Expression and functional characterization of missense mutations in ATP8A2 linked to severe neurological disorders
Abstract
ATP8A2 is a P4-ATPase (adenosine triphosphate) that actively flips phosphatidylserine and phosphatidylethanolamine from the exoplasmic to the cytoplasmic leaflet of cell membranes to generate and maintain phospholipid asymmetry. Mutations in the ATP8A2 gene have been reported to cause severe autosomal recessive neurological diseases in humans characterized by intellectual disability, hypotonia, chorea, and hyperkinetic movement disorders with or without optic and cerebellar atrophy. To determine the effect of disease-associated missense mutations on ATP8A2, we expressed six variants with the accessory subunit CDC50A in HEK293T cells. The level of expression, cellular localization, and functional activity were analyzed by western blot analysis, immunofluorescence microscopy, and ATPase activity assays. Two variants (p.Ile376Met and p.Lys429Met) expressed at normal ATP8A2 levels and preferentially localized to the Golgi-recycling endosomes, but were devoid of ATPase activity. Four variants (p.Lys429Asn, pAla544Pro, p.Arg625Trp, and p.Trp702Arg) expressed poorly, localized to the endoplasmic reticulum, and lacked ATPase activity. The expression of these variants was increased twofold by the addition of the proteasome inhibitor MG132. We conclude that the p.Ile376Met and p.Lys429Met variants fold in a native-like conformation, but lack key amino acid residues required for ATP-dependent lipid transport. In contrast, the p.Lys429Asn, pAla544Pro, p.Arg625Trp, and p.Trp702Arg variants are highly misfolded and undergo rapid proteosomal degradation.
1 INTRODUCTION
ATP8A2 is a member of the P4-ATPase subfamily of P-type ATPases which is highly enriched in brain, testes, retina, and spinal cord (Cacciagli et al., 2010; Coleman, Kwok, & Molday, 2009; Onat et al., 2012; Wang et al., 2018; Zhu et al., 2012). It uses the energy from adenosine triphosphate (ATP) hydrolysis to transport or flip phosphatidylserine (PS) and phosphatidylethanolamine (PE) from the exoplasmic to the cytoplasmic leaflet of biological membranes. This generates and maintains phospholipid asymmetry, a property that has been implicated in such cellular processes as vesicle trafficking, membrane curvature, axonal elongation, sensory physiology, and neural development and neuron survival (Andersen et al., 2016; Lopez-Marques, Theorin, Palmgren, & Pomorski, 2014; Sebastian, Baldridge, Xu, & Graham, 2012).
Like other P-type ATPases, ATP8A2 consists of a large catalytic or α-subunit nominally called ATP8A2 and a smaller accessory or β-subunit known as CDC50A or TMEM30A (Andersen et al., 2016). The α-subunit contains four functional domains: a nucleotide or N-domain that binds ATP; a phosphorylation or P-domain containing an aspartic acid residue within the conserved DKTGT motif that undergoes transient phosphorylation as part of the catalytic cycle; the actuator or A-domain harboring a DGET motif that functions in the dephosphorylation of the phosphorylated intermediate; and a membrane or M-domain consisting of 10 transmembrane segments that serves as the pathway for translocation of PS and PE across the cell membrane (Figure 1a). In addition, ATP8A2 has a relatively long C-terminal segment that has been implicated in the proper folding of ATP8A2 and regulation of its phospholipid flippase activity (Chalat, Moleschi, & Molday, 2017). The CDC50A β-subunit consisting of two transmembrane domains flanking a highly glycosylated exoplasmic loop plays an essential role in the proper folding of ATP8A2, its exit from the endoplasmic reticulum (ER), and the active transport of PS and PE across membranes (Coleman & Molday, 2011; Lenoir, Williamson, Puts, & Holthuis, 2009; Paulusma et al., 2008; van der Velden et al., 2010).
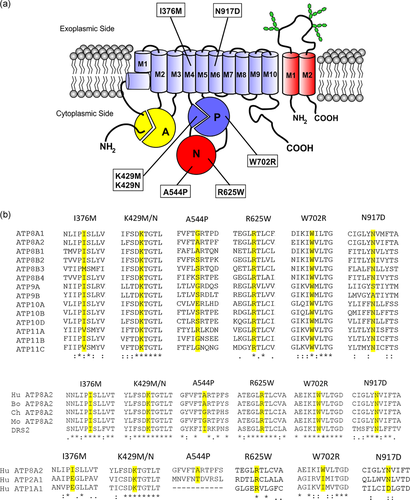
Topological model and sequence alignment of ATP8A2. (a) Schematic of the ATP8A2-CDC50A complex showing various domains and sites of disease-causing missense mutations. ATP8A2 domains include the membrane domain containing 10 transmembrane segments, an actuator or A domain, a phosphorylation or P domain, and a nucleotide-binding or N domain. The CDC50A subunit contains two transmembrane segments and a highly glycosylated exocytoplasmic domain. (b) The degree of conservation of residues (yellow/grey) replaced with disease-causing mutations is shown in multiple sequence alignments along with flanking amino acid residues for the 13 other human P4-ATPases and ATP8A2 from other species including bovine (Bo), chicken (CH), and mouse (Mo), Drs2 from yeast, and human ATP2A1 (SERCA) and ATP1A1 (Na/K ATPase). Multiple sequence alignments were performed using Clustral Omega with (*) designating conserved residues and (:) designating highly conserved residues. Mutations are: (p.I376M; p.K429M; p.K429N; p.A544P; p.R625W; p.W702R; p.N917D)
Mutations in the gene encoding ATP8A2 have been reported to cause severe autosomal recessive neurological diseases in rodents and humans. The wabbler-lethal (wl) mouse harboring a seven amino acid deletion in the N-domain of ATP8A2 and an Atp8a2 knockout mouse show progressive ataxia, pronounced neurodegeneration, and visual and auditory defects (Coleman et al., 2014; Zhu et al., 2012). Genetic analysis of four members of a consanguineous Turkish family led to the discovery that a p.Ile376Met (p.I376M) mutation in ATP8A2 caused cerebellar ataxia, mental retardation, and disequilibrium (CAMRQ) syndrome (Onat et al., 2012). More recently, missense, nonsense, and splice-site mutations have been found in individuals with intellectual disability, severe hypotonia, chorea, hyperkinetic movement disorders, and optic atrophy with or without cerebellar atrophy (Alsahli, Alrifai, Al Tala, Mutairi, & Alfadhel, 2018; Cacciagli et al., 2010; Martín-Hernández et al., 2016; McMillan et al., 2018).
The functional activity of two disease-associated variants in ATP8A2 (c1128C>G, p.Ile376Met (p.I376M) missense mutation in transmembrane segment M4 and c2749A>G, p.Asn917Asp (p.N917D) mutation in transmembrane segment M6) have been examined at a biochemical level in the bovine orthologue (Mikkelsen et al., 2019; Vestergaard et al., 2014; Figure 1). Both variants were found to be devoid of PS and PE activated ATPase activity (Mikkelsen et al., 2019; Vestergaard et al., 2014). The p.I376M variant was also shown to lack ATP-dependent phospholipid flippase activity. The expression and activity of other recently identified disease-causing missense mutations in ATP8A2, however, have not been examined. Here, we compare the expression, cellular localization, and functional activity of six disease-causing variants (c.1287G>T, p.Lys429Asn (p.K429N); c.1286A>T, p.Lys429Met (p.K429M); c.1630G>C, p.Ala544Pro (p.A544P); c.1873C>T, p.Arg625Trp (p.R625W); c.2104T>C p.Trp702Arg (p.W702R); and c.1128C>G, p.Ile376Met (p.I376M)) with normal ATP8A2.
2 MATERIALS AND METHODS
2.1 Materials
The following lipids were purchased from Avanti Polar lipids (Alabaster, AL): 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1, 2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), and 1,2-dioleoyl-sn-glycero-3-phosphoserine (DOPS), and egg PC (ePC). ATP and MG132 were purchased from MilliporeSigma (Oakville, ON), 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS) from Anatrace (Maumee, OH), Protease Arrest from G-Biosciences (St Louis, MO), and 1D4 peptide from Celtek Peptides (Franklin, TN). Rho1D4 antibody generated in-house (MacKenzie, Arendt, Hargrave, McDowell, & Molday, 1984) was purchased from UBC through Flintbox (www.rho1d4.com/) for the preparation of the immunoaffinity column. Primary antibodies against calnexin and β-tubulin were from Abcam (Toronto, ON); GM130 antibody was from MilliporeSigma (Oakville, ON); fluorescent-tagged secondary antibodies for immunofluorescence imaging were from Molecular Probes-Invitrogen (Carlsbad, CA); and anti-CDC50A monoclonal antibody Cdc50-7F4 was produced in-house as previously described (Coleman & Molday, 2011). Restriction enzymes, Instant sticky-end ligase, Antarctic Phosphatase, Phusion polymerase, and Q5 DNA polymerase were procured from New England Biolabs (Whitby, ON).
2.2 Generation of ATP8A2 mutant constructs
All variants were denoted based on the NCBI reference sequence for human ATP8A2 (NM_016529.6). Point mutations in ATP8A2 containing a sequence encoding the C-terminal 1D4 tag were introduced by using the Q5 site-directed mutagenesis kit (NEB E0552S) from New England Biolabs (Whitby, ON) with primers specific to each point mutation in ATP8A2. The mutated constructs were verified by DNA sequencing of the entire coding sequence and promoter region.
2.3 Expression and purification of ATP8A2 from HEK293T cells
HEK293T cells (American Type Culture Collection, Manassas, VA) were transfected in 10 cm dishes at 50% confluence using 5 μg of pcDNA3.1 containing the ATP8A2-1D4 construct and 5 μg pcDNA3.1 containing the CDC50A construct using 15 μg the polyethylenimine (PEI) as the transfection agent. For negative control experiments, cells were transfected with empty pcDNA3.1. The cells were harvested after 48 hr. For expression studies, cells were lysed with either 4% sodium dodecyl sulfate (SDS) or 20 mM CHAPS in 50 mM HEPES/NaOH, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM dithiothreitol (DTT) and 1× Protease arrest with stirring on a magnetic stirrer for 30 min at 4°C. The CHAPS solubilized samples were centrifuged at 40,000gav for 10 min to remove aggregated protein. Samples were analyzed on SDS polyacrylamide gels and on Western blots labeled for ATP8A2 and β-tubulin.
ATP8A2-CDC50A complex was purified from transfected HEK293T cells as previously described (Coleman & Molday, 2011). Briefly, cells overexpressing ATP8A2-CDC50A were harvested from culture plates and resuspended in 50 mM HEPES/NaOH, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM dithiothreitol (DTT), 20 mM CHAPS, 0.5 mg/ml DOPC, and 1× Protease Arrest (lysis buffer) with stirring for 30 min at 4°C. The CHAPS-insoluble pellet was removed by centrifugation at 40,000gav for 10 min. The supernatant was either directly analyzed by SDS buffer. After incubation on a rotator for 30 min at 4°C, the immunoaffinity matrix was washed six times each with 500 μl of buffer containing 50 mM HEPES/NaOH, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 1 mM DTT, 10 mM CHAPS, and 0.5 mg/ml ePC (column wash buffer). Bound protein was then eluted twice from the matrix each with 75 µl 1D4 elution buffer (column wash buffer containing 0.4 mg/ml 1D4 peptide) for 30 min at 18°C. The concentration of purified protein was measured on a Coomassie blue–stained sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel using bovine serum albumin of known concentrations as previously described (Coleman, Vestergaard, Molday, Vilsen, & Andersen, 2012). The Rho1D4 immunoaffinity column was prepared by coupling the Rho1D4 monoclonal antibody to CNBr-activated Sepharose 2B as described previously (Molday, Cook, Kaupp, & Molday, 1990).
2.4 RNA expression
HEK293T cells grown in six-well culture plates were transfected with 0.5 µg of the ATP8A2-1D4 variant and 0.5 µg of CDC50A per well as described above. Total RNA was extracted from the transfected or untransfected control cells using RNeasy Mini Kit (Qiagen, Toronto, ON). One microgram of RNA was reverse-transcribed using the QuantiTect Reverse Transcription Kit (Qiagen, Toronto, ON). For conventional reverse-transcription polymerase chain reaction (RT-PCR), ATPA2 was amplified using the forward primer: 5′-CTCTTCCCCAGATGAAGCTG-3′ and reverse primer: 5'GATACCAATCGGCAGGAATACC-3′ to generate a 600 bp fragment and the loading control glyceraldehyde 3-phosphate dehydrogenase glyceraldehyde gene (GAPDH) was amplified using: forward primer: 5′-GGAGCCAAAAGGGTCATCAT-3′ and reverse primer: TTGAAGTCAGAGGAGACCAC-3′ using Taq Polymerase for analysis on a 1.5% agarose gel. PCR conditions for ATP8A2 were as follows: 95°C 30 s followed by 30 cycles of 95°C for 30 s, 53°C for 45 s, and 68°C for 45 s and finally 68°C for 5 min. mRNA expression was further analyzed by quantitative polymerase chain reaction (qPCR) for ATP8A2 (forward primer: 5′-CCTTCAAGCAGGAGTTCCAG-3′ and reverse primer: 5′-GATACCAATCGGCAGGAATACC-3′) and GADPH (forward primer: 5′-TGCCGTCTAGAAAAACCTGC-3′ and reverse primer: TTGAAGTCAGAGGAGACCAC-3′). The primers efficiency was tested for to use 1/10 dilution for cDNA originally at ~1 µg. All qPCR reactions were performed in 20 µl mixture containing 2 µl of 10× ThermoPol buffer, 0.4 µl of 10 mM dNTPs, 9 µl dH2O, 0.5 µl 20× SYBR Green I Invitrogen, S7585; Carlsbad, CA), 0.1 µl of Taq polymerase (M0267L; New England Biolabs, Whitby, ON), 1 µl 1/10 dilution cDNA, and 5 µl of 5 µM forward and reverse primers.
2.5 ATPase activity assay
The ATPase activity of immunoaffinity purified ATP8A2 variants was measured as previously described (Coleman et al., 2009) using a colorimetric method (Chifflet, Torriglia, Chiesa, & Tolosa, 1988). Briefly, ∼1 ng of purified protein was mixed with 5 mM ATP and 2.5 mg/ml lipid (a mixture of DOPC and DOPS combined at different predetermined ratios) to obtain a final volume of 25 μl. Both ATP and lipids were prepared in ATPase assay buffer containing 50 mM HEPES/NaOH, pH 7.5, 150 mM NaCl, 12.5 mM MgCl2, 1 mM DTT, and 10 mM CHAPS. The reaction was carried out at 37°C for 30 min and terminated by the addition of 25 μl of 12% SDS. The phosphate released from ATP hydrolysis was measured by treating the reaction mix with 75 μl of solution I (6% ascorbic acid, 1% ammonium molybdate in 1 N HCl) for 5 min, followed by 120 μl of solution II (2% sodium citrate, 2% sodium meta-arsenite, 2% acetic acid). The intensity of the resultant color was measured from the absorbance at 850 nm in a microplate reader. This measured intensity was compared with those of known phosphate concentrations on a standard curve to calculate the reaction velocity (micromoles of Pi released/minute/milligram protein) for each PS concentration used in the assay. The resultant data were fitted to a Michaelis–Menten equation to calculate the maximum reaction velocity (Vmax) and PS-activation constant (Ka). Each measurement was recorded in triplicate, and every experiment was repeated at least four times independently.
2.6 SDS-PAGE and western blot analysis
Proteins were separated by SDS gel electrophoresis on 9% polyacrylamide gels and either stained with Coomassie Blue or transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA) in buffer containing 25 mM Tris, 192 mM glycine, 10% methanol, pH 8.3. Membranes were blocked with 1% milk in phosphate-buffered saline (PBS) for 45 min and incubated with hybridoma cell culture fluid containing either the Rho1D4 or Cdc50-7F4 monoclonal antibodies. The Rho1D4 hybridoma culture fluid was diluted 1:100 and the Cdc50-7F4 culture fluid was diluted 1:13 in PBS. In some cases, the blots were also labeled with a rabbit anti-tubulin antibody as a loading control. The blots were labeled for 40 min, washed stringently with PBST (PBS containing 0.05% Tween 20), incubated for 40 min with secondary antibody (goat anti-mouse conjugated with IR dye 680 or donkey anti-rabbit conjugated with IR dye 800 (LI-COR, Lincoln, NE)) diluted 1:20,000 in PBST containing 0.5% milk, and washed with PBST before data collection on a LI-COR Odyssey infrared imaging system (LI-COR, Lincoln, NE).
2.7 Immunofluorescence microscopy
COS7 cells grown on glass coverslips in six-well tissue culture plates were cotransfected at 50% confluence with 1.25 µg of ATP8A2-1D4 and 1.25 µg of CDC50A plasmids using the calcium phosphate method. After 48 hr, the cells were washed in PBS and fixed with 4% paraformaldehyde for 15 min. After blocking and permeabilization for 1 hr with 10% normal goat serum (NGS) and 0.2% Triton X-100, cells were treated for 2 hr with primary antibody diluted in 100 mM phosphate buffer (pH 7.4) containing 2.5% NGS and 0.05% Triton X-100. The primary antibodies were Rho1D4 hybridoma culture fluid (1:50 dilution); Cdc50-7F4 hybridoma culture fluid (1:12 dilution); rabbit polyclonal anti-calnexin antibody (1:2,000 dilution) or rabbit anti-GM130 antibody (1:2,000). The coverslips were washed and labeled with goat anti-mouse immunoglobulin tagged with Alexa 488 and goat anti-rabbit immunoglobulin tagged with Alexa 594 for 0.5 hr and labeled with 4′,6-diamidino-2-phenylindole (DAPI) nuclear stain. Coverslips were washed and mounted with Mowiol medium on glass slides before imaging. The reagents for fixing, permeabilizing, and labeling were prepared in 100 mM phosphate buffer (pH 7.4). The secondary antibodies were used at 1:1,000 dilution. Fluorescence images were acquired using a 40 × objective with a numerical aperture of 0.8 on a Zeiss LSM 700 confocal microscope equipped with Zen Image analysis software (Carl Zeiss Canada, Toronto, ON). Composite figures were prepared using Photoshop software. Colocalization of ATP8A2 variants to the ER and Golgi complex was carried out using Coloc2 plugin from ImageJ (National Institutes of Health, Bethesda, MD, https://imagej.nih.gov/ij/, 1997-2018). Cells which were not labeled with the Rho1D4 antibody were not considered for analysis. At least 15 cells were analyzed to obtain colocalization data for obtaining Manders colocalization coefficient (Dunn, Kamocka, & McDonald, 2011).
2.8 MG132 inhibitor studies
HEK293T cells were grown for 48 hr after transfection and cell culture media was replaced with fresh media. After 15 min, 10 µl of 1 mM MG132 was added to each plate to obtain a final concentration of 10 µM inhibitor. The cells were incubated for 5 hr at 37oC and 5% CO2, before harvesting the cells for solubilization in SDS or CHAPS detergent as described above.
3 RESULTS
3.1 Sequence alignment of disease-associated mutations
To date, seven missense mutations in ATP8A2 have been reported to cause severe neurological disorders in humans (Alsahli et al., 2018; Martín-Hernández et al., 2016; McMillan et al., 2018). These include p.K429M, p.K429N, and p.W702R mutations in the phosphorylation domain (P domain), p.A544P and p.R625W mutations in the nucleotide-binding domain (N domain), and the p.I376M and p.N917D mutations in the membrane domain of ATP8A2 (Figure 1a). Sequence alignments show that these substitutions generally occur in residues that are highly conserved in the other 13 members of the human P4-ATPase subfamily, vertebrate orthologues of ATP8A2, and the yeast homologue Drs2 with the exception of alanine in position 544 which shows a high degree of sequence variation (Figure 1b). More limited conservation is observed for these residues and flanking sequences in other P-type ATPases with the exception of lysine at position 429 within the DKTGTLT motif which is highly conserved in other P-type ATPases including the Na/K ATPase α-subunit (ATP1A1) and SERCA (ATP2A1) (Andersen et al., 2016; Lopez-Marques et al., 2014).
3.2 Expression of ATP8A2 disease-causing missense mutations
To determine the effect of the disease-associated mutations on the expression of ATP8A2, we coexpressed ATP8A2 and six variants p.K429M, p.K429N, p.A544P, p.R625W, p.W702R, and p.I376M all containing a C-terminal 1D4 tag together with the β-subunit CDC50A in HEK293T cells. The cells were treated with either SDS, a strong detergent which solubilizes essentially all membrane proteins, or CHAPS, a mild detergent widely used to solubilize and purify membrane proteins for functional characterization. The expression levels of the variants were then analyzed on western blots labeled for ATP8A2. The p.I376M and p.K429M variants were detected at levels above control ATP8A2 in cell extracts solubilized in SDS (Figure 2a). In contrast, the p.K429N, p.R625W, and p.W702R variants expressed at low levels, less than 25% control ATP8A2, and the p.A544P variant was barely detectable. The profile of the variants solubilized in CHAPS is shown in Figure 2b. The p.I376M and p.K429M variants were detected at a level comparable to WT ATP8A2, whereas the p.K429N mutant was present at 10% control ATP8A2. The p.A544P, p.R625W, and p.W702R mutants were present at exceedingly low levels limiting the molecular characterization of these mutants.
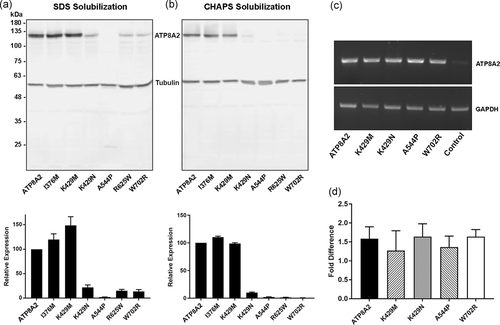
The effect of disease-causing mutations on ATP8A2 expression and solubility. (a) Upper panel: An example of a western blot labeled for ATP8A2 showing the expression profiles of nonmutated control ATP8A2 (ATP8A2) and the different ATP8A2 variants (p.I376M; p.K429M; p.K429N; p.A544P; p.R625W; p.W702R) expressed in HEK293T cells after solubilization in sodium dodecyl sulfate. Tubulin was used as a loading control for the cell lysate. Lower panel: Quantification of disease-associated mutants relative to control ATP8A2. Data show the mean ± SD for n = 10. (b) Upper panel: Western blot labeled for ATP8A2 shows the expression profiles of the different ATP8A2 variants expressed in HEK293T cells after solubilization in CHAPS. Lower panel: Quantification of disease-associated mutants relative to nonmutated control ATP8A2. Data show the mean ± SD for n = 10. (c) mRNA expression for ATP8A2 variants by reverse-transcription polymerase chain reaction (RT-PCR). GAPDH was used as loading control. Control lane is for RNA expression from nontransfected HEK293T cells. (d) mRNA expression of ATP8A2 variants as measured by real-time PCR. Data show the mean ± SD for n = 3. Differences in expression of the variants relative to the nonmutated ATP8A2 were not significant (p > 0.10). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; mRNA, messenger RNA; standard deviation SD
To determine if the low expression of some disease-associated variants was due to RNA instability, we compared the mRNA levels of transfected ATP8A2 with those of the disease-associated variants. As shown in Figure 2c, the mRNA levels as measured by RT-PCR were similar for all the ATP8A2 constructs. These results were confirmed quantitatively using real-time qPCR in which small differences observed was not statistically significant (Figure 2d). These results show that the low level of protein expression of some disease-associated variants was not due to RNA instability.
3.3 Localization of ATP8A2 variants by immunofluorescence microscopy
The low expression levels of certain ATP8A2 mutants could arise in part from the retention of misfolded protein in the endoplasmic reticulum (ER) by the quality control system of the cell for eventual proteolytic degradation. To address this possibility, we expressed ATP8A2 and the various mutants together with CDC50A in COS7 cells for analysis by immunofluorescence microscopy using anti-GM130 and anticalnexin antibodies as markers for Golgi-recycling endosomes and the ER, respectively. Typical micrographs of labeled cells are shown in Figure 3. As previously reported (Coleman & Molday, 2011), ATP8A2 showed significant colocalization with GM130. The p.I376M and p.K429M variants also showed a high degree of colocalization with GM130, while the p.K429N, p.R625W, and p.W702R variants preferentially colocalized with calnexin in the ER. The p.A544P mutant could not be confidently localized by immunofluorescence microscopy due to the low number of cells expressing this variant.
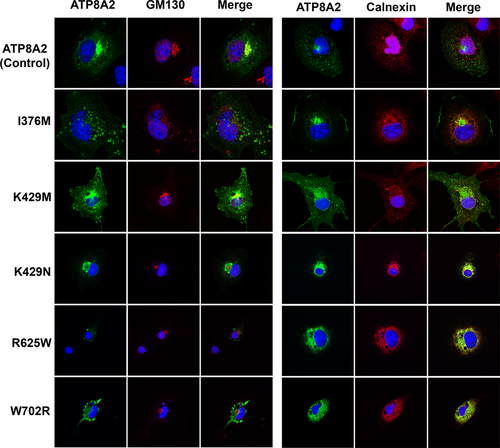
Immunofluorescence localization of ATP8A2 variants. COS7 cells were cotransfected with nonmutated control ATP8A2 or ATP8A2 variants (p.I376M; p.K429M; p.K429N; p.R625W; p.W702R) and CDC50A. Fixed and permeabilized transfected cells were labeled with the Rho1D4 monoclonal antibody for the detection of ATP8A2 variants (green) and either the Golgi marker GM130 or the ER marker calnexin (red) and counter stained with the nuclear stain DAPI (blue) for analysis by confocal scanning microscopy. DAPI, 4′,6-diamidino-2-phenylindole; ER, endoplasmic reticulum
The extent of colocalization of the ATP8A2 variants with the GM130 and calnexin was further investigated using the Manders split coefficient analysis with ATP8A2 as a reference (Dunn et al., 2011). As summarized in Table 1, the p.I376M and p.K429M variants showed relatively high colocalization with the GM130 and lower colocalization with calnexin, whereas the p.K429N, p.R625W, and p.W702R variants showed the reverse. The preferential localization of the p.I376M and p.K429M variants in the Golgi-recycling endosomal compartment correlated with their relatively high level of expression observed after CHAPS detergent solubilization (Table 1).
| ATP8A2 variant | Colocalization with GM130 | Colocalization with calnexin | Ratio GM130/calnexin | % Relative colocalization | % CHAPS expression |
|---|---|---|---|---|---|
| ATP8A2 | 28.30 ± 4.2 | 48.90 ± 6.6 | 0.58 | 100 | 100 |
| p.I376M | 37.90 ± 7.5 | 57.40 ± 4.1 | 0.66 | 114.1 | 110.4 |
| p.K429M | 30.60 ± 9.4 | 58.20 ± 7.3 | 0.53 | 90.8 | 98.9 |
| p.K429N | 6.30 ± 2.7 | 79.80 ± 5.0 | 0.08 | 13.6 | 10.0 |
| p.R625W | 1.30 ± 1.9 | 83.80 ± 9.5 | 0.02 | 2.7 | 1.5 |
| p.W702R | 0.95 ± 1.2 | 78.10 ± 7.1 | 0.01 | 2.1 | 0.6 |
- Note: Normal ATP8A2 and disease-causing variants were coexpressed with CDC50A. Manders coefficient colocalization of ATP8A2 variants with the GM130 Golgi marker and calnexin ER marker was determined by ImageJ plugin Coloc2 relative to normal ATP8A2. Percent expression of the ATP8A2 variants solubilized in CHAPS detergent was obtained from Western blots labeled for ATP8A2.
3.4 Purification and functional activity of the ATP8A2 disease variants
For functional analysis, ATP8A2 and the p.I376M, p.K429M, and p.K429N mutants were coexpressed with CDC50A in HEK293T cells, solubilized in CHAPS detergent, and purified on a Rho1D4-Sepharose immunoaffinity matrix. Figure 4a shows a SDS gel stained with Coomassie blue and western blots labeled for ATP8A2 and CDC50A. All variants migrated as a single Coomassie blue stained protein with an apparent molecular mass of 129 kDa corresponding to ATP8A2 as confirmed by western blot analysis. The CDC50A subunit was not evident on the Coomassie blue-stained gel due to the high content and heterogeneous nature of glycosylation of this subunit. However, this subunit coimmunoprecipitated with the ATP8A2 variants as revealed by the broad band observed on western blots labeled for CDC50A (Figure 4a). These studies indicate that the ATP8A2 variants assembled with CDC50A as a heteromeric complex.
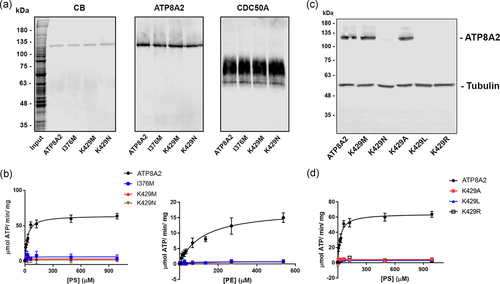
Purification and ATPase activity of ATP8A2 variants. ATP8A2 and ATP8A2 variants (p.I376M; p.K429M; p.K429N; p.K429A; p.K429L; p.K429R) coexpressed with CDC50A were solubilized in CHAPS detergent and purified on a Rho1D4 immunoaffinity column for the analysis of their ATPase activity. (a) SDS gel stained with Coomassie blue (CB) and western blots labeled for ATP8A2 with the Rho1D4 antibody and CDC50A with the Cdc50-7F4 monoclonal antibody. (b) ATPase activity as a function of increasing phosphatidylserine (PS) or phosphatidylethanolamine (PE). (c) The expression of various variants in which lysine at position 429 was replaced with the indicated amino acid. β-Tubulin was used as a loading control. (d) ATPase activity of the ATP8A2 and variants as a function of increasing PS. None of the mutants showed detectable ATPase activity relative to control ATP8A2. ATP, adenosine triphosphate; SDS, sodium dodecyl sulfate
The ATPase activities of the purified ATP8A2 variants were measured as a function of increasing PS or PE (Figure 4b). As previously reported for bovine ATP8A2 (Coleman et al., 2009; Vestergaard et al., 2014), both PS and PE strongly activated the ATPase activity of human ATP8A2. The activation constants (Ka) were 24.9 µM for PS and 117 µM for PE. In contrast, the p.K429M and p.K429N variants like the p.I376M variant were devoid of activity. The p.A544P, p.R625W, and p.W702R mutants expressed at levels too low for reliable purification and functional analysis.
The lysine residue at position 429 is located within the conserved sequence DKTGTLT. To further determine the importance of lysine at position 429, we replaced this residue with amino acids having different properties including alanine with a small side chain (p.K429A), leucine with a bulky hydrophobic side chain (p.K429L), and arginine with a positively charged side chain (p.K429R). As shown in Figure 4c, only the p.K429A mutant expressed at control ATP8A2 levels. By increasing the number of transfected cells, sufficient protein could be isolated to measure the ATPase activity of the low expressing p.K429L and p.K429R variants. These variants lacked PS-activated ATPase activity.
3.5 The effect of the proteasome inhibitor MG132 on the expression of ATP8A2 disease-causing variants
Most of the ATP8A2 variants examined in this study expressed at exceedingly low levels. This suggests that these variants are highly misfolded such that they undergo rapid degradation in the cell. A major mechanism to degrade misfolded proteins in cells is through the proteasome (Bard et al., 2018). This was investigated by treating transfected HEK293T cells with or without the proteasome inhibitor MG132, and subsequently comparing the level of expression of the ATP8A2 variants by western blot analysis after solubilization with SDS or CHAPS. As shown in Figure 5a,b, the p.K429N, p.A544P, p.R625W, and p.W792R variants generally showed a twofold increase in expression for cells solubilized in SDS after treatment with MG132. In contrast, no significant increase in protein levels was observed when treated cells were solubilized with CHAPS. These studies are consistent with the effect of MG132 in inhibiting the proteasome degradation of highly misfolded protein observed after SDS solubilization. These misfolded proteins most likely aggregate in CHAPS and are removed by high-speed centrifugation before analysis by western blot analysis.
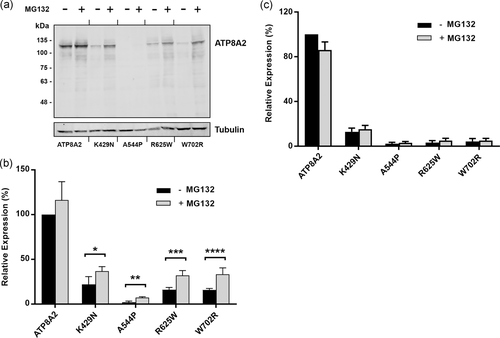
The effect of the proteasome inhibitor MG132 on the expression of ATP8A2 variants. HEK293T cells cotransfected with the ATP8A2 variants and CDC50A were incubated in the presence or absence of MG132. The cells were then solubilized in SDS or CHAPS and the expression level of the ATP8A2 was determined by western blot analysis. (a) Typical western blot of ATP8A2 and variants (p.K429N; p.A544P; p.R625W; p.W702R) labeled with the Rho1D4 antibody for the detection of ATP8A2 and anti-β-tubulin antibody as a loading control. (b) The expression of ATP8A2 variants solubilized in SDS was quantified from western blots. Data are represented as mean ± SD for n = 4. Difference in expression of ATP8A2 in the presence and absence of MG132 is not significant. p values for ATP8A2 variants ± MG132 are: *p.K429N = 0.027; **p.A544P = 0.0008; ***p.R625W = 0.002; and ****p.W702R = 0.0029. (c) Quantification of ATP8A2 expression for variants solubilized in CHAPS is shown. Data is the mean ± SD for n = 4. The difference in expression of variants ± MG132 was not significant for CHAPS studies. CHAPS, 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate
4 DISCUSSION
In this study, we analyzed the expression and phospholipid-dependent ATPase activity of six missense variants in ATP8A2 that have been recently reported to cause severe neurological disorders characterized by intellectual disability, hypotonia, and hyperkinetic movement disorders, with or without cerebellar and optic nerve atrophy. These variants can be separated into two categories based on their effect on protein expression.
Four disease mutants (p.K429N, p.A544P, p.R625W, and p.W702R) expressed at very low levels indicating that these amino acid replacements caused significant protein misfolding leading to rapid degradation in cells. This is supported by immunofluorescence microscopy showing that these ATP8A2 mutants are retained in the ER by the quality control system of the cell (Figure 3 and Table 1) and inhibitor studies showing increased expression of protein variants from cells treated with the proteasome inhibitor MG132 and solubilized in SDS (Figure 5). In an earlier study, the expression of missense mutations in the P4-ATPase ATP8B1 that cause progressive familial intrahepatic cholestasis Type 1 and benign recurrent intrahepatic cholestasis type 1 was examined (Folmer, van der Mark, Ho-Mok, Oude Elferink, & Paulusma, 2009). The level of expression of these disease variants was significantly lower than the nonmutated protein. Furthermore, the addition of MG132 enhanced the expression level of these variants as found in our studies.
Two disease-associated ATP8A2 variants (p.I376M and p.K429M), on the other hand, expressed at or near normal ATP8A2 levels. Like the non-mutated ATP8A2, these variants preferentially localized to the Golgi/recycling endosomal system in cells indicating that these amino acid substitutions did not affect the global folding or stability of the protein. However, these mutants were devoid of functional activity as measured by PS and PE stimulated ATPase activity (Figure 4). The p.I376M has been shown previously to be devoid of ATP-dependent phospholipid flippase activity as well as PS and PE stimulated ATPase activity (Lee et al., 2015; Vestergaard et al., 2014). The p.N917D disease-causing mutation (p.N905D in the bovine orthologue) can be added to this category since it expresses at a level comparable to non-mutated ATP8A2 but lacks ATPase activity (Mikkelsen et al., 2019). Although these two categories of mutations display significant differences in levels of protein expression, they both can be considered as loss-in-function mutations since neither category displayed phospholipid activated ATPase activity. This is in agreement with clinical studies in which patients with these different missense mutations all show similar severe neurological phenotypes as found in patients homozygous for premature stop codons and frameshift mutations in the ATP8A2 gene (Alsahli et al., 2018; Martín-Hernández et al., 2016; McMillan et al., 2018; Onat et al., 2012).
The disease-causing missense mutations with the exception of p.A544P are located in highly conserved regions of ATP8A2. The p.I376M and p.N917D mutations are present in transmembrane segments 4 and 6, respectively. Mutational studies together with molecular modeling and molecular simulation indicate that isoleucine 376 may be part of a hydrophobic gate that controls the translocation of PS and PE through a groove formed by transmembrane segments M1, M2, M4 and M6 of ATP8A2 (Andersen et al., 2016; Vestergaard et al., 2014). Asparagine 917, on the other hand, plays a crucial role in phospholipid induced dephosphorylation of ATP8A2 during phospholipid translocation (Mikkelsen et al., 2019). The p.K429M, p.K429N, p.A544P, p.R625W, and p.W702R mutations are located within domains important for the catalytic cycle. Lysine 429 is part of the highly conserved DK429TGTLT motif within the P-domain and adjacent to aspartic acid 428 which undergoes transient phosphorylation during the ATPase catalytic cycle. Replacement of lysine 429 with amino acids varying in side chains including methionine, asparagine, alanine, leucine, and arginine all resulted in proteins devoid of ATPase activity indicating that the lysine residue plays a crucial role in the function of ATP8A2 as a phospholipid transporter. Interestingly, only alanine and methionine substitutions yielded proteins with expression levels comparable to the non-mutated protein. Previous studies have investigated the importance of the corresponding lysine residue in the DKTGTLT motif of the Ca-ATPase SERCA (Maruyama et al., 1989; McIntosh, Woolley, MacLennan, Vilsen, & Andersen, 1999). These studies found that substitutions in this lysine residue resulted in an inactive protein and substitution of lysine for arginine resulted in low SERCA expression consistent with our studies on ATP8A2. The SERCA crystal structure with bound nucleotide shows that this lysine is critical to ATP binding. Its interaction with the oxygen of the juxtaposed threonine side-chain positions the latter for binding the gamma-phosphate of the nucleotide (Toyoshima & Mizutani, 2004). Arginine 625 corresponds to an arginine shown in both SERCA and Na, K-ATPase to be crucial for interaction with the β-phosphate of the bound ATP (Clausen, McIntosh, Vilsen, Woolley, & Andersen, 2003; Jacobsen, Pedersen, & Jorgensen, 2002; Toyoshima & Mizutani, 2004). Moreover, tryptophan 702 corresponds to an isoleucine in SERCA and Na, K-ATPase that is located in a β-strand that leads right into the highly conserved threonine-glycine-aspartate motif that contributes to the binding of the γ-phosphate of ATP (Toyoshima & Mizutani, 2004). Replacement of the tryptophan is thus also predicted to disturb ATP binding. It seems no coincidence that three of the disease-causing mutations studied here interfere with ATP binding, suggesting that the misfolding of the mutant proteins could be a consequence of the inability to bind ATP.
Although severe neurological phenotypes including hypotonia, cognitive impairment, and hyperkinetic movement disorders have been observed in all reported patients with mutations in ATP8A2, some phenotypic traits such as progressive optic atrophy, hearing loss, dystonia, and cerebral and cerebellar atrophy were only observed in some patients (McMillan et al., 2018). Variations in these clinical features do not appear to be associated with differences in the activity of the ATP8A2 variants since all mutations result in a loss-in-function protein. Instead, clinical variations may be due to other genetic differences in patients. One possibility is that other P4-ATPases with similar substrate specificities as ATP8A2 compensate for the loss in function of ATP8A2 in certain tissues. ATP8A1, ATP11A, ATP11B and ATP11C like ATP8A2 actively flip PS and PE across cell membranes (Liou, Molday, Wang, Andersen, & Molday, 2019; Takatsu et al., 2014; Wang et al., 2018). Of these P4-ATPases, ATP8A1 is most similar to ATP8A2 sharing 67% identity in sequence to ATP8A2 and it is widely expressed in various tissues. The overlapping function of ATP8A2 and ATP8A1 is inferred from the analysis of knockout mice. ATP8A1 deficient mice appear generally normal except for aberrant hippocampus-dependent behavior. ATP8A2 deficient mice which can live up to 6 month display distal axonal degeneration in spinal motor neurons, degeneration of photoreceptors, optic and spiral ganglion cells (Coleman et al., 2014; Zhu et al., 2012). Loss of both P4-ATPases results in neonatal lethality in mice (Zhu et al., 2012).
Although ATP8A2 is known to transport PS and PE to generate membrane lipid asymmetry (Coleman et al., 2009), its role in neuronal cells is not well understood. However, previous studies have shown that ATP8A2 promotes axonal elongation in PC12 cells and hippocampal neurons (Xu et al., 2012). Furthermore, trafficking of transferrin receptors to the plasma membrane of neurons isolated from Atp8a2 knockout mice is significantly reduced (Lee et al., 2015). These studies suggest that ATP8A2 may function in the trafficking of key neuronal cell surface proteins via the endosomal recycling system. Interestingly, mutations in very-low-density lipoprotein receptor (VLDLR) found on the surface of neuronal cells, like mutations in ATP8A2, are known to cause cerebellar ataxia, mental retardation, and disequilibrium (CAMRQ) syndrome (Moheb et al., 2008). Hence ATP8A2 may play a key role in the trafficking of VLDLR to cell surfaces such that either reduction in the function of ATP8A2 or VLDLR may result in similar disease phenotypes. Further studies are required to determine if ATP8A2 and VLDLR are linked through the same cellular trafficking pathway in neuronal cells.
ACKNOWLEDGMENTS
The authors would like to thank Alex Harrison and Laurie Molday with technical assistance and Dr. Eric Jan for helpful discussion related to mRNA expression. This study was supported by Canadian Institutes of Health Research Grant PJT 148649 and National Institutes of Health Grant EY002422 (RSM). Danish Council for Independent Research grant DFF-6110-00271 (JPA).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
DATA ACCESSIBILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.




