Comprehensive variant spectrum of the CNGA3 gene in patients affected by achromatopsia
Abstract
Achromatopsia (ACHM) is a congenital cone photoreceptor disorder characterized by impaired color discrimination, low visual acuity, photosensitivity, and nystagmus. To date, six genes have been associated with ACHM (CNGA3, CNGB3, GNAT2, PDE6C, PDE6H, and ATF6), the majority of these being implicated in the cone phototransduction cascade. CNGA3 encodes the CNGA3 subunit of the cyclic nucleotide-gated ion channel in cone photoreceptors and is one of the major disease-associated genes for ACHM. Herein, we provide a comprehensive overview of the CNGA3 variant spectrum in a cohort of 1060 genetically confirmed ACHM patients, 385 (36.3%) of these carrying “likely disease-causing” variants in CNGA3. Compiling our own genetic data with those reported in the literature and in public databases, we further extend the CNGA3 variant spectrum to a total of 316 variants, 244 of which we interpreted as “likely disease-causing” according to ACMG/AMP criteria. We report 48 novel “likely disease-causing” variants, 24 of which are missense substitutions underlining the predominant role of this mutation class in the CNGA3 variant spectrum. In addition, we provide extensive in silico analyses and summarize reported functional data of previously analyzed missense, nonsense and splicing variants to further advance the pathogenicity assessment of the identified variants.
1 BACKGROUND
Achromatopsia (ACHM; rod monochromacy) is a rare autosomal recessively inherited retinal disorder characterized by impaired cone photoreceptor function. The disease is estimated to affect one in 30,000–50,000 live births worldwide (François, 1961). Signs and symptoms of the disorder manifest in early infancy and include marked photosensitivity, nystagmus, severely reduced visual acuity (<0.1), and the inability to discriminate colors (Hirji et al., 2018; Michalakis et al., 2017; Sun & Zhang, 2019).
ACHM is a phenotypically and genetically heterogeneous inherited retinal disorder. To date, pathogenic variants in six genes have been identified to cause ACHM. Five of these genes, namely CNGA3 (MIM# 600053) (Kohl et al., 1998), CNGB3 (MIM# 605080) (Kohl et al., 2000; Sundin et al., 2000), GNAT2 (MIM# 139340) (Aligianis et al., 2002; Kohl et al., 2002), PDE6C (MIM# 600827) (Chang et al., 2009; Thiadens et al., 2009), and PDE6H (MIM# 601190) (Kohl et al., 2012), encode key components of the cone phototransduction cascade. Only ATF6 (MIM# 605537) is not a part of this pathway, but codes for a transcription factor involved in the unfolded protein response (Ansar et al., 2015; Kohl et al., 2015; Xu et al., 2015).
Molecular genetic analyses in ACHM patients revealed that a large proportion of these cases carry causative variants in the CNGA3 gene though its prevalence varies among different ethnic populations. While biallelic CNGA3 mutations are the most common cause of ACHM among patients of Chinese (80%) or Israeli/Palestinian (84%) origin (Liang et al., 2015; Sun et al., 2020; Zelinger et al., 2015), its prevalence ranges between 5% and 44% in European and US ACHM patients of European origin, thus representing the second most commonly afflicted gene in the latter countries after CNGB3 (Johnson et al., 2004; Thiadens et al., 2009; Varsányi et al., 2005; Wissinger et al., 2001).
CNGA3 encodes for the A3 subunit of the cyclic nucleotide-gated (CNG) channel in cone photoreceptors outer segments, a key component of the phototransduction cascade (Kaupp & Seifert, 2002). Cone CNG channels are ligand-activated, nonselective cation channels forming heterotetrameric complexes via assembly of three CNGA3 and one CNGB3 subunit (Barret et al., 2022; Biel & Michalakis, 2009; Shuart et al., 2011; Zheng et al., 2022; Zhong et al., 2002). Both subunits are structurally homologous proteins comprising six transmembrane helices (TM1–TM6) which are connected via intracellular and extracellular loops (also compare Figure 3b). The pore region including the pore helix is located between TM5 and TM6. The cyclic nucleotide-binding domain (CNBD) consists of three α-helices (αA–αC) and eight β-sheets (β1–β8), and is connected via the C-linker—formed by six α-helices (αA′–αF′)–to TM6 (Gofman et al., 2014). Furthermore, the CNGA3 subunit comprises a C-terminal leucine zipper (CLZ) domain important for subunit assembly (Dai et al., 2013; Zagotta & Siegelbaum, 1996).
Nonsense and frameshift variants likely result in the loss of the CNGA3 subunit, while some missense variants in the CNGA3 subunit have been shown to impair or even abolish CNG channel function and/or protein folding and trafficking leading to impaired phototransduction and impaired or lost cone function (Burkard et al., 2018; Dai & Varnum, 2013; Duricka et al., 2012; Koeppen et al., 2008, 2010; Kuniyoshi et al., 2016; Liu & Varnum, 2005; Matveev et al., 2010; Meighan et al., 2015; Michalakis et al., 2017; Muraki-Oda et al., 2007; Patel et al., 2005; Reuter et al., 2008; Shaikh et al., 2015; Täger et al., 2018; Tränkner et al., 2004).
ACHM caused by pathogenic variants in CNGA3 is of strong current interest as a first gene supplementation therapy trial has been completed for CNGA3-ACHM (CNGA3: NCT02610582; Fischer et al., 2020; Reichel et al., 2021), and a number of such therapy trials are ongoing at several sites all over the world for both CNGA3- and CNGB3-ACHM (NCT03758404, NCT02935517, and NCT02599922). Although the primary endpoint of such a trial is safety—which was confirmed, the functional benefits were limited but persistent for the adult treated patients for a period of 3 years (Fischer et al., 2020; Reichel et al., 2021). These included slight improvement in color contrast sensitivity, improvements in the ability to fixate, to recognize letters and numbers as well as in photoaversion. It has been discussed whether treatment of younger patients may result in greater functional benefit by avoiding amblyopia as a potential limiting factor (Reichel et al., 2021). The emergence of therapeutic studies highlights the necessity to classify the pathogenicity of disease-associated variants as unambiguously as possible to enable the right selection of patients for these trials.
In the present study, we aim to provide a complete and comprehensive spectrum overview of variants in the CNGA3 gene. The CNGA3 variant database combines the variants of our Tübingen in-house investigated ACHM cohort, variants from literature, and mutation databases and is complemented by extensive in silico analysis of the identified variants for assessing their pathogenic effect. In addition, we provide an overview of clinical and diagnostic aspects of ACHM and the animal models of CNGA3-associated ACHM.
2 MATERIAL AND METHODS
2.1 Patient cohort, clinical examination, and ethical considerations
Patients with a clinical diagnosis of ACHM were recruited over a period of 25 years (1996–2020) in various ophthalmic centers predominantly located in Europe (Austria, Belgium, Czech Republic, Denmark, France, Germany, Greece, Hungary, Italy, the Netherlands, Spain, Sweden, and United Kingdom), but also in Brazil, Israel, Palestine, Turkey, and in the USA. In total, our entire Tübingen ACHM cohort of genetically confirmed ACHM patients includes 1060 affected individuals diagnosed with ACHM originating from 889 independent families.
Clinical diagnosis was established by standard clinical ophthalmologic examination and depended on the local clinical protocol (i.e., color vision testing, best-corrected visual acuity, electroretinography [ERG], fundus photography, and optical coherence tomography).
The study was conducted in accordance with the principles of the Declaration of Helsinki. Blood, saliva, or DNA samples of the ACHM patients and family members were collected over a period of 25 years after informed consent approved by the respective local research and ethical boards and/or dependent on the local regulatory bodies and regulations. Specifically, the study was approved by the Ethics Board of the University of Tübingen under the study no. 349/2003V and 116/2015BO2.
2.2 Variant screening
Peripheral blood, saliva or DNA samples from patients diagnosed with ACHM and family members were sent to the Institute for Ophthalmic Research, Tübingen (Germany) for genetic testing. Extraction of whole genomic DNA was performed according to standard procedures. Molecular genetic screening of the CNGA3 gene was executed as previously described (Kohl et al., 1998; Wissinger et al., 2001). Primers used for PCR amplification and Sanger sequencing are provided in Table S1. If two potential disease-causing CNGA3 alleles were identified in cases that were analyzed within a research setting, no further genetic analysis in other ACHM genes was performed. In case DNA samples from additional family members were available, segregation analysis was performed to validate the presence and the independent segregation of two mutant CNGA3 alleles using either restriction fragment length polymorphism analysis or Sanger sequencing.
2.3 Variant nomenclature and establishment of the CNGA3 variant database
Variant nomenclature of the identified CNGA3 sequence alterations was based on the NCBI reference sequence NM_001298.2 and the Ensembl transcript reference sequence ENST00000272602.7 (GRCh38/hg38 genome assembly) comprising seven coding exons. The first nucleotide of the translation initiation codon ATG is denoted as nucleotide 1. Moreover, all variants located in the alternatively spliced exon 3b (formerly named exon 2b; Wissinger et al., 2001; now renamed exon 3b) and its flanking intronic sequences were denoted based on NCBI reference sequence XM_011510554.2 (GRCh38/hg38). Validation of the variant nomenclature was performed using the web-based Mutalyzer name checker tool (https://mutalyzer.nl/name-checker).
To establish a comprehensive CNGA3 variant database, a literature search was conducted on the free search engine PubMed® (https://pubmed.ncbi.nlm.nih.gov/) (last queried on January 11, 2021). In addition, web-based databases including the Human Gene Mutation Database (HGMD®) (http://www.hgmd.cf.ac.uk/ac/index.php) (Stenson et al., 2017), the Leiden Open Variation Database (LOVD) for CNGA3 (https://www.lovd.nl/CNGA3), and the public archive of human genetic variants ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) (Landrum et al., 2014) were searched for entries regarding CNGA3 disease-associated variants (searches conducted on January 11, 2021 and March 18, 2021). Variants retrieved from public databases (i.e., LOVD and ClinVar) that have not been associated with a retinal phenotype (i.e., ACHM or allied disease) were not included and not validated. Finally, all CNGA3 variants identified in our ACHM cohort were entered into our in-house CNGA3 variant database. Variants identified in the ACHM cohort genetically characterized in Tübingen that were not yet published in a journal listed on PubMed® are referred to as “novel.” All novel variants were submitted to the ClinVar database with accession numbers from SCV001571262 to SCV001571322. Additionally, these variants will be submitted to the Global Variome shared LOVD database and can be accessed using the following URL: databases.lovd.nl/shared.
2.4 In silico variant assessment
Sequence alterations listed in the CNGA3 variant database were subjected to in silico analysis to evaluate their pathogenic impact. The Combined Annotation Dependent Depletion (CADD) tool was used to score the deleteriousness of both coding and noncoding single-nucleotide variants in the CNGA3 gene (https://cadd.gs.washington.edu/). CADD provides PHRED-scaled scores ranging from 1 to 99. A scaled CADD score of 10 thereby indicates a variant being amongst the top 10% of deleterious variants in the human genome whereas a score of 20 is used to describe variants that are amongst the top 1% of deleterious variants and so on (Kircher et al., 2014). To score the identified variants, we used the CADD model GRCh38-v1.6 and applied a PHRED-scaled score of 20 as a cutoff for deleteriousness as suggested by the authors (Rentzsch et al., 2021). Three in silico prediction algorithms for the evaluation of missense variants were used as embedded in Alamut Genova v.1.4/Alamut Visual v.2.14.0: SIFT (Sorting Intolerant From Tolerant, v4.0.3/v6.2.0) applies a score range of 0.0 for a deleterious effect to 1.0 for the substitution being tolerated (http://sift.bii.a-star.edu.sg/) (Kumar et al., 2009), whereas MutationTaster (v2013) classifies the variants either as “disease-causing” or as a “polymorphism” with the p value indicating the certainty of the prediction (http://www.mutationtaster.org/) (Schwarz et al., 2010). The third prediction algorithm, Align-GVGD (v2007), provides prediction classes defining a spectrum of classifications with C0 suggesting least likely to interfere with protein function to C65 being most likely to affect functionality of the protein (https://agvgd.iarc.fr/index.php) (Tavtigian et al., 2006). In addition, phyloP scores and the Grantham distance were computed as embedded in Alamut Genova v.1.4/Alamut Visual v.2.14.0 to further elucidate the possible pathogenic effect of missense variants. PhyloP determines the evolutionary conservation and acceleration of a given nucleotide. Positive scores are assigned to sites predicted to be conserved whereas negative scores indicate a fast-evolving site (Pollard et al., 2010). The Grantham distance scores missense substitutions regarding the physicochemical difference between the exchanged amino acids with scores ranging from 0 to 215 (Grantham, 1974).
To further assess the conservation of amino acid residues of missense substitutions, we, in addition, performed a protein sequence alignment of Homo sapiens CNGA3 against its orthologues from seven other species, the Homo sapiens CNGA1 protein and its orthologues from additional five species using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) (Sievers et al., 2011) and the alignment visualization tool BoxShade version 3.21 (https://embnet.vital-it.ch/software/BOX_form.html) (Figure S2).
Minor allele frequencies were retrieved from the Genome Aggregation Database (gnomAD) (v2.0.2/v2.1) (https://gnomad.broadinstitute.org/).
In silico evaluation of variants affecting either canonical or noncanonical splice sites was conducted using the splicing algorithms SpliceSiteFinder-like [0-100] (Shapiro & Senapathy, 1987), MaxEntScan [donor sites: 0-12 & acceptor sites: 0-16] (Yeo & Burge, 2004), NNSplice [0-1] (Reese et al., 1997), GeneSplicer [0-15] (Pertea et al., 2001) as embedded in Alamut Genova v.1.4/Alamut Visual v.2.14.0, and SpliceAI [0-1] (Jaganathan et al., 2019) (https://spliceailookup.broadinstitute.org/). Furthermore, variants affecting noncanonical exonic splice site sequences (cutoff: first/last five nucleotides of an exon) were also evaluated using the above-mentioned splicing algorithms. All in silico tools were used following the guidelines for the use of prediction tools (Vihinen, 2013).
2.5 Variant classification
The pathogenic impact of the CNGA3 variants listed in this study was automatically and/or semi-automatically classified according to the standards and guidelines provided by the American College of Medical Genetics and Genomics (ACMG) and the Association for Molecular Pathology (AMP) (Richards et al., 2015) using the web-based variant interpretation tool Franklin (Genoox Ltd, https://franklin.genoox.com/). The criterion PP1 was thereby used for sequence alterations showing co-segregation of the variant(s) with disease in multiple affected family members, either performed in-house or documented in previously published studies. CNGA3 variants which were functionally analyzed in previously published studies and proven to have a damaging effect on protein function were assigned the criterion PS3. The criterion PP3 was used for missense variants that were predicted to be deleterious by CADD and MutationTaster, and for canonical/noncanonical splice site variants unanimously predicted to affect splicing using the above-mentioned in silico tools. If the applied in silico tools did not suggest a pathogenic effect, the criterion BP4 was assigned. For CNGA3 variants having a minor allele frequency of <0.1% on gnomAD, the criterion PM2 was applied. In contrast, variants with a minor allele frequency of >0.1% were assigned the criterion BS1.
3 OVERVIEW OF CNGA3 VARIANTS UNDERLYING ACHM
3.1 Spectrum of CNGA3 gene variants in our ACHM study cohort genetically characterized in Tübingen
Our total ACHM cohort genetically characterized in Tübingen comprises 1060 affected individuals from 889 independent families carrying “likely disease-causing” variants in the six genes associated with ACHM. Among these, 385 patients from 304 distinct families carry likely biallelic ACHM-related variants in the CNGA3 gene, hereby revealing that CNGA3 is the second most commonly mutated gene in our study cohort (36.3% patients/34.2% families, Figure S1). Of note, 216 of these families have not been published previously.
In total, we detected 221 distinct genotypes of CNGA3 sequence alterations in our patient cohort (Table S2). Segregation analysis was performed in both parents for 181 affected individuals and in a single parent and/or in other family members (i.e., siblings, cousins, or offspring) for 88 ACHM patients confirming the presence and independent inheritance of two mutant biallelic CNGA3 alleles. In 116 cases no relatives were available for segregation analysis which corresponds to 116 families out of 304 families in the cohort missing segregation analysis (38.2%). One hundred and forty-seven affected individuals carry possibly disease-causing CNGA3 variants in an (apparent) homozygous state whereas 238 solved cases harbor two (likely compound) heterozygous variants expected to explain the ACHM phenotype. Homozygosity for the c.1641C>A/p.(Phe547Leu) or the c.847C>T/p.(Arg283Trp) CNGA3 variant were by far the most common genotypes in our cohort (n = 23 and n = 22 affected individuals, respectively; Table S2).
Moreover, we observed one family in our study cohort with pseudo-dominant inheritance involving two mutant CNGA3 alleles. In this family, the affected mother was homozygous for the c.847C>T/p.(Arg283Trp) variant and the clinically unaffected spouse was a heterozygous carrier of the c.488C>T/p.(Pro163Leu) variant. Both affected children in this family were compound heterozygous for the two CNGA3 variants inherited from the parents (Table S2).
One patient in our study cohort was shown to harbor two heterozygous missense variants (c.353A>G/p.(Gln118Arg) and c.1615G>A/p.(Val539Met); Table S2), of which the c.353A>G variant was classified as likely benign and the c.1615G>A alteration as a variant of uncertain significance (VUS) according to ACMG/AMP guidelines. Notably, this male patient was in the meantime shown to carry an alteration at the OPN1LW/OPN1MW gene cluster compatible with a genetic diagnosis of blue cone monochromacy, an X-linked incomplete form of ACHM. In one patient, only a single heterozygous CNGA3 variant was identified. Of note, in this patient screening of all other ACHM-associated genes did not identify any other likely causative variants. Both above-mentioned cases were excluded from the group of genetically solved ACHM cases.
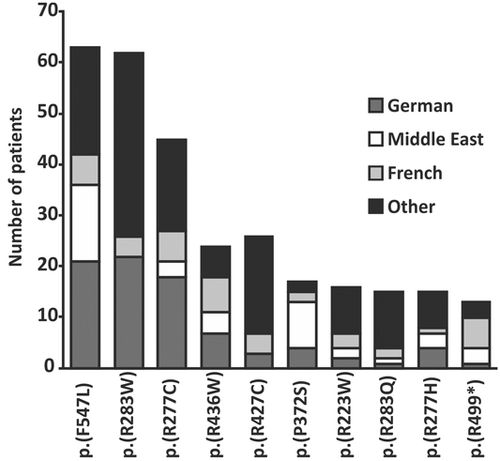
In the ACHM cohort genetically characterized in Tübingen, we identified 140 different potential disease-associated variants. The ten most common CNGA3-ACHM-related alleles detected in our patient cohort are presented in Table 1. All but one of these are missense sequence alterations. The two missense substitutions c.1641C>A/p.(Phe547Leu) and c.847C>T/p.(Arg283Trp) are the by far most commonly observed CNGA3-associated alleles for ACHM in our cohort, both with a similar allele frequency (n = 86 chromosomes for c.1641C>A/p.(Phe547Leu) and n = 84 chromosomes for c.847C>T/p.(Arg283Trp)). Both alleles are frequently seen in ACHM patients originating from Germany (c.1641C>A/p.(Phe547Leu): n = 21 affected individuals; c.847C>T/p.(Arg283Trp): n = 22) and in the case of the c.1641C>A/p.(Phe547Leu) variant also in the Middle East (n = 15). Further common CNGA3 alleles associated with ACHM include the missense substitution c.829C>T/p.(Arg277Cys) (n = 48 chromosomes) which is mainly seen in German (n = 18) and US ACHM patients (n = 6). Similarly, we observed some descent-specific enrichment for c.1279C>T/p.(Arg427Cys) (total n = 26 chromosomes) in US patients (n = 9), as well as for the c.848G>A/p.(Arg283Gln) variant (total n = 20 chromosomes) observed in 5 out of 15 individuals who originated from Great Britain. Furthermore, the c.1495C>T/p.(Arg499Ter) variant (total n = 19 chromosomes) is the only nonsense variant detected in the spectrum of commonly observed CNGA3-ACHM-related alleles and was predominantly identified in French patients (n = 6) (Table 1 and Figure 1).
| Alteration nucleotide sequencea | Alteration polypeptide | Total number of chromosomes |
|---|---|---|
| c.1641C>A | p.(Phe547Leu) | 86 |
| c.847C>T | p.(Arg283Trp) | 84 |
| c.829C>T | p.(Arg277Cys) | 48 |
| c.1306C>T | p.(Arg436Trp) | 32 |
| c.1279C>T | p.(Arg427Cys) | 26 |
| c.1114C>T | p.(Pro372Ser) | 25 |
| c.667C>T | p.(Arg223Trp) | 20 |
| c.848G>A | p.(Arg283Gln) | 20 |
| c.830G>A | p.(Arg277His) | 19 |
| c.1495C>T | p.(Arg499Ter) | 19 |
- Abbreviation: ACHM, achromatopsia.
- a Variant designation is based on NM_001298.2 (GRCh38/hg38).
3.2 Comprehensive CNGA3 variant database
To create a comprehensive CNGA3 variant database, we combined all CNGA3 variants identified in our ACHM cohort with all CNGA3 sequence alterations obtained after an extensive literature search on PubMed®, and database search in HGMD®, LOVD and ClinVar. The so established CNGA3 variant database currently comprises 316 variants (Tables 2 and S3). Of these, four variants are located in the alternate exon 3b and its flanking intronic sequences. As the functional relevance of this sequence remains unclear and no association with disease has ever been reported, these variants were not further analyzed and were classified as “likely benign.”
| Location CNGA3 | Alteration nucleotide sequencea | Alteration polypeptide | Location protein domainb | References | ACMG/AMP classification |
|---|---|---|---|---|---|
| Likely disease-causing variants | |||||
| Intron 1 | c.-37-1G>C | p.(?) | - | Burkard et al. (2018) | Pathogenic |
| Exon 2 | c.1A>T | p.(?) | N-Term. | This study | VUS |
| Exon 2 | c.40del | p.(Thr14ProfsTer5) | N-Term. | Liang et al. (2015) | Pathogenic |
| Exon 2 | c.62C>G | p.(Ser21Ter) | N-Term. | Li et al. (2014) | Pathogenic |
| Exon 2 | c.67C>T | p.(Arg23Ter) | N-Term. | Johnson et al. (2004) | Pathogenic |
| Intron 2 | c.101+1G>A | p.(?) | - | Abdelkader et al. (2018) | Pathogenic |
| Exon 3 | c.107_110del | p.(His36ArgfsTer136) | N-Term. | Thomas et al. (2012) | Pathogenic |
| Exon 3 | c.128C>A | p.(Ser43Ter) | N-Term. | Sun et al. (2020) | Likely pathogenic |
| Exon 3 | c.130_151dup | p.(Ala51ValfsTer16) | N-Term. | Zelinger et al. (2015) | Pathogenic |
| Exon 3 | c.139C>T | p.(Gln47Ter) | N-Term. | Zobor et al. (2017) | Likely pathogenic |
| Exon 3 | c.147dup | p.(Ile50AspfsTer10) | N-Term. | Wissinger et al. (2001) | Likely pathogenic |
| Exon 3 | c.162_163insT | p.(Arg55Ter) | N-Term. | Ezquerra-Inchausti et al. (2018) | Pathogenic |
| Exon 4 | c.248G>A | p.(Trp83Ter) | N-Term. | Ellingford et al. (2016) | Pathogenic |
| Exon 4 | c.332_333delinsAA | p.(Ser111Ter) | N-Term. | This study | Pathogenic |
| Exon 4 | c.340G>T | p.(Glu114Ter) | N-Term. | This study | Pathogenic |
| Exon 4 | c.384_387del | p.(Asp128GlufsTer44) | N-Term. | This study | Likely pathogenic |
| Exon 4 | c.387del | p.(Arg131GlufsTer42) | N-Term. | This study | Likely pathogenic |
| Intron 4 | c.395+1G>T | p.(?) | - | Abdelkader et al. (2018) | Likely pathogenic |
| Intron 4 | c.396-2_398dup | p.(?) | - | This study | Likely pathogenic |
| Intron 4 | c.396-11C>G | p.(?) | - | Li et al. (2014) | VUS |
| Intron 5 | c.450-1G>A | p.(?) | - | Georgiou et al. (2019) | Likely pathogenic |
| Intron 5 | c.450-15T>G | p.(?) | - | This study | VUS |
| Exon 6 | c.464del | p.(Lys155ArgfsTer18) | N-Term. | This study | Pathogenic |
| Exon 6 | c.478G>A | p.(Val160Met) | N-Term. | ClinVar | VUS |
| Exon 6 | c.479T>G | p.(Val160Gly) | N-Term. | This study | VUS |
| Exon 6 | c.485A>T | p.(Asp162Val) | N-Term. | Wissinger et al. (2001) | Likely pathogenic |
| Exon 6 | c.488C>T | p.(Pro163Leu) | N-Term. | Kohl et al. (1998) | Pathogenic |
| Exon 6 | c.489_492del | p.(Ser164AlafsTer8) | N-Term. | LOVD | Pathogenic |
| Exon 6 | c.499del | p.(Leu167CysfsTer6) | N-Term. | Weisschuh et al. (2020) | Pathogenic |
| Exon 6 | c.512G>A | p.(Trp171Ter) | TM1 | Li et al. (2014) | Pathogenic |
| Exon 6 | c.513G>T | p.(Trp171Cys) | TM1 | Li et al. (2014) | VUS |
| Exon 6 | c.513G>A | p.(Trp171Ter) | TM1 | This study | Pathogenic |
| Exon 6 | c.536T>A | p.(Val179Asp) | TM1 | Dubis et al. (2014) | VUS |
| Exon 6 | c.542A>G | p.(Tyr181Cys) | TM1 | Wissinger et al. (2001) | Pathogenic |
| Exon 6 | c.544A>G | p.(Asn182Asp) | TM1 | This study | Likely pathogenic |
| Exon 6 | c.544A>T | p.(Asn182Tyr) | TM1 | Wissinger et al. (2001) | Likely pathogenic |
| Exon 6 | c.556C>T | p.(Leu186Phe) | TM1 | Wissinger et al. (2001) | Likely pathogenic |
| Exon 6 | c.560T>C | p.(Ile187Thr) | TM1 - TM2 | Taylor et al. (2017) | Likely pathogenic |
| Exon 6 | c.566G>A | p.(Arg189Lys) | TM1 - TM2 | This study | VUS |
| Exon 7 | c.572G>A | p.(Cys191Tyr) | TM1 - TM2 | Wissinger et al. (2001) | Likely pathogenic |
| Exon 7 | c.580G>A | p.(Glu194Lys) | TM1 - TM2 | Wissinger et al. (2001) | Likely pathogenic |
| Exon 7 | c.584T>C | p.(Leu195Pro) | TM1 - TM2 | ClinVar | VUS |
| Exon 7 | c.586C>T | p.(Gln196Ter) | TM1 - TM2 | Johnson et al. (2004) | Likely pathogenic |
| Exon 7 | c.589T>C | p.(Ser197Pro) | TM2 | ClinVar | Likely pathogenic |
| Exon 7 | c.591del | p.(Glu198SerfsTer3) | TM2 | This study | Likely pathogenic |
| Exon 7 | c.608G>A | p.(Trp203Ter) | TM2 | Georgiou et al. (2019) | Likely pathogenic |
| Exon 7 | c.609G>A | p.(Trp203Ter) | TM2 | Sun et al. (2020) | Likely pathogenic |
| Exon 7 | c.609G>T | p.(Trp203Cys) | TM2 | This study | VUS |
| Exon 7 | c.624C>G | p.(Tyr208Ter) | TM2 | Hull et al. (2020) | Likely pathogenic |
| Exon 7 | c.633T>A | p.(Asp211Glu) | TM2 | Liang et al. (2015) | VUS |
| Exon 7 | c.649G>C | p.(Asp217His) | TM2 | ClinVar | VUS |
| Exon 7 | c.661C>T | p.(Arg221Ter) | TM2 | Johnson et al. (2004) | Pathogenic |
| Exon 7 | c.667C>T | p.(Arg223Trp) | TM2 | Wissinger et al. (2001) | Pathogenic |
| Exon 7 | c.667C>G | p.(Arg223Gly) | TM2 | Wiszniewski et al. (2007) | Likely pathogenic |
| Exon 7 | c.668G>A | p.(Arg223Gln) | TM2 | Li et al. (2014); Greenberg et al. (2014) | Pathogenic |
| Exon 7 | c.671C>G | p.(Thr224Arg) | TM2 - TM3 | Wissinger et al. (2001) | Likely pathogenic |
| Intron 7 | c.674-2A>C | p.(?) | - | Li et al. (2014) | Likely pathogenic |
| Exon 8 | c.674-1935_*1838del (Deletion Exon 8) | p.(?) | - | This study | Pathogenic |
| Exon 8 | c.682G>A | p.(Glu228Lys) | TM2 - TM3 | Reuter et al. (2008) | Pathogenic |
| Exon 8 | c.704A>T | p.(Asp235Val) | TM2 - TM3 | Weisschuh et al. (2020) | VUS |
| Exon 8 | c.742C>T | p.(Gln248Ter) | TM3 | This study | Likely pathogenic |
| Exon 8 | c.746T>C | p.(Phe249Ser) | TM3 | Nishiguchi et al. (2005) | VUS |
| Exon 8 | c.754G>A | p.(Asp252Asn) | TM3 | Koeppen et al. (2008) | Likely pathogenic |
| Exon 8 | c.755A>C | p.(Asp252Ala) | TM3 | Sun et al. (2020) | VUS |
| Exon 8 | c.772C>G | p.(Pro258Ala) | TM3 | This study | VUS |
| Exon 8 | c.773C>G | p.(Pro258Arg) | TM3 | Li et al. (2014) | VUS |
| Exon 8 | c.778G>A | p.(Asp260Asn) | TM3 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.778G>C | p.(Asp260His) | TM3 | This study | Likely pathogenic |
| Exon 8 | c.778dup | p.(Asp260GlyfsTer27) | TM3 | LOVD | Pathogenic |
| Exon 8 | c.787T>G | p.(Tyr263Asp) | TM3 | Nishiguchi et al. (2005) | Likely pathogenic |
| Exon 8 | c.800G>A | p.(Gly267Asp) | TM3 - TM4 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.811C>G | p.(Pro271Ala) | TM3 - TM4 | Zelinger et al. (2015) | Likely pathogenic |
| Exon 8 | c.811C>A | p.(Pro271Thr) | TM3 - TM4 | This study | Likely pathogenic |
| Exon 8 | c.811C>T | p.(Pro271Ser) | TM3 - TM4 | Georgiou et al. (2019) | Likely pathogenic |
| Exon 8 | c.822G>T | p.(Arg274Ser) | TM4 | Azam et al. (2010) | Likely pathogenic |
| Exon 8 | c.821G>A | p.(Arg274Lys) | TM4 | Li et al. (2014) | Likely pathogenic |
| Exon 8 | c.827A>G | p.(Asn276Ser) | TM4 | Saqib et al. (2011) | Likely pathogenic |
| Exon 8 | c.829C>T | p.(Arg277Cys) | TM4 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.829C>G | p.(Arg277Gly) | TM4 | Koeppen et al. (2010) | Likely pathogenic |
| Exon 8 | c.830G>A | p.(Arg277His) | TM4 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.833T>C | p.(Leu278Pro) | TM4 | Li et al. (2014) | Likely pathogenic |
| Exon 8 | c.838A>T | p.(Lys280Ter) | TM4 | Wang et al. (2019) | Likely pathogenic |
| Exon 8 | c.847C>T | p.(Arg283Trp) | TM4 | Kohl et al. (1998) | Pathogenic |
| Exon 8 | c.848G>A | p.(Arg283Gln) | TM4 | Kohl et al. (1998) | Pathogenic |
| Exon 8 | c.851del | p.(Leu284ProfsTer78) | TM4 | This study | Likely pathogenic |
| Exon 8 | c.869G>A | p.(Arg290His) | TM4 | Burkard et al. (2018) | Pathogenic |
| Exon 8 | c.872C>G | p.(Thr291Arg) | TM4 - TM5 | Kohl et al. (1998) | Pathogenic |
| Exon 8 | c.872_873del | p.(Thr291ArgfsTer77) | TM4 - TM5 | Li et al. (2014) | Likely pathogenic |
| Exon 8 | c.902_903delinsAA | p.(Phe301Ter) | TM5 | This study | Likely pathogenic |
| Exon 8 | c.904A>G | p.(Arg302Gly) | TM5 | Zelinger et al. (2015) | Likely pathogenic |
| Exon 8 | c.906G>T | p.(Arg302Ser) | TM5 | Weisschuh et al. (2020) | Likely pathogenic |
| Exon 8 | c.907A>T | p.(Ile303Phe) | TM5 | ClinVar | Likely pathogenic |
| Exon 8 | c.940_942del | p.(Ile314del) | TM5 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.945C>G | p.(His315Gln) | TM5 | Patel et al. (2019) | Likely pathogenic |
| Exon 8 | c.947G>A | p.(Trp316Ter) | TM5 | Wissinger et al. (2001) | Likely pathogenic |
| Exon 8 | c.952G>A | p.(Ala318Thr) | TM5 | Li et al. (2017) | Likely pathogenic |
| Exon 8 | c.955T>C | p.(Cys319Arg) | TM5 | Shaikh et al. (2015) | Pathogenic |
| Exon 8 | c.955_963delinsACCAATGAAATGGAAAT | p.(Cys319ThrfsTer46) | TM5 | This study | Likely pathogenic |
| Exon 8 | c.965T>C | p.(Phe322Ser) | TM5 | Li et al. (2014) | Likely pathogenic |
| Exon 8 | c.967G>C | p.(Ala323Pro) | TM5 | Carss et al. (2017) | Likely pathogenic |
| Exon 8 | c.968C>A | p.(Ala323Asp) | TM5 | Li et al. (2015) | Likely pathogenic |
| Exon 8 | c.983T>C | p.(Ile328Thr) | TM5 | This study | Likely pathogenic |
| Exon 8 | c.985G>T | p.(Gly329Cys) | TM5 - pore helix | Genead et al. (2011) | Pathogenic |
| Exon 8 | c.989T>C | p.(Phe330Ser) | TM5 - pore helix | Li et al. (2014) | Likely pathogenic |
| Exon 8 | c.991G>C | p.(Gly331Arg) | TM5 - pore helix | Saqib et al. (2015) | Likely pathogenic |
| Exon 8 | c.992G>A | p.(Gly331Glu) | TM5 - pore helix | This study | Likely pathogenic |
| Exon 8 | c.997_998del | p.(Asp333LeufsTer35) | TM5 - pore helix | Kuniyoshi et al. (2016) | Likely pathogenic |
| Exon 8 | c.1001C>T | p.(Ser334Phe) | TM5 - pore helix | Sundaram et al. (2014) | Likely pathogenic |
| Exon 8 | c.1004G>A | p.(Trp335Ter) | TM5 - pore helix | This study | Likely pathogenic |
| Exon 8 | c.1006G>T | p.(Val336Phe) | TM5 - pore helix | Liang et al. (2015) | Likely pathogenic |
| Exon 8 | c.1010_1012delinsCAATCCCAGTG | p.(Tyr337SerfsTer28) | TM5 - pore helix | Li et al. (2014) | Likely pathogenic |
| Exon 8 | c.1021T>C | p.(Ser341Pro) | TM5 - pore helix | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1030G>T | p.(Glu344Ter) | TM5 - pore helix | Nishiguchi et al. (2005) | Likely pathogenic |
| Exon 8 | c.1034A>G | p.(His345Arg) | TM5 - pore helix | ClinVar | Likely pathogenic |
| Exon 8 | c.1039C>T | p.(Arg347Cys) | TM5 - pore helix | Sun et al. (2020) | Likely pathogenic |
| Exon 8 | c.1048A>G | p.(Arg350Gly) | TM5 - pore helix | ClinVar | VUS |
| Exon 8 | c.1058T>C | p.(Ile353Thr) | Pore helix | Liang et al. (2015) | Likely pathogenic |
| Exon 8 | c.1061A>G | p.(Tyr354Cys) | Pore helix | This study | Likely pathogenic |
| Exon 8 | c.1063A>G | p.(Ser355Gly) | Pore helix | This study | Likely pathogenic |
| Exon 8 | c.1070A>G | p.(Tyr357Cys) | Pore helix | Vincent et al. (2011) | Likely pathogenic |
| Exon 8 | c.1074G>A | p.(Trp358Ter) | Pore helix | Li et al. (2014) | Likely pathogenic |
| Exon 8 | c.1076C>T | p.(Ser359Phe) | Pore helix | This study | Likely pathogenic |
| Exon 8 | c.1085C>T | p.(Thr362Ile) | Pore helix | ClinVar | Likely pathogenic |
| Exon 8 | c.1088T>C | p.(Leu363Pro) | Pore helix | Koeppen et al. (2010) | Pathogenic |
| Exon 8 | c.1100G>T | p.(Gly367Val) | pore helix -TM6 | Koeppen et al. (2010) | Pathogenic |
| Exon 8 | c.1106C>G | p.(Thr369Ser) | pore helix -TM6 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1114C>T | p.(Pro372Ser) | pore helix -TM6 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1114C>G | p.(Pro372Ala) | pore helix -TM6 | Sharon et al. (2020) | Likely pathogenic |
| Exon 8 | c.1115C>T | p.(Pro372Leu) | pore helix -TM6 | This study | Likely pathogenic |
| Exon 8 | c.1116del | p.(Val373Ter) | pore helix -TM6 | Zobor et al. (2017) | Likely pathogenic |
| Exon 8 | c.1116dup | p.(Val373ArgfsTer4) | pore helix -TM6 | Li et al. (2014) | Pathogenic |
| Exon 8 | c.1117G>A | p.(Val373Met) | pore helix -TM6 | This study | Likely pathogenic |
| Exon 8 | c.1124A>G | p.(Asp375Gly) | TM6 | Sun et al. (2020) | Likely pathogenic |
| Exon 8 | c.1126G>A | p.(Glu376Lys) | TM6 | Koeppen et al. (2010) | Pathogenic |
| Exon 8 | c.1129_1131del | p.(Glu377del) | TM6 | Fahim et al. (2013) | Likely pathogenic |
| Exon 8 | c.1137_1139del | p.(Phe380del) | TM6 | Liang et al. (2015) | Likely pathogenic |
| Exon 8 | c.1139T>C | p.(Phe380Ser) | TM6 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1146dup | p.(Val383ArgfsTer36) | TM6 | Li et al. (2014) | Likely pathogenic |
| Exon 8 | c.1163G>A | p.(Gly388Asp) | TM6 | Sun et al. (2020) | Likely pathogenic |
| Exon 8 | c.1163G>T | p.(Gly388Val) | TM6 | Sun et al. (2020) | Likely pathogenic |
| Exon 8 | c.1190G>T | p.(Gly397Val) | TM6 | Ahuja et al. (2008) | Likely pathogenic |
| Exon 8 | c.1201T>C | p.(Ser401Pro) | TM6 | Nishiguchi et al. (2005) | Likely pathogenic |
| Exon 8 | c.1217T>C | p.(Met406Thr) | TM6 | Wissinger et al. (2001) | Likely pathogenic |
| Exon 8 | c.1228C>T | p.(Arg410Trp) | TM6 - C-linker | Kohl et al. (1998) | Pathogenic |
| Exon 8 | c.1235_1236del | p.(Glu412ValfsTer6) | TM6 - C-linker | Méjécase et al. (2019) | Likely pathogenic |
| Exon 8 | c.1243G>C | p.(Ala415Pro) | C-linker αA' | Stone et al. (2017) | Likely pathogenic |
| Exon 8 | c.1254T>G | p.(Asp418Glu) | C-linker αA' | ClinVar | Likely pathogenic |
| Exon 8 | c.1255T>C | p.(Ser419Pro) | C-linker αA' | Weisschuh et al. (2020) | Likely pathogenic |
| Exon 8 | c.1256C>T | p.(Ser419Phe) | C-linker αA' | Patel et al. (2019) | Likely pathogenic |
| Exon 8 | c.1262del | p.(Lys421SerfsTer44) | C-linker αA' | Rim et al. (2017) | Likely pathogenic |
| Exon 8 | c.1267dup | p.(Tyr423LeufsTer20) | C-linker αA' | Sun et al. (2020) | Likely pathogenic |
| Exon 8 | c.1268A>C | p.(Tyr423Ser) | C-linker αA' | This study | Likely pathogenic |
| Exon 8 | c.1270A>G | p.(Met424Val) | C-linker αA' | Kuniyoshi et al. (2016) | Pathogenic |
| Exon 8 | c.1271T>C | p.(Met424Thr) | C-linker αA' | ClinVar | Likely pathogenic |
| Exon 8 | c.1273C>T | p.(Gln425Ter) | C-linker αA' | This study | Likely pathogenic |
| Exon 8 | c.1279C>T | p.(Arg427Cys) | C-linker αA' - αB' | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1280G>T | p.(Arg427Leu) | C-linker αA' - αB' | ClinVar | Likely pathogenic |
| Exon 8 | c.1286T>C | p.(Val429Ala) | C-linker αA' - αB' | Georgiou et al. (2019) | Likely pathogenic |
| Exon 8 | c.1294G>T | p.(Asp432Tyr) | C-linker αB' | Sun et al. (2020) | Likely pathogenic |
| Exon 8 | c.1294del | p.(Asp432ThrfsTer33) | C-linker αB' | Zelinger et al. (2015) | Likely pathogenic |
| Exon 8 | c.1298T>G | p.(Leu433Trp) | C-linker αB' | Koeppen et al. (2008) | Pathogenic |
| Exon 8 | c.1306C>T | p.(Arg436Trp) | C-linker αB' | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1307G>A | p.(Arg436Gln) | C-linker αB' | Li et al. (2014) | Likely pathogenic |
| Exon 8 | c.1315C>T | p.(Arg439Trp) | C-linker αB' | Reuter et al. (2008) | Pathogenic |
| Exon 8 | c.1319G>C | p.(Trp440Ser) | C-linker αB' | Georgiou et al. (2019) | Likely pathogenic |
| Exon 8 | c.1320del | p.(Trp440CysfsTer25) | C-linker αB' | This study | Pathogenic |
| Exon 8 | c.1319G>A | p.(Trp440Ter) | C-linker αB' | Li et al. (2014) | Likely pathogenic |
| Exon 8 | c.1320G>A | p.(Trp440Ter) | C-linker αB' | Wissinger et al. (2001) | Likely pathogenic |
| Exon 8 | c.1351dup | p.(Val451GlyfsTer3) | C-linker αB' - αC' | Wissinger et al. (2001) | Likely pathogenic |
| Exon 8 | c.1360A>T | p.(Lys454Ter) | C-linker αC' | Sundaram et al. (2014) | Likely pathogenic |
| Exon 8 | c.1366del | p.(Val456CysfsTer9) | C-linker αC' | This study | Likely pathogenic |
| Exon 8 | c.1379del | p.(Leu460ProfsTer5) | C-linker αC' - αD' | This study | Likely pathogenic |
| Exon 8 | c.1391T>G | p.(Leu464Arg) | C-linker αD' | Greenberg et al. (2014) | Likely pathogenic |
| Exon 8 | c.1395_1412del | p.(Lys465_Ile470del) | C-linker αD' | This study | Likely pathogenic |
| Exon 8 | c.1405G>A | p.(Ala469Thr) | C-linker αD' | Reuter et al. (2008) | Pathogenic |
| Exon 8 | c.1435_1436delinsGT | p.(Lys479Val) | C-linker αE' | Sun et al. (2020) | VUS |
| Exon 8 | c.1443dup | p.(Ile482HisfsTer6) | C-linker αF' | Johnson et al. (2004) | Likely pathogenic |
| Exon 8 | c.1454A>T | p.(Asp485Val) | C-linker αF' | Wissinger et al. (2001) | Likely pathogenic |
| Exon 8 | c.1457G>A | p.(Cys486Tyr) | C-linker αF' - CNBD αA | Abouelhoda et al. (2016) | Likely pathogenic |
| Exon 8 | c.1466G>T | p.(Gly489Val) | CNBD αA | This study | Likely pathogenic |
| Exon 8 | c.1495C>T | p.(Arg499Ter) | CNBD β1 | Burgueño-Montañés et al. (2014) | Likely pathogenic |
| Exon 8 | c.1513C>G | p.(Pro505Ala) | CNBD β1 - β2 | Huang et al. (2016) | Likely pathogenic |
| Exon 8 | c.1519del | p.(Asp507IlefsTer47) | CNBD β1 - β2 | This study | Likely pathogenic |
| Exon 8 | c.1520A>G | p.(Asp507Gly) | CNBD β1 - β2 | LOVD | Likely pathogenic |
| Exon 8 | c.1529G>C | p.(Cys510Ser) | CNBD β2 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1535A>T | p.(Lys512Met) | CNBD β2 - β3 | Carrigan et al. (2016) | Likely pathogenic |
| Exon 8 | c.1537G>C | p.(Gly513Arg) | CNBD β2 - β3 | Huang et al. (2016) | Likely pathogenic |
| Exon 8 | c.1537G>A | p.(Gly513Arg) | CNBD β2 - β3 | This study | Likely pathogenic |
| Exon 8 | c.1538G>A | p.(Gly513Glu) | CNBD β2 - β3 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1540G>A | p.(Asp514Asn) | CNBD β2 - β3 | Arshad et al. (2019) | Likely pathogenic |
| Exon 8 | c.1541A>T | p.(Asp514Val) | CNBD β2 - β3 | Genead et al. (2011) | Likely pathogenic |
| Exon 8 | c.1547G>A | p.(Gly516Glu) | CNBD β2 - β3 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1556T>C | p.(Met519Thr) | CNBD β3 | Huang et al. (2016) | Likely pathogenic |
| Exon 8 | c.1557G>A | p.(Met519Ile) | CNBD β3 | Ellingford et al. (2016) | Likely pathogenic |
| Exon 8 | c.1565T>C | p.(Ile522Thr) | CNBD β3 | Wissinger et al. (2001) | Likely pathogenic |
| Exon 8 | c.1569_1577del | p.(Asn523_Gly525del) | CNBD β3 | Sun et al. (2020) | VUS |
| Exon 8 | c.1573G>A | p.(Gly525Ser) | CNBD β3 - β4 | Patel et al. (2016) | Likely pathogenic |
| Exon 8 | c.1574G>A | p.(Gly525Asp) | CNBD β3 - β4 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1579C>A | p.(Leu527Met) | CNBD β4 | Wang et al. (2011) | Likely pathogenic |
| Exon 8 | c.1580T>G | p.(Leu527Arg) | CNBD β4 | Lam et al. (2011) | Likely pathogenic |
| Exon 8 | c.1585G>A | p.(Val529Met) | CNBD β4 | Kohl et al. (1998) | Pathogenic |
| Exon 8 | c.1597G>C | p.(Asp533His) | CNBD β4 - β5 | Li et al. (2014) | Pathogenic |
| Exon 8 | c.1609C>T | p.(Gln537Ter) | CNBD β4 - β5 | Wissinger et al. (2001) | Likely pathogenic |
| Exon 8 | c.1621C>T | p.(Leu541Phe) | CNBD β5 | Greenberg et al. (2014) | Likely pathogenic |
| Exon 8 | c.1627_1635del | p.(Asp543_Ser545del) | CNBD β5/β5 - β6 | Li et al. (2014) | VUS |
| Exon 8 | c.1640T>G | p.(Phe547Cys) | CNBD β6 | Zelinger et al. (2015) | Likely pathogenic |
| Exon 8 | c.1641C>A | p.(Phe547Leu) | CNBD β6 | Kohl et al. (1998) | Pathogenic |
| Exon 8 | c.1642G>A | p.(Gly548Arg) | CNBD β6 | Johnson et al. (2004) | Pathogenic |
| Exon 8 | c.1645G>T | p.(Glu549Ter) | CNBD β6 | This study | Likely pathogenic |
| Exon 8 | c.1658T>A | p.(Leu553Gln) | CNBD β6 - β7 | This study | Likely pathogenic |
| Exon 8 | c.1669G>A | p.(Gly557Arg) | CNBD β6 - β7 | Kohl et al. (1998) | Pathogenic |
| Exon 8 | c.1679C>T | p.(Ser560Leu) | CNBD β6 - β7 | ClinVar | Likely pathogenic |
| Exon 8 | c.1682G>A | p.(Gly561Glu) | CNBD β6 - β7 | Zobor et al. (2017) | Likely pathogenic |
| Exon 8 | c.1682_1683insACGCG | p.(Asn562ArgfsTer47) | CNBD β6 - β7 | This study | Likely pathogenic |
| Exon 8 | c.1686C>A | p.(Asn562Lys) | CNBD β6 - β7 | ClinVar | Likely pathogenic |
| Exon 8 | c.1687C>T | p.(Arg563Cys) | CNBD β6 - β7 | Koeppen et al. (2008) | Pathogenic |
| Exon 8 | c.1688G>A | p.(Arg563His) | CNBD β6 - β7 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1694C>T | p.(Thr565Met) | CNBD β6 - β7 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1705C>T | p.(Arg569Cys) | CNBD β7 | This study | Likely pathogenic |
| Exon 8 | c.1706G>A | p.(Arg569His) | CNBD β7 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1708A>G | p.(Ser570Gly) | CNBD β7 | This study | Likely pathogenic |
| Exon 8 | c.1709G>A | p.(Ser570Asn) | CNBD β7 | Li et al. (2014) | Likely pathogenic |
| Exon 8 | c.1709G>T | p.(Ser570Ile) | CNBD β7 | Thiadens et al. (2010) | Likely pathogenic |
| Exon 8 | c.1712T>C | p.(Ile571Thr) | CNBD β7 - β8 | Jinda et al. (2021) | Likely pathogenic |
| Exon 8 | c.1717T>C | p.(Tyr573His) | CNBD β7 - β8 | This study | Likely pathogenic |
| Exon 8 | c.1718A>G | p.(Tyr573Cys) | CNBD β7 - β8 | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1719C>G | p.(Tyr573Ter) | CNBD β7 - β8 | Holtan et al. (2020) | Likely pathogenic |
| Exon 8 | c.1736T>G | p.(Leu579Arg) | CNBD β8 | This study | Likely pathogenic |
| Exon 8 | c.1768G>A | p.(Glu590Lys) | CNBD αB | Nishiguchi et al. (2005) | Pathogenic |
| Exon 8 | c.1771T>C | p.(Tyr591His) | CNBD αB - αC | This study | Likely pathogenic |
| Exon 8 | c.1775C>T | p.(Pro592Leu) | CNBD αB - αC | This study | Likely pathogenic |
| Exon 8 | c.1777G>A | p.(Glu593Lys) | CNBD αC | Wissinger et al. (2001) | Pathogenic |
| Exon 8 | c.1793T>G | p.(Leu598Arg) | CNBD αC | Sun et al. (2020) | VUS |
| Exon 8 | c.1805G>A | p.(Gly602Glu) | CNBD αC | Georgiou et al. (2019) | Likely pathogenic |
| Exon 8 | c.1810C>T | p.(Gln604Ter) | CNBD αC | Taylor et al. (2017) | Likely pathogenic |
| Exon 8 | c.1811del | p.(Gln604ArgfsTer3) | CNBD αC | Sun et al. (2020) | Likely pathogenic |
| Exon 8 | c.1898T>C | p.(Leu633Pro) | CLZ | Goto-Omoto et al. (2006) | Likely pathogenic |
| Exon 8 | c.1931T>C | p.(Phe644Ser) | CLZ | Ellingford et al. (2016) | VUS |
| Exon 8 | c.1937G>A | p.(Arg646His) | CLZ | Li et al. (2014) | VUS |
| Exon 8 | c.1949A>C | p.(Glu650Ala) | CLZ | LOVD | VUS |
| Exon 8 | c.1963C>T | p.(Gln655Ter) | CLZ | Wissinger et al. (2001) | Likely pathogenic |
| Exon 8 | c.1975A>T | p.(Lys659Ter) | CLZ | Li et al. (2014) | Likely pathogenic |
| Exon 8 | c.1981C>A | p.(Arg661Ser) | CLZ | Yang et al. (2014) | Likely pathogenic |
| Exon 8 | c.1982G>A | p.(Arg661His) | CLZ | Sun et al. (2020) | Likely pathogenic |
| Variants of uncertain significance | |||||
| Exon 2 | c.68G>Ac | p.(Arg23Gln) | N-Term. | ClinVar | VUS |
| Exon 2 | c.80G>Ac | p.(Arg27His) | N-Term. | ClinVar | VUS |
| Exon 3 | c.110C>Tc | p.(Ser37Leu) | N-Term. | ClinVar | VUS |
| Exon 3 | c.154A>Gc | p.(Met52Val) | N-Term. | ClinVar | VUS |
| Exon 3 | c.211G>Ac | p.(Ala71Thr) | N-Term. | ClinVar | VUS |
| Exon 4 | c.316G>Ac | p.(Glu106Lys) | N-Term. | Sharon et al. (2020) | VUS |
| Exon 4 | c.358A>Gc | p.(Asn120Asp) | N-Term. | Li et al. (2014) | VUS |
| Exon 5 | c.440C>Gc | p.(Thr147Arg) | N-Term. | LOVD | VUS |
| Exon 6 | c.471T>Gc | p.(Asp157Glu) | N-Term. | ClinVar | VUS |
| Exon 6 | c.473C>Tc | p.(Ala158Val) | N-Term. | LOVD | VUS |
| Exon 6 | c.553C>G | p.(Leu185Val) | TM1 | Kim et al. (2019) | VUS |
| Exon 7 | c.587A>G | p.(Gln196Arg) | TM1 - TM2 | Sun et al. (2020) | VUS |
| Exon 7 | c.625T>C | p.(Ser209Pro) | TM2 | Weisschuh et al. (2020) | VUS |
| Exon 7 | c.664G>C | p.(Ala222Pro) | TM2 | Reuter et al. (2008) | VUS |
| Exon 7 | c.670A>G | p.(Thr224Ala) | TM2 - TM3 | This study | VUS |
| Exon 7 | c.671C>T | p.(Thr224Ile) | TM2 - TM3 | Li et al. (2014) | VUS |
| Exon 8 | c.743A>G | p.(Gln248Arg) | TM3 | This study | VUS |
| Exon 8 | c.784G>C | p.(Ala262Pro) | TM3 | Zobor et al. (2017) | VUS |
| Exon 8 | c.796G>A | p.(Val266Met) | TM3 - TM4 | Thiadens et al. (2010) | VUS |
| Exon 8 | c.1343A>G | p.(Lys448Arg) | C-linker αB' - αC' | This study | VUS |
| Exon 8 | c.1615G>Ad | p.(Val539Met) | CNBD β5 | This study | VUS |
| Exon 8 | c.1784A>Gc | p.(Lys595Arg) | CNBD αC | ClinVar | VUS |
| Exon 8 | c.1845G>Tc | p.(Glu615Asp) | CNBD αC - CLZ | ClinVar | VUS |
| Exon 8 | c.1877T>G | p.(Leu626Arg) | CLZ | Huang et al. (2016) | VUS |
| Exon 8 | c.1889T>C | p.(Val630Ala) | CLZ | This study | VUS |
| Exon 8 | c.1968G>Ac | p.(Met656Ile) | CLZ | ClinVar | VUS |
| Exon 8 | c.2005G>Cc | p.(Val669Leu) | CLZ | ClinVar | VUS |
| Exon 8 | c.2050G>Ac | p.(Gly684Arg) | C-Term. | Patel et al. (2016) | VUS |
| Likely benign variants | |||||
| Exon 2 | c.59C>T | p.(Thr20Ile) | N-Term. | ClinVar | Benign |
| Exon 2 | c.66C>T | p.(=) | N-Term. | This study | VUS |
| Exon 2 | c.72T>C | p.(=) | N-Term. | This study | Benign |
| Exon 2 | c.81C>T | p.(=) | N-Term. | Nishiguchi et al. (2005) | Likely benign |
| Intron 2 | c.102-16A>G | p.(?) | - | This study | Benign |
| Exon 3 | c.129G>A | p.(=) | N-Term. | ClinVar | Likely benign |
| Exon 3 | c.143C>T | p.(Pro48Leu) | N-Term. | Nishiguchi et al. (2005) | Benign |
| Intron 3 | c.215+11A>G | p.(?) | - | LOVD | Benign |
| Intron 3 | c.215+151T>C | p.(?) | - | This study | Benign |
| Exon 3 | c.198C>T | p.(=) | N-Term. | This study | Likely benign |
| Exon 3be | c.336G>Ae | p.(Met112Ile) | - | This study | n.a. |
| Exon 3be | c.360C>Te | p.(=) | - | This study | n.a. |
| Intron Exon 3be | c.215+46T>Ge | p.(?) | - | This study | n.a. |
| Intron Exon 3be | c.216-60A>Te | p.(?) | - | This study | n.a. |
| Exon 4 | c.225C>T | p.(=) | N-Term. | ClinVar | VUS |
| Exon 4 | c.238C>T | p.(=) | N-Term. | ClinVar | VUS |
| Exon 4 | c.284C>Tf | p.(Pro95Leu) | N-Term. | Thiadens et al. (2010) | VUS |
| Exon 4 | c.353A>Gd | p.(Gln118Arg) | N-Term. | This study | Likely benign |
| Intron 4 | c.395+9C>T | p.(?) | - | ClinVar | VUS |
| Intron 4 | c.396-4G>A | p.(?) | - | ClinVar | Benign |
| Intron 5 | c.449+13A>G | p.(?) | - | ClinVar | VUS |
| Exon 6 | c.458C>T | p.(Thr153Met) | N-Term. | Kohl et al. (1998) | Benign |
| Intron 6 | c.566+6C>T | p.(?) | - | ClinVar | Likely benign |
| Intron 6 | c.566+14G>A | p.(?) | - | ClinVar | VUS |
| Exon 7 | c.592G>A | p.(Glu198Lys) | TM2 | This study | Benign |
| Exon 8 | c.715C>T | p.(=) | TM2 - TM3 | ClinVar | VUS |
| Exon 8 | c.734C>Tf | p.(Thr245Met) | TM2 - TM3 | Johnson et al. (2004) | VUS |
| Exon 8 | c.740C>T | p.(Thr247Met) | TM3 | Li et al. (2014) | Likely benign |
| Exon 8 | c.777C>A | p.(=) | TM3 | ClinVar | VUS |
| Exon 8 | c.1116C>T | p.(=) | pore helix - TM6 | ClinVar | Likely benign |
| Exon 8 | c.1347G>A | p.(=) | C-linker αB' - αC' | This study | VUS |
| Exon 8 | c.1412A>Gf | p.(Asn471Ser) | C-linker αD' | Wissinger et al. (2001) | VUS |
| Exon 8 | c.1413C>T | p.(=) | C-linker αD' | Nishiguchi et al. (2005) | VUS |
| Exon 8 | c.1569C>T | p.(=) | CNBD β3 | Nishiguchi et al. (2005) | Likely benign |
| Exon 8 | c.1611G>A | p.(=) | CNBD β4 - β5 | ClinVar | VUS |
| Exon 8 | c.1618G>Af | p.(Val540Ile) | CNBD β5 | Thiadens et al. (2010) | VUS |
| Exon 8 | c.1626C>T | p.(=) | CNBD β5 | ClinVar | Benign |
| Exon 8 | c.1695G>A | p.(=) | CNBD β6 - β7 | Nishiguchi et al. (2005) | Likely benign |
| Exon 8 | c.1746C>T | p.(=) | CNBD αB | LOVD | Likely benign |
| Exon 8 | c.1767C>T | p.(=) | CNBD αB | LOVD | Likely benign |
| Exon 8 | c.1856C>Tf | p.(Ala619Val) | CNBD αC - CLZ | Thiadens et al. (2010) | VUS |
| Exon 8 | c.1862C>Af | p.(Ala621Glu) | CNBD αC - CLZ | Wiszniewski et al. (2007) | VUS |
| Exon 8 | c.1863G>A | p.(=) | CNBD αC - CLZ | LOVD | Likely benign |
| Exon 8 | c.1866C>T | p.(=) | CNBD αC - CLZ | ClinVar | VUS |
- Note: All 316 variants were classified following ACMG/AMP criteria and subsequently revised manually and expert-categorized into “likely benign,” “VUS,” and “likely disease-causing.”
- Abbreviation: ACHM, achromatopsia.
- a Variant designation is based on NM_001298.2 (GRCh38/hg38).
- b Functional domains of CNGA3 according to Gofman et al., 2014 and Shuart et al., 2011: N-Term., N-terminus; TM1 to TM6, transmembrane domains; C-linker having six alpha helices (αA'–αF'); CNBD, cyclic-nucleotide binding domain having three alpha helices (αA–αC) and eight beta-sheets (β1–β8); CLZ, C-terminal leucin zipper; C-Term., C-terminus; -, intermediate amino acid residue between two domains.
- c Variants classified as VUS: no genotype could be established from the literature or database data.
- d Variants observed in an affected individual with an OPN1MW/OPN1LW-associated genotype indicative for and compatible with a clinical diagnosis of blue cone monochromatism, X-linked.
- e Variant designation of variants located in the alternate exon 3b (formerly named exon 2b (Wissinger et al., 2001), now renamed exon 3b) and its flanking intronic sequences is based on XM_011510554.2 (GRCh38/hg38).
- f Variants only observed in a heterozygous state in the absence of a second likely disease-causing allele.
Following the standards and guidelines of ACMG/AMP, 67 out of the remaining 312 variants were classified as pathogenic, 152 as likely pathogenic, and 71 were categorized as variants of uncertain significance (VUS). Furthermore, 12 out of 312 sequence alterations were classified as likely benign and 10 as benign (Tables 2 and S3).
The 316 CNGA3 variants listed in our CNGA3 variant database were revised manually and expert-categorized into “likely benign,” “VUS,” and “likely disease-causing” applying the following criteria. Variants categorized as “likely benign” (n = 44 variants; Tables 2 and S3) included: (1) variants classified as likely benign or benign according to ACMG/AMP guidelines, (2) synonymous variants that are not predicted to affect splicing, (3) missense variants with a minor allele frequency (MAF) higher than 0.1% and predicted to be tolerated by at least two in silico algorithms, (4) missense variants predicted to be tolerated by at least two in silico tools and only observed in a heterozygous state in the absence of a second “likely disease-causing” allele, (5) noncanonical splice variants not predicted to affect splicing and (6) variants located in the alternate exon 3b and its flanking intronic sequences.
Variants classified as “VUS” (n = 28; Tables 2 and S3) comprised missense variants that were classified as VUS following the ACMG/AMP guidelines and were predicted to be tolerated by at least two in silico tools. In addition, 15 variants were grouped in this category since no genotype could be established from the literature or database data. If the following criteria for a “VUS” were fulfilled, we considered patients carrying such variants with respect to the phenotype as solved cases: (1) observation of the variant in a heterozygous state with a (known) “likely disease-causing” variant and/or (2) segregation analysis demonstrated independent segregation of two variants and/or co-segregation of the variant with the disease.
All variants in the CNGA3 variant database not meeting the above-mentioned criteria were categorized as “likely disease-causing” (n = 244; Tables 2 and S3). Thus, we included the following variants in this “likely disease-causing” category: (1) variants classified as pathogenic or likely pathogenic following the ACMG/AMP guidelines, (2) variants representing likely null alleles (nonsense, start loss, canonical splice site, and frameshift variants), (3) missense variants classified as VUS following ACMG/AMP guidelines, but predicted to be deleterious by at least three in silico tools, (4) missense variants for which functional analysis revealed a deleterious effect on protein function, (5) noncanonical splice site variants classified as VUS following ACMG/AMP guidelines, but predicted to have an effect on splicing by at least three in silico tools, and (6) in-frame deletions affecting (completely) conserved amino acid residues. Consequently, all patients carrying CNGA3 variants categorized as “likely disease-causing” were designated as solved cases with respect to the ACHM phenotype.
Among 316 CNGA3 variants listed in the CNGA3 variant database, 251 variants were already published in the literature and in public databases. From these, we classified 196 variants as “likely disease-causing”, 23 as “VUS” and 32 as “likely benign” following manual and expert-based revision (Table S3). Only a small number of previously published missense variants were categorized as “likely benign” including the variants p.(Thr20Ile), p.(Pro48Leu), p.(Thr153Met), and p.(Val540Ile) based on the following observations: (1) All but one of these are located in the N-terminal region affecting residues which are neither conserved within CNGA3 orthologues nor the rod homolog CNGA1—only the p.(Val540Ile) variant affects a conserved residue in the CNBD. (2) All above-mentioned missense variants have a MAF higher than 0.1% and were predicted to be tolerated by in silico tools underlining their presumably nonpathogenic impact.
Within our CNGA3 variant database, we report 48 novel “likely disease-causing” variants detected in our ACHM cohort with six variants classified as pathogenic, 36 as likely pathogenic, and six as VUS following ACMG/AMP classification (Table S3). These previously unpublished variants comprise 24 missense substitutions, 14 small indel variants, and 6 nonsense substitutions creating a premature stop codon.
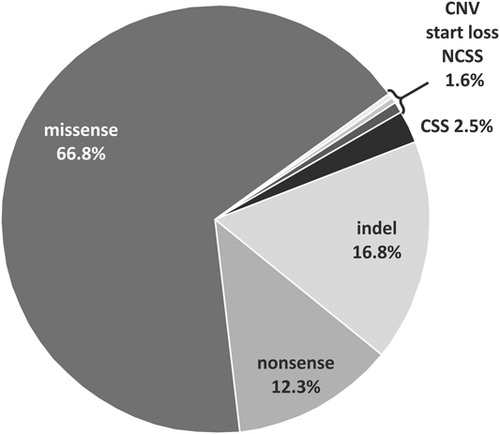
Overall, out of the 244 “likely disease-causing” variants in the CNGA3 variant database, missense substitutions were the most frequently observed variant type (66.8%) followed by small indel variants (16.8%) and nonsense alterations (12.3%) creating a premature stop codon (Figure 2). An overview of the mutation types and the respective frequencies of these “likely disease-causing” CNGA3 variants within our database is provided in Figure 2.
In addition, we observed one novel start loss variant affecting the first nucleotide of the translation initiation codon ATG (c.1A>T/p.(?)) and one copy number variation leading to the complete deletion of exon 8 (NC_000002.12:g.98393909_98399093del (GRCh38/hg38)). Actually, this deletion is the first large structural variation/copy number variation observed and reported for the CNGA3 gene and was identified in compound heterozygous state with the variant c.847C>A/p.(Arg283Trp) on the second allele in one patient. Following the standard variant screening, the variant c.847C>A/p.(Arg283Trp) was initially identified apparent homozygously in this index patient. Segregation analysis failed to detect the variant in the father of the patient. Microsatellite marker analysis confirmed paternity and deletion mapping via PCR and primer walking identified the heterozygous 5.19 kb deletion.
Copy number variations (CNVs) are an important mutation type associated with ACHM and are commonly seen in the CNGB3 gene as well as in GNAT2 and ATF6. Whereas ten out of 98 disease-associated CNGB3 variants identified in a large cohort of ACHM patients are CNVs, the mutation spectrum of ATF6 and GNAT2 includes two CNVs each (Felden et al., 2019; Kohl et al., 2002; Lee et al., 2020; Mayer et al., 2017). In contrast, it seems that this kind of structural variation is not common in CNGA3. This notion is further supported by the fact, that the gene had not been pinpointed by a CNV-study focusing on genes in inherited retinal dystrophies (Van Schil et al., 2018). In addition, single heterozygous cases are rarely found in CNGA3 compared to the CNGB3 gene where 44 out of 552 patients with CNGB3 variants were identified to harbor only a single heterozygous pathogenic variant following standard variant screening and CNV analysis (Mayer et al., 2017). This further suggests that most variants in CNGA3 are exonic indicating that there are not many hidden pathogenic variants—such as deep intronic variants—left.
Regarding the localization of “likely disease-causing” CNGA3 variants, 236 sequence alterations are located in the coding exons whereas only eight variants are located in the intronic sequences of the gene (Figure 3). Of these intronic variants, seven variants represent single nucleotide substitutions affecting either the canonical splice sites (i.e., the first two and last two nucleotides of an intron) (n = 5) or are located in the vicinity of a highly conserved splice acceptor site (n = 2). All variants affecting the canonical splice sites are predicted to lead to the loss of the respective donor or acceptor site based on five in silico algorithms. Additionally, the previously published variant c.396-11C>G/p.(?) and the novel sequence alteration c.450-15T>G/p.(?), both located in the vicinity of a highly conserved splice acceptor site, are predicted to activate an intronic cryptic acceptor site potentially affecting splicing (SpliceAI Δ scores: acceptor loss 0.87 and 0.65, respectively; acceptor gain 0.70 and 0.53, respectively; Table S3). Furthermore, one small duplication is localized at the exon/intron border of exon 5 (c.396-2_398dup/p.(?)). Although only one of the in silico tools predicts a minor effect on transcript splicing, the variant is still considered to be “likely disease-causing” (SpliceAI Δ score: acceptor loss 0.00; acceptor gain 0.22). The novel missense variant c.566G>A/p.(Arg189Lys) affecting the last nucleotide of exon 6 was also predicted to have an effect—though minor—on transcript splicing (SpliceAI Δ score: donor loss 0.08; donor gain 0.32). Consequently, it remains unclear whether this variant needs to be considered a splicing or missense variant, as it may also act both ways.
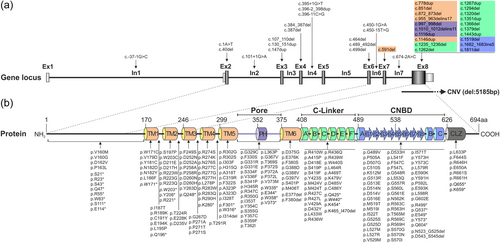
The majority of the exonic ACHM-associated CNGA3 variants classified as “likely disease-causing” within the CNGA3 variant database are located in exon 8 including 140 missense substitutions, 46 nonsense/small indel variants, and the single copy number variation (deletion of complete exon 8) (Table 2 and Figure 3). The remaining 49 variants are distributed across exon 2 to exon 7, with exons 6 and 7 (encoding TM1 and TM2) each harboring triple the number of variants as exons 2 to exon 5. On protein level, the majority of CNGA3 variants (n = 82) are spread across the six transmembrane domains and their connecting loops. Another major group of “likely disease-causing” CNGA3 variants (n = 57) clusters within the CNBD whereas only few variants (n = 8) reside in the adjacent CLZ domain more closely located to the C-terminus. The C-linker of the CNGA3 subunit and the pore region harbor a similar fraction of “likely disease-causing” CNGA3 variants (n = 36 and n = 30, respectively). The pore region spanning around 46 amino acids, is thereby rendered to have the highest mutation density within the CNGA3 subunit. Whereas the overall majority of variants within the CNGA3 protein are missense substitutions, the sequence changes within the N-terminal region mainly comprise nonsense variants or small indels (17 out of 22) (Figure 3). Of 163 “likely disease-causing” missense variants listed in our database, 94 affect amino acid positions which are completely conserved across CNGA3 orthologues and its rod equivalent CNGA1 (Table S3 and Figure S2).
3.3 Prevalence of CNGA3 variants in ACHM
From our in-house genetic investigations, we report disease-associated CNGA3 variants in 385 affected individuals descending from 304 independent families representing 36.3% of our ACHM cohort which predominantly comprises Western European and North American patients (Figure S1). This observation is in line with our previous and other studies reporting a prevalence of 5%–41% for CNGA3 variants in cohorts of Dutch and British ACHM patients making CNGA3 the second most frequently mutated gene in European ACHM patients or patients of European descent (Johnson et al., 2004; Thiadens et al., 2009; Wissinger et al., 2001).
The vast majority of all “likely disease-causing” CNGA3 variants listed in our CNGA3 database represent amino acid substitutions (66.8%) which are completely in line with several previously conducted studies where missense variants showed a frequency of 61.5%–71.4% (Li et al., 2014; Liang et al., 2015; Wissinger et al., 2001). In contrast, 16 out of 98 sequence changes (16%) in the CNGB3 subunit of the cone photoreceptor CNG channel identified in a large cohort of ACHM patients are missense variants (Mayer et al., 2017). This high prevalence of missense substitutions in the CNGA3 gene may indicate a reduced tolerance of CNGA3 for amino acid substitutions regarding the functional maintenance of the channel protein.
All “likely disease-causing” variants listed in our CNGA3 database except the c.682G>A/p.(Glu228Lys) variant have a minor allele frequency (MAF) lower than 0.1% in gnomAD (Table S3). In contrast, the mentioned variant displays a MAF of 0.13% (Table S3). In the South Asian population, the c.682G>A/p.(Glu228Lys) variant displays an even higher allele frequency of 0.99% in concordance with CNGA3 variants being more common here than in the European population. The c.682G>A/p.(Glu228Lys) variant has been observed in four patients from two families of South Asian origin in our cohort, but since it is part of complex alleles in these patients (homozygous for c.[67C>T;682G>A] or c.[682G>A;1315C>T]) the contribution of the c.682G>A/p.(Glu228Lys) variant to the ACHM phenotype in these families remains unclear—it may exert a cumulative effect (Table S2). In addition, one ACHM patient carrying the c.682G>A/p.(Glu228Lys) variant homozygously was described in a previously published study (Sun & Zhang, 2019). Furthermore, the variant was described to be part of a triallelic inheritance pattern involving the CNGB3 c.1208G>A/p.(Arg403Gln) variant (Burkard et al., 2018; Thiadens et al., 2010). Due to the deleterious effect predicted by two out of three in silico analysis tools and functional studies demonstrating impaired functionality of CNG channels harboring CNGA3Glu228Lys subunits (Reuter et al., 2008; Täger et al., 2021), we classified this variant as “likely disease-causing.” A complete overview of the outcomes of the in silico prediction algorithms and previously published functional studies are included in Table S3.
3.4 Functional studies on CNGA3 variants
As a first gene therapy trial for ACHM resulting from defects in CNGA3 has already been completed (Fischer et al., 2020), and several trials are ongoing (ClinicalTrials.gov Identifier: NCT02610582, NCT03278873, NCT03758404, and NCT02935517), the genotypes of patients with CNGA3 missense variants classified as “VUS” unfortunately hamper the inclusion of such patients eligible for these gene therapeutic trials. Therefore, functional studies are needed to validate the possible pathogenic effect of the respective variants. Commonly used methods to characterize the functionality of mutant CNG channels involve calcium imaging and patch-clamp recordings of heterologously expressed mutant channels (Burkard et al., 2018; Dai & Varnum, 2013; Koeppen et al., 2008, 2010; Kuniyoshi et al., 2016; Liu & Varnum, 2005; Matveev et al., 2010; Meighan et al., 2015; Muraki-Oda et al., 2007; Patel et al., 2005; Reuter et al., 2008; Shaikh et al., 2015; Täger et al., 2018; Tränkner et al., 2004). Furthermore, a recently developed functional assay proposes a fluorescence-based, high-throughput calcium influx-based approach for analyzing mutant CNGA3 channels (Jacobson et al., 2019). In total, 54 missense substitutions, two nonsense and one canonical splice site variant have already been functionally characterized (Table S3), and this information was used to classify 44 variants as pathogenic, 11 as likely pathogenic, one as VUS—due to contradictory results in functional approaches—and one as benign according to ACMG/AMP guidelines.
Following functional characterization of heterologously expressed mutant channels in previously conducted studies, 27 out of 54 missense variants completely abolished formation of functional channels (Koeppen et al., 2008, 2010; Liu & Varnum, 2005; Matveev et al., 2010; Muraki-Oda et al., 2007; Patel et al., 2005; Shaikh et al., 2015; Tränkner et al., 2004). Seventeen previously analyzed missense substitutions allowed the formation of functional channels but with impaired/altered functionality such as impaired protein folding and/or channel trafficking or altered biophysical properties, for example, altered apparent cGMP-sensitivity or ion selectivity (Burkard et al., 2018; Dai & Varnum, 2013; Koeppen et al., 2008; Liu & Varnum, 2005; Meighan et al., 2015; Muraki-Oda et al., 2007; Patel et al., 2005; Reuter et al., 2008; Tränkner et al., 2004). Furthermore, an improvement in the mutant channel functionality was achieved in a total of ten missense variants by using chemical chaperones or co-expressing the CNGB3 subunit and/or reducing the cell cultivation temperature from the standard 37°C to 27/28°C, a procedure that is known to enhance protein folding and trafficking (Koeppen et al., 2010; Kuniyoshi et al., 2016; Patel et al., 2005; Reuter et al., 2008). A complete overview of CNGA3 variants previously analyzed in functional studies and the respective channel functionality is summarized in Table S3.
4 CLINICAL AND DIAGNOSTIC ASPECTS OF ACHM
ACHM usually becomes clinically manifest at early infancy. The most typical signs and symptoms are marked photosensitivity, nystagmus, severely reduced visual acuity, and the inability to discriminate colors. The presented nystagmus is mostly of low amplitude and in many affected individuals improves with older age. However, the most pronounced and subjectively difficult symptom photophobia usually remains for the lifetime of the patient.
In a complete achromat, the best-corrected visual acuity is stable at around 20/200. Patients suffering from incomplete ACHM present with a milder phenotype with retained residual color discrimination, better visual acuity, and/or residual photopic ERG responses (Hirji et al., 2018; Michalakis et al., 2017; Sun & Zhang, 2019).
ACHM is clinically diagnosed in childhood due to the typical symptoms of nystagmus, photophobia, and low vision. Parents of affected children usually notice nystagmus in the first months of life, but in some patients the diagnosis is only made in preschool age. In the case of incomplete achromats, the diagnosis is occasionally made even later (Felden et al., 2019; Weisschuh et al., 2018).
The differential diagnosis of ACHM also includes treatable ophthalmological diseases of infancy, but due to the low cooperation of children at ophthalmological examination, the final confirmation is usually given by molecular genetic testing. The most important differential diagnoses for ACHM not only include other rare genetic retinal diseases such as blue cone monochromacy, bradyopsia, or early onset cone dystrophy, but also include other diseases of early childhood connected to photophobia such as juvenile glaucoma or neuroophthalmological causes of nystagmus.
ACHM is genetically heterogeneous encompassing six known causative genes, but the multimodal ophthalmological diagnostics, especially retinal imaging, almost always reveal a very similar clinical picture: a funduscopic normal-appearing retina (Figure 4d) with a normal or close to normal fundus autofluorescence pattern (Figure 4e). In some patients, changes in the foveal autofluorescence are obvious, corresponding to granular changes in the ellipsoid zone of the cones or fovea thinning (Felden et al., 2019; Zobor et al., 2017). Optical coherence tomography (OCT) shows a normal layering of the central retina, especially in children and young adults (Figure 4a), but disruptions of the outer segments become obvious with increasing age (Figures 4b,c). Furthermore, hypoplasia of the fovea has been described in many ACHM patients. However, the image quality is frequently diminished due to nystagmus (Tekavcic Pompe et al., 2021; Zobor et al., 2017).
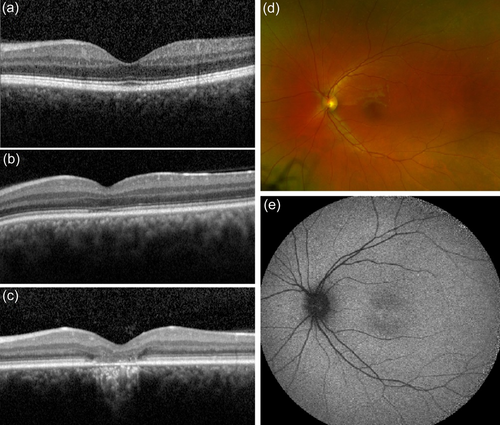
Achromatopsia has been classically considered to be a stationary disease. But nowadays using high-resolution retinal imaging we know that progressive cone cell loss and degenerative changes occur in the retinal morphology in ACHM patients (Georgiou et al., 2020; Thiadens et al., 2010; Thomas et al., 2012). Four stages of morphological degenerative changes have been described in ACHM patients using high-resolution OCT (Tekavcic Pompe et al., 2021; Zobor et al., 2017): preserved inner segment ellipsoid, disrupted inner segment ellipsoid, inner segment ellipsoid loss, and inner segment & retinal pigment epithelium loss (Figure 4a–c).
Another very important tool for the diagnostics of ACHM is retinal functional testing. As already mentioned above, the best-corrected visual acuity is usually in the range of 20/400–20/200 but can be higher in incomplete achromats. Color vision testing supporting the subjective loss of color discrimination shows a rod-dominated mixed color perception disorder (Zobor et al., 2017). Cone-driven perimetric and microperimetric techniques reveal reduced light sensitivity especially in the macular region (Zobor et al., 2017).
Essential diagnostic tests are also those that can reliably and objectively distinguish between rod and cone function thereby detecting normal rod functionality while revealing missing or markedly reduced cone functionality. Thus, the full-field ERG (ffERG) is a well-established diagnostic tool for ACHM. In ffERG the scotopic responses, especially the rod-driven dark-adapted flash-elicited responses, are in the normal range, but the standard mixed flashes (3 and 10 cd/m2) can elicit either reduced or close to normal responses (Maguire et al., 2018; Zobor et al., 2017). The standard photopic recordings in the ffERG including the 30 Hz flicker are reduced below the noise level and therefore clinically not measurable. However, in some incomplete achromats reduced photopic responses can be observed (Maguire et al., 2018; Tekavcic Pompe et al., 2021; Zobor et al., 2017). A recent study using time-frequency analysis of the standard ffERG in ACHM patients compared to age-matched controls showed a strongly reduced but not absent activity of oscillatory potentials in a frequency range mostly driven by cones and only in a small part by rods (Righetti et al., 2021). This manifestation of the lack of cone modulation on the retinal network gives insights into interactions between both types of photoreceptors and the inner retinal network (Righetti et al., 2021). Similarly, pupillometric measures are objective tests of the retinal function that are able to address cones and rods independently from each other and can reveal reduced cone functionality and preserved or increased rod function due to the missing interaction of cones and rods in the retina of ACHM patients (Kelbsch et al., 2019; Lisowska et al., 2017). Due to the lack of rod inhibition by cones, as is the case in normal eyes, the affected individuals experience severe photophobia. Rods are not directly affected by defects in ACHM genes and accordingly the dark-adapted thresholds are also in a normal range, not only for white and blue stimuli, but also for red stimuli (Zobor et al., 2017).
5 ANIMAL MODELS OF CNGA3-ASSOCIATED ACHM
Both small and large animal models of CNGA3-associated ACHM exist enabling the characterization of the pathogenesis of the disease. The first described small animal model was the Cnga3 knockout (KO) mouse which was generated by a targeted deletion of exon 7 of the murine Cnga3 gene (Biel et al., 1999). Furthermore, a naturally occurring mouse model of ACHM, the so-called cpfl5 mouse, was discovered harboring a missense variant c.493A>G/p.(Thr165Ala) in exon 5 of the murine Cnga3 gene (Pang et al., 2012). Both mouse models exhibited a similar ocular phenotype including the selective loss of cone-mediated light responses which was accompanied by a progressive degeneration of cone photoreceptors. Additionally, both mouse models presented a defect in the cone opsin transport by failing to target cone opsins to the outer segments. In contrast, the rod photoreceptors remained both structurally and functionally intact (Biel et al., 1999; Pang et al., 2012). A recent study exploring the disease mechanism in Cnga3 KO mice revealed an accumulation of cGMP in Cnga3 KO cones and a transient upregulation of the cGMP-dependent protein kinase 2 (Prkg2). Knockout of Prkg2 counteracted the cone degeneration by normalizing the endoplasmic reticulum homeostasis thereby pinpointing Prkg2 as a novel key mediator of cone degeneration (Koch et al., 2020).
A large animal model of ACHM includes the naturally occurring Awassi sheep model presenting with congenital day blindness and severely diminished cone function (Ezra-Elia et al., 2014; Shamir et al., 2010). The affected lambs are homozygous for a nonsense variant p.(Arg236Ter) in the ovine CNGA3 gene (Reicher et al., 2010). Furthermore, the day-blind sheep show no responses to bright red light in chromatic pupil light reflex measurement underlining the loss of cone function (Ross et al., 2020). Similar to human ACHM patients, the scotopic responses are unaffected (Shamir et al., 2010). In addition, a second Awassi sheep model was described by Gootwine and colleagues harboring a missense variant in CNGA3 (c.1618G>A/p.(Gly540Ser)) resulting in day blindness (Gootwine et al., 2017).
Finally, two naturally occurring canine models of CNGA3-associated channelopathy were described. German Shepherds were identified to carry the missense variant c.1270C>T/p.(Arg424Trp) homozygously in the canine CNGA3 gene whereas Labrador Retrievers presented with a three-nucleotide deletion in exon 7 of CNGA3 (c.1931_1933del/p.(Val644del)) in a homozygous state (Tanaka et al., 2015). Both canine models were characterized by a complete loss of cone function at 8–10 weeks of age, further underlined by the lack of light-induced cone responses under photopic conditions. In contrast, the scotopic ERG remained unaffected indicating normal rod function (Tanaka et al., 2015).
6 CONCLUSIONS AND FUTURE DIRECTIONS
In conclusion, the present study provides a comprehensive overview of the CNGA3 variant spectrum by combining variants identified in our large cohort of 889 independent families with ACHM, including variants retrieved from 216 unpublished families, as well as all previously published CNGA3 variants, both in literature and mutation databases. We thereby expand the allele spectrum of CNGA3 variants by 48 novel “likely disease-causing” variants to a total of 316 variants emphasizing the role of CNGA3 as a major disease-causing gene for ACHM. We further provide data on the extensive in silico analysis demonstrating the importance of in silico variant assessment and provide ACMG/AMP classification for 312 CNGA3 variants. Furthermore, we summarize clinical and diagnostic aspects of ACHM and provide an overview of several animal models of CNGA3-associated ACHM. However, our study demonstrates the need for functional assays to finalize the pathogenicity assessment of variants still assigned as “VUS.” This would contribute to the improvement of genetic diagnostics and facilitate the selection of patients with CNGA3-associated ACHM eligible for future gene therapeutic trials.
WEB RESOURCES
Mutalyzer name checker tool: https://mutalyzer.nl/name-checker
PubMed®: https://pubmed.ncbi.nlm.nih.gov/
Human Gene Mutation Database (HGMD®): http://www.hgmd.cf.ac.uk/ac/index.php
Leiden Open Variation Database (LOVD) for CNGA3: https://www.lovd.nl/CNGA3
ClinVar: https://www.ncbi.nlm.nih.gov/clinvar/
Combined Annotation Dependent Depletion (CADD): https://cadd.gs.washington.edu/
Sorting Intolerant From Tolerant (SIFT): http://sift.bii.a-star.edu.sg/
MutationTaster: http://www.mutationtaster.org/
Align-GVGD: https://agvgd.iarc.fr/index.php
Clustal Omega: https://www.ebi.ac.uk/Tools/msa/clustalo/
BoxShade version 3.21: https://embnet.vital-it.ch/software/BOX_form.html
Genome Aggregation Database (gnomAD): https://gnomad.broadinstitute.org/
SpliceAI: https://spliceailookup.broadinstitute.org/
Franklin by Genoox Ltd: https://franklin.genoox.com/
ACKNOWLEDGMENTS
The authors would like to thank all patients and their families for participating in this study. We would also like to acknowledge the contributions of Agnes Farkas (Budapest, Hungary) and the deceased Christian Hamel (Montpellier, France). Jana Moravíková performed segregation analysis in samples of Czech origin. This study was funded by the Deutsche Forschungsgemeinschaft as part of the SPP 2127: Gene and cell-based therapies to counteract neuroretinal degeneration, project numbers: KO 2176/3-1, 398539671 to S. Kohl and STI 727/1-1, 399487883 to K. Stingl. In addition, S. Kohl has received reimbursement for CNGA3 variant deposition at the LOVD, which is supported by a Research Core Award of the Foundation Fighting Blindness Inc. (award number BR-GE-0120-0775-LUMC) to J. T. den Dunnen, F. P. M. Cremers, I. F. A. C. Fokkema, and S. Roosing. S. Kohl, K. Stingl, I. Audo, I. Meunier, H. Dollfus, E. De Baere, P. Liskova, and B. Lorenz are members of ERN-EYE. This study was supported by the Instituto de Salud Carlos III (ISCIII) of the Spanish Ministry of Health (FIS PI19/00321). I. Audo was supported by LabEx LifeSenses, IHU FOReSIGHT, and Foundation Fighting Blindness grant (BR-GE-0619-0761-INSERM). Samples from I. Audo originate from NeuroSensCol, a biobank for research in neuroscience (PI: J. A. Sahel, coPI I. Audo, partner with Centre Hospitalier National d'Ophtalmologie des Quinze-Vingts, INSERM, and CNRS). The authors are thankful to the patients and family members participating in the study and clinical staff from the Centre d'Investigation Clinique CIC1438. P. L. was supported by the Ministry of Health of the Czech Republic (AZV NU20-07-00182 research grant). Open access funding enabled and organized by Projekt DEAL.
CONFLICTS OF INTEREST
S. H. Tsang is salaried by the National Institute of Health 5P30CA013696, U01 EY030580, R01EY033770, R01EY018213, R01EY024698, R24EY028758, R24EY027285, 5P30EY019007, U54OD020351, and R21AG050437. S. H. Tsang receives grant support from Abeona Therapeutics, Inc and Emendo. He is also the founder of Rejuvitas and is on the scientific and clinical advisory board for Nanoscope Therapeutics. The other authors declared no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in ClinVar at https://www.ncbi.nlm.nih.gov/clinvar/, reference number SCV001571262 to SCV001571322 and will be submitted to the Global Variome shared LOVD database at databases.lovd.nl/shared.




