levels in cancerous than in benign tissue, their expression was reduced in aggressive cancers.
Since the largest differences in mRNA levels between benign and cancerous prostatic tissues were observed in KLK12 and KLK15 levels, and their functions in PCa have not been well established, we investigated the effects of KLK12 and KLK15 down-regulation by RNAi. To this end, we used LNCaP cells, known to express both KLK12 and -1547, 50 and, as models for tumor growth, colony formation and proliferation. We observed that KLK15, but not KLK12, down-regulation consistently and significantly reduced colony formation of LNCaP cells grown on Matrigel basement membrane preparation. Some of the KLK12 and KLK15 siRNAs also reduced the proliferation of the cells grown on plastic surface. There was rather poor correlation between the knock-down efficiency and colony formation, the reason for which we can only speculate. Apart from experimental variation, one possibility is that there is some kind of feedback regulation, via alternative protease or other mechanism that regulates the colony formation or cell proliferation, and prevents direct comparison of mRNA levels and functional consequences. The observed effects were modest and, especially regarding proliferation, high variation was observed between siRNAs. However, our results are in line with the observed increased mRNA expression of these KLKs in cancer compared to benign tissue, and with previous reports showing that siRNA-mediated KLK12 down-regulation reduces proliferation of MKN-45 human gastric cancer cells,57 and inhibits cell viability and promotes apoptosis of HT-29 colorectal cancer cells.58 KLK12 has also been proposed to promote angiogenesis.20
It has been reported that KLK15 may have a role in cancer development by cleaving extracellular matrix proteins.59 This is highly relevant since our expression studies suggest a role for KLK15 in postoperative relapse, albeit rather in suppressing formation of metastases than promoting it. To study whether KLK12 and KLK15 are functionally associated with cell invasion, a prerequisite for metastatic dissemination, we used, instead of poorly invasive LNCaP cells, an invasion model with invasive PC-3 cells overexpressing KLK12 and KLK15. Although in patients the expression of KLK12 and KLK15 was associated with metastasis-free survival, no significant differences in chemotactic cell invasion or migration were found upon overexpression of these KLKs. To note, the activity of these overexpressed KLKs remains to be shown. This was complicated by the relatively low expression and presence of other proteases, like trypsins, showing high activity toward small peptide substrates for KLK12 and KLK15 (results not shown). However, the same furin-activable propeptide we used here has previously been successfully used for activation of KLK3 in PC-3 cells,36 suggesting that KLK12 and KLK15 should also be activated. Further, it is challenging to predict the clinical outcomes based on artificial and simplistic cellular models, lacking full tumor microenvironment, like stromal cells, and showing variable expression of protease inhibitors and substrates.52 Additionally, individual cell lines, or prostate cancer cell lines in general, are not able to recapitulate intratumoral or intertumoral heterogeneity of multifocal prostate cancer.60 While PC-3 cells are useful for invasion studies, those do not express AR or most of the KLKs.10 KLKs are known to act in cascades20, 24 and it is possible that the PC-3 cells lack KLK and other substrates needed to mediate effects of KLK12 and KLK15. Perhaps apart from our finding showing that KLK15 reduce formation of cell colonies on Matrigel, suggesting a role in tumor growth, the prostate cancer relevant functions of KLK12 and KL15 remains rather speculative.
In conclusion, we analyzed mRNA expression of all 15 KLKs and four PARs in PCa, in intermediate and high-risk cancers which did or did not progress during postoperative follow-up. The greatest difference in expression levels were found in KLK12 and -15, the former being up-regulated, and latter down-regulated in aggressive cancers. Our results strongly suggested, that adding KLK and PAR expression data to traditional clinical parameters improves discrimination of those GG2-4 PCa patients, who will develop metastatic disease. Strong KLK12 immunostaining and low mRNA expression of KLK15 were associated with poor survival. The knock-down of KLK15 was found to reduce colony formation of LNCaP cells. Our findings further support the involvement of several KLKs in PCa progression and highlight that, in addition to classical prostatic KLKs, other KLKs may serve as prognostic PCa biomarkers and should be addressed more thoroughly in functional and clinical biomarker studies.
AUTHOR CONTRIBUTIONS
The work reported in the paper has been performed by the authors, unless clearly specified in the text. Timo-Pekka K. Lehto: Data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review & editing. Ruusu-Maaria Kovanen: Formal analysis, investigation, visualization, writing—original draft, writing—review & editing. Susanna Lintula: Investigation, writing—review & editing. Adrian Malén: Data curation, writing—review & editing. Carolin Stürenberg: Writing—review & editing. Andrew Erickson: Data curation, writing—review & editing. Olli-Pekka Pulkka: Investigation, writing—review & editing. Ulf-Håkan Stenman: Writing—review & editing. Eleftherios P. Diamandis: Resources, Writing—review & editing. Antti Rannikko: Funding acquisition, supervision, writing—review & editing. Tuomas Mirtti: Conceptualization, funding acquisition, project administration, resources, supervision, writing—review & editing. Hannu Koistinen: Conceptualization, funding acquisition, methodology, project administration, resources, supervision, validation, writing—original draft, writing—review & editing.
ACKNOWLEDGEMENTS
We would like to thank Sanna Iikkanen, Jenni Niinimäki, Susanna Lauttia and Annikki Löfhjelm for excellent technical assistance. We would also like to thank Lars Paulin for the help in Nanostring nCounter analysis and FIMM Digital Microscopy and Molecular Pathology Unit supported by HiLIFE and Biocenter Finland for tissue microarray slide scanning services. We thank the Institute for Molecular Medicine Finland FIMM Technology Centre for authentication of the cell lines. We would like to thank Antoninus Soosaipillai for performing KLK12 and KLK15 ELISA experiments.
FUNDING INFORMATION
This work was supported by grants from Sigrid Jusélius Foundation (Hannu Koistinen), Magnus Ehrnrooth Foundation (Hannu Koistinen), Finnish Society of Clinical Chemistry (Hannu Koistinen), Laboratoriolääketieteen edistämissäätiö (Hannu Koistinen), Cancer Society Finland (Tuomas Mirtti and Antti Rannikko), Academy of Finland (Tuomas Mirtti), Jane and Aatos Erkko Foundation (Antti Rannikko) and State funding for university level health research (Tuomas Mirtti and Antti Rannikko). The funding sources did not have any role in study design, execution, data analyses, writing of the report or submission of the article for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
The study was performed following the principles of good clinical practice and in accordance with the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008). The study protocol was approved by Helsinki University Hospital Ethical Committee (HUS/1439/2018) and the National Supervisory Agency for Health and Welfare (Dnro V/38176/2018). No express consent was required from the studied patients as per national legislation, since the studied patient data were registry data.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are available from the corresponding author upon reasonable request.