TAB2 c.1398dup variant leads to haploinsufficiency and impairs extracellular matrix homeostasis
Abstract
Transforming growth factor β-activated kinase 1 (TAK1) mediates multiple biological processes through the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and the mitogen-activated protein kinase (MAPK) signaling pathways. TAK1 activation is tightly regulated by its binding partners (TABs). In particular, binding with TAB2 is crucial for cardiovascular development and extracellular matrix (ECM) homeostasis. In our previous work, we reported a novel multisystem disorder associated with the heterozygous TAB2 c.1398dup variant. Here, we dissect the functional effects of this variant in order to understand its molecular pathogenesis. We demonstrate that TAB2 c.1398dup considerably undergoes to nonsense-mediated messenger RNA decay and encodes a truncated protein that loses its ability to bind TAK1. We also show an alteration of the TAK1 autophosphorylation status and of selected downstream signaling pathways in patients’ fibroblasts. Immunofluorescence analyses and ECM-related polymerase chain reaction-array panels highlight that patient fibroblasts display ECM disorganization and altered expression of selected ECM components and collagen-related pathways.
In conclusion, we deeply dissect the molecular pathogenesis of the TAB2 c.1398dup variant and show that the resulting phenotype is well explained by TAB2 loss-of-function. Our data also offer initial insights on the ECM homeostasis impairment as a molecular mechanism probably underlying a multisystem disorder linked to TAB2.
1 INTRODUCTION
TAK1 signaling is linked with major proinflammatory, proapoptotic, and profibrotic pathways, such as the mitogen-activated protein kinase (MAPK)-p38 and MKK4-C-Jun N-terminal kinase (JNK) signaling, as well as with the cytoprotective autophagy process (Kim et al., 2012). TAK1 activation is tightly regulated by the formation of a complex with TAK1-binding proteins (TABs) 1 (TAB1), 2 (TAB2), and 3 (TAB3). The TAK1-TABs complex is crucial for connective tissue and, in particular, extracellular matrix (ECM) homeostasis, as TAK1 signaling stimulates type I and IV collagen and fibronectin (FN) production in mesangial cells and fibroblasts (Chin et al., 2001; Hocevar, Prunier, and Howe, 2005; Kim et al., 2007; Ono, Ohtomo, Ninomiya-Tsuji, and Tsuchiya, 2003; Wang, Ma, Flavell, and Choi, 2002). In addition, TAK1-null phenotype is lethal early in mice embryos (Shim et al., 2005) and this corroborates a key role of the TAK1 signaling in development.
Among the TABs, TAB2 acts as an adapter protein in TAK1 activation by linking TAK1 to TNF receptor-associated factor 6 (TRAF6; Walsh, Kim, Maurizio, Molnar, and Choi, 2008). TAB2 may also play alone as an inhibitor of endogenous autophagy by directly inactivating Beclin1 (Takaesu, Kobayashi, and Yoshimura, 2012), an autophagy-related protein (Kang, Zeh, Lotze, and Tang, 2011), in unstimulated conditions. Similarly to TAK1, TAB2 also has a role in early development, since tab2 haploinsufficiency causes developmental defects in zebrafish embryos (Thienpont et al., 2010). Tab2 expression is ubiquitous during early development and subsequently shows a more restricted pattern in vasculogenesis, as it is particularly expressed in the developing cardiac outflow tract, dorsal aorta, and posterior cardinal vein (Thienpont et al., 2010). In humans, TAB2 haploinsufficiency has been proposed as the master molecular finding in the recurrent 6q25.1 microdeletion syndrome, which is characterized by cardiomyopathy, congenital heart defect, and satellite features (Cheng et al., 2017). In addition, rare TAB2 point variants have been associated with frontometaphyseal dysplasia type 3 (Wade et al., 2016) and isolated cardiomyopathy or congenital heart defects (Caulfield et al., 2018; Thienpont et al., 2010).
We recently described a multigeneration family with the novel TAB2 heterozygous c.1398dup variant and a systemic phenotype resembling in part the 6q25.1 microdeletion syndrome (Ritelli et al., 2018). The observed phenotype recapitulates the multiple biological functions of TAB2, as this family shows a protean cardiac phenotype coupled with multiple connective tissue features mirroring a perturbed ECM homeostasis.
In this work, we explored, at the molecular level, the effects of the TAB2 c.1398dup variant. We found that TAB2-truncated protein impairs its ability to physically bind TAK1, which, in turn, is demonstrated to alter the activity of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and mitogen-activated protein kinase (MAPK) signaling. By employing multiple investigative strategies, we also showed that patients’ fibroblasts displayed altered ECM homeostasis.
2 MATERIALS AND METHODS
2.1 Patients and samples
This study focused on the TAB2 (RefSeq NM_015093) variant found in Family 1 in our previous work (Ritelli et al., 2018). The variant has been submitted to Leiden Open Variation Database (https://databases.lovd.nl/shared/phenotypes/0000175660, patient ID 00235348). This family originally included three available affected individuals (i.e., patients 1 [Pt 1], 2 [Pt 2], and 3 [Pt 3]). Unfortunately, Pt 3 (father of Pt 2 and older brother of Pt 1) suddenly died at 60 years of age and was therefore not available for this study. Pt 1, Pt 2, and the unaffected mother of Pt 2 (control) underwent blood sampling and skin biopsy for the following investigations. All signed the informed consent. This study was approved by the local ethics committee (protocol no. GTB12001). This study is in accordance with the Helsinki declaration and its following modifications. Both patients presented a pleiotropic syndrome with generalized joint hypermobility, facial dysmorphism, abnormal skin texture, short limbs, hearing loss, recurrent otitis media, and polyvalvular heart dystrophy. In addition, Pt 1, a 48-year-old woman, also showed progressive mitral valve disease, which requested surgery, dilated cardiomyopathy, cardiac arrhythmias, and atrial septum aneurysm. Differently, Pt 2, a 28-year-old woman, presented disproportionate short stature, short hands and feet, bicuspid aortic valve, and myocardial noncompaction.
2.2 Conservation of TAB2 (Thr467) amino acid
Evolutionary conservation of TAB2 p.Thr467 amino acid was investigated with protein sequence alignment generated by Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) and compared with data provided by UCSC Database (https://genome.ucsc.edu).
2.3 Cell cultures
Primary dermal fibroblasts were established from skin biopsies of Pt 1, Pt 2, and the unaffected mother of Pt 2 (control) and cultured in Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEMF12; Thermo Fisher Scientific), plus 10% fetal bovine serum (FBS; Thermo Fisher Scientific) and 1% streptomycin and penicillin (Thermo Fisher Scientific). Fibroblast cultures were deposited into Genomic and Genetic Disorders Biobank at the Fondazione IRCCS Casa Sollievo della Sofferenza, San Giovanni Rotondo, Foggia, Italy. Human embryonic kidney (HEK) 293 cell lines were maintained in DMEM with Glutamax supplemented with 10% FBS and 1% penicillin (100 U/ml) and streptomycin (100 μg/ml; Thermo Fisher Scientific). HEK293 and fibroblast cultures were grown in a 5% CO2 incubator at 37°C.
2.4 Total RNA and nonsense-mediated messenger RNA decay assay
Nonsense-mediated mRNA decay (NMD) was assayed by treating cell cultures with cycloeximide (Sigma Aldrich, Germany) at a concentration of 200 μg/ml. After 4 hr of incubation, total RNA was extracted using RNeasy Mini Kit (Qiagen, Hilden, Germany), treated with DNase-RNase free (Qiagen), quantified by Nanodrop (Thermo Fisher Scientific) and reverse-transcribed using QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer's instructions. Polymerase chain reaction (PCR) amplification and Sanger sequencing were carried out with primers listed in Table S1.
2.5 Quantitative real-time polymerase chain reaction
Oligos for quantitative PCR were designed using the Primer express program (Rozen and Skaletsky, 2000) with default parameters. Primers were blasted against human genome by BLAST to ensure specificity. GAPDH and EEF1A were used as reference genes. The reactions were run in triplicate in 10 µl of final volume with 10 ng of sample complementary DNA (cDNA), 0.3 mM of each primer, and 1× Power SYBR Green PCR Master Mix (Thermo Fisher Scientific). Reactions were set up in a 384-well plate format with a Biomeck 2000 (Beckmann Coulter) and run in an ABI Prism 7900HT (Thermo Fisher Scientific) with default amplification conditions. Raw Ct values were obtained using SDS 2.3 (Applied Biosystems). Calculations were carried out by the comparative Ct method as reported in Micale et al. (2014). Significance was determined by a two-tailed unpaired t-test for means.
2.6 Generation of expression constructs
The p.(Thr467Tyrfs*6) TAB2 variant was generated by using the QuickChange II site-directed mutagenesis kit (Stratagene), according to the manufacturer's instructions. The mutant construct was confirmed by Sanger sequencing. Primer pairs used are listed in Table S1.
2.7 Coimmunoprecipitation assay and western blot analysis
HEK293 cells were plated in 100-mm culture dishes at a density of 5 × 105 cells/ml, and then transfected with FLAG-TAK1 and/or FLAG empty vector, HA-TAB2, and/or HA-TAB2 p.(Thr467Tyrfs*6) plasmids. Plasmid transfections were performed using Lipofectamine® LTX (Thermo Fisher Scientific), according to the manufacturer's instructions. After 48 hr, cells were lysed and coimmunoprecipitated with anti-FLAG (#F3165, Sigma Aldrich) using Dynabeads magnetic beads (Thermo Fisher Scientific) following the manufacturer's instructions. Complexes were resolved by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by transfer to a nitrocellulose membrane. The membrane was incubated with anti-FLAG and anti-HA (MMS-101P-500; Eurogentec, Belgium) antibodies (Abs), as reported in Micale et al. (2014) and Venuto et al. (2019) followed by secondary horseradish peroxidase-conjugated anti-mouse and anti-rabbit (Santa Cruz) Abs. The chemiluminescence system (GE Healthcare, UK) was used for detection.
2.8 Quantification of phosphorylated protein
Pt 1 and Pt 2 and control (unaffected mother of patient 2) fibroblasts were lysed in 1× Dulbecco's phosphate buffered saline (D-PBS), 0.025% NP-40, protease inhibitors (Roche), and phospho-inhibitors (Roche). The supernatant was cleared by centrifugation. Proteins were resolved by electrophoresis on 10–12% SDS-PAGE, followed by transfer to nitrocellulose membrane. Membranes were probed with anti-NF-κB (#8242; Cell Signaling, The Netherlands), anti-MAPK (#4695; Cell Signaling), anti-phospho-MAPK (#4370; Cell Signaling), anti-TAK1 (#S828A; MRC PPU Reagent, UK), anti-phospho-TAK1 (#9339; Cell Signaling) and anti-GAPDH (#sc-47724; Santa Cruz) Abs. Detection was carried out as described above. To analyze the reactive MAPK or TAK1 phosphorylation level, band intensity of phosphorylated (p)-MAPK or -TAK1 and total MAPK or TAK1 was quantified using ImageJ software, and the ratio of p-MAPK or p-TAK1 to total MAPK or TAK1 was calculated, as previously reported (Palumbo et al., 2019).
2.9 Flow cytometric analysis
Skin fibroblasts of Pt 1 and Pt 2 and unaffected control were cultured in vitro for 10 days and subsequently fixed in 80% ethanol for 10 min at −20°C, washed, and resuspended in D-PBS (Thermo Fisher Scientific). Afterward, fixed cells were treated with RNase 20U (Sigma Aldrich) for 30 min at 37°C and stained with 10 μg/ml propidium iodide (PI; Sigma Aldrich) in D-PBS to determine the cell-cycle distribution by DNA content. The flow cytometry data were acquired using MoFlo®AstriosTM instrument (Beckman Coulter) and analyzed by FlowJo software (TreeStar).
2.10 Extracellular matrix remodeling and collagen disease polymerase chain reaction arrays
Total RNA was reverse-transcribed using iScript gDNA clear cDNA Synthesis kit (Biorad, UK), according to the manufacturer's instructions. Resulting cDNA from each sample was aliquoted in both ECM remodeling (#10025274; Biorad, UK) and collagen disease (#10037956; Biorad, UK) predesigned PCR array panels using 1× SsoAvanced Universal SYBR Green Supermix (Biorad, UK). The panel of ECM remodeling includes matrix metalloproteinases (collagenases, gelatinases, stromelysins, matrilysins), inhibitors of metalloproteinases, cell-surface heparin sulphate proteoglycans, and collagens. The panel of collagen disease analyzed a number of genes found differentially expressed within specified collagen pathologies. The reactions were run in triplicate in 10 µl of final volume. Real-time PCRs were performed on an ABI 7900 (Thermo Fisher Scientific). GAPDH, TBP, and HPRT1 were used as reference genes. Data were analyzed by using PCR Array Data Analysis software (www.SABiosciencies.com/pcrarraydataanalysis.php), provided by Biorad.
2.11 Immunofluorescence microscopy
To analyze the ECM organization of type III collagen (COLLIII), type V collagen (COLLV), and FN, fibroblasts derived either from Pt 1 and Pt 2 or from the unaffected mother of Pt 2 and an unrelated healthy individual, grown for 72 hr, were immunoreacted as previously reported (Chiarelli et al., 2016; Zoppi, Gardella, De Paepe, Barlati, and Colombi, 2004; Zoppi, Barlati, and Colombi, 2008). In brief, cold methanol fixed fibroblasts were immunoreacted with 1:100 anti-COLLIII, anti-COLLV (MerkMillipore-Chemicon Int.), and anti-FN (Sigma Aldrich) Abs. For the analysis of α2β1 and α5β1 integrin receptors, cells were fixed in 3% paraformaldehyde/60 mM sucrose and permeabilized with 0.5% (v/v) Triton X-100 as previously reported (Zoppi et al., 2004; Zoppi et al., 2008). In particular, fibroblasts were reacted for 1 hr at room temperature with 4 μg/ml of anti-α5β1 (clone JBS5) and anti-α2β1 (clone BHA.2) integrin monoclonal Abs. Cells were incubated for 1 hr with Alexa Fluor 488 anti-rabbit and Alexa Fluor 594 anti-mouse Abs (Thermo Fisher Scientific), or with rhodamine-conjugated anti-goat IgG (Calbiochem-Merck, Germany). Immunofluorescence signals were acquired by a black-and-white CCD TV camera (SensiCam-PCO Computer Optics GmbH, Germany) mounted on a Zeiss fluorescence Axiovert microscope and digitalized by Image-Pro Plus software (Media Cybernetics).
2.12 Statistical analysis
Densitometry analysis of immunoblots was performed using the ImageJ software. Statistical analysis of immunoblotting was performed using unpaired, two-tailed Student's t test (*p < .05, **p < .01).
3 RESULTS
3.1 The TAB2 c.1398dup variant is a substrate of NMD
The TAB2 c.1398dup variant was predicted to introduce a premature stop codon [p.(Thr467Tyrfs*6)] (Figure 1a) located in the last exon more than 50–55 nucleotides upstream of the 3′-most exon-exon junction, a condition that generally triggers NMD. To verify whether this frameshift variant leads to NMD activation, we measured the levels of TAB2 mRNA in the patient's cells before and after treatment with cycloheximide, a well-known indirect inhibitor of NMD (Micale et al., 2010). First, quantitative PCR analysis performed in patient skin fibroblasts showed reduced TAB2 transcriptional levels compared to control cells (Figure 1b). After cycloheximide treatment, we observed a significant 1.8–2.0-fold increase in the TAB2 transcript levels in patients’ fibroblasts (Pt 2 and Pt 1, respectively), compared with untreated cells (Figure 1c). The reduced amount of TAB2 mRNA levels in mutated cell lines and its partial recovery after cycloheximide treatment indicate that endogenous TAB2 mutant transcripts are subject to NMD. The physiological NMD substrate SC-35 1.7 kb (Figure 1d) was included as the positive control, as previously reported (Micale et al., 2014). These results were supported by direct sequencing of RT-PCR products of total RNA derived from patient's and control fibroblasts, treated or not with cycloheximide, for the region encompassing TAB2 exons 4–6. NMD inhibition restores the expression of the TAB2-mutated allele in patient cells treated with cycloheximide compared to untreated cells (Figure 1e). Without cycloheximide, mutant alleles were observed at a lower level than the wild-type allele in the patient's cells.
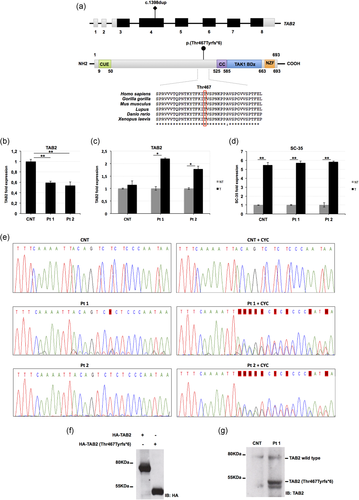
TAB2 c.1398dup variant undergoes nonsense-mediated messenger RNA (mRNA) decay and encodes a truncated protein. (a) Schematic representation of TAB2 and its domains, and conservation of the involved residue p.Thr467 among species. Coding regions are in black, UTR sequences are in gray; introns are not to scale. Amino acid positions of each domain are indicated below the structures. The variant here described is located on the gene and protein structure. (b) Quantitative PCR analysis of TAB2 mRNA levels in control and patients’ fibroblasts. (c) Quantitative PCR analysis of TAB2 transcript levels in control and patients’ fibroblasts after treatment (T) or not (NT) with cycloheximide. TAB2 mRNA levels in control and patients’ fibroblasts not treated were set as 1. (d) Quantitative PCR analysis of the physiological NMD substrate SC-35 included as positive control. (b-d) Values are expressed as mean ± SEM (*p < .05; **p < .01, n = 2). (e) Electropherograms showing complementary DNA (cDNA) sequencing analysis of PCR products amplified with primers targeting exons 4 and 6 of TAB2. Nucleotide sequences are provided. Unaffected mother of patient 2 (CNT) and patient fibroblast cell lines (Pt 1 and Pt 2) were treated or not with cycloheximide. (f) Whole protein lysates of HEK293 cells transfected with vectors encoding HA-TAB2 or HA-TAB2 [p.(Thr467Tyrfs*6)] clones were separated on 10% sodium dodecyl sulphate (SDS)-gel and subjected to immunoblotting with anti-HA Ab. (g) Whole protein lysates of primary skin fibroblasts cell lines from Pt 1 and control (CNT) were separated on 10% SDS-gel and subjected to immunoblotting with anti-TAB2 Ab. CC, coiled coil region; CUE, coupling of ubiquitin conjugation to endoplasmic reticulum degradation; NZF, Npl4 zinc finger; TAK1 BD, TAK1 binding domain
3.2 The TAB2 c.1398dup variant encodes a truncated protein
The TAB2 c.1398dup variant was predicted to lack 222 amino acids, including the C-terminal zinc finger domain (Figure 1a), which is essential for the activation of TAK1-dependent pathways. In order to confirm the translational impact of the TAB2 c.1398dup variant, we analyzed the total lysate of HEK293 cells transfected with plasmids encoding either the TAB2 wild-type allele or the p.(Thr467Tyrfs*6) mutant. Immunoblot analysis confirmed that cells with the plasmid carrying the TAB2 c.1398dup variant expressed an aberrant protein of about 50 kDa (Figure 1f). Consistently, a truncated TAB2 protein was also detected in cell lysates from Pt 1's skin fibroblasts (Figure 1g). These findings suggested that a portion of the mutated transcripts could escape the NMD and result in a shortened protein.
3.3 The TAB2 truncated protein which impairs its binding to TAK1
We hypothesized that, in this family, the pathogenic cascade leading to the multiple observed congenital anomalies was related to the fact that the TAB2-truncated protein loses the capability to interact with TAK1. Accordingly, previous studies demonstrated that the region of TAB2 comprised between residues 574 and 653 is sufficient to bind TAK1 (Figure 1a). To assess our hypothesis, we tested the interaction of TAK1 with TAB2 wild-type and/or TAB2 mutant expressed proteins by performing coimmunoprecipitation assays. HEK293 cells were transfected with vector expressing the wild-type FLAG-tagged-TAK1 and the mutated HA-tagged-TAB2. As shown in Figure 2a (lane 2), TAK1 and TAB2 wild-type proteins efficiently associate to each other. Upon TAB2 mutant protein expression, the association between TAB2 and TAK1 is strongly affected (Figure 2a, lane 3), and this indicates that the TAB2 disease-causing variant impairs the formation of the TAK1-TAB2 complex.
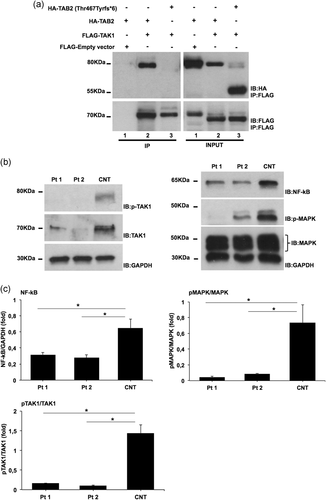
Molecular findings of TAB2 c.1398dup variant. (a) HEK293 cells were transfected with vectors encoding HA-TAB2 or HA-TAB2 [p.(Thr467Tyrfs*6)] in combination with FLAG-TAK1. Cells were lysed and protein extracts subjected to coimmunoprecipitation using anti-FLAG Ab. Immunopurified complexes and total lysates were analyzed by immunoblotting using indicated Abs. (b) Cell lysates were obtained from skin fibroblasts after stimulation with transforming growth factor-β (TGFβ; 10 ng/ml) during 2 hr in serum-free medium. Whole protein lysates were separated on 10% SDS-gel and subjected to immunoblotting with indicated Abs. (c) Level of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and phospho-mitogen-activated protein kinase (MAPK)/MAPK were quantified by densitometry using ImageJ analysis software. Relative NF-κB and phospho-MAPK/MAPK levels in control cells were set as 1. Graphs show averages calculated on three different experiments and scale bars represent standard errors. Values are expressed as mean ± SEM (*p < .05, n = 3). CNT, control individual; Pt 1, patient 1; Pt 2, patient 2; SDS, sodium dodecyl sulphate
3.4 Functional impact of the TAB2 c.1398dup variant on patients’ fibroblasts
The formation of the TAK1-TABs complex is required for the autophosphorylation-induced activation of TAK1 (Cheung, Nebreda, and Cohen, 2004; Ishitani et al., 2003). Upon activation, TAK1 is sequentially autophosphorylated within its kinase domain (Scholz et al., 2010), starting at the Ser192 residue and followed by the phosphorylation of Thr178, Thr187, and Thr184 residues. This, in turn, triggers the phosphorylation of a number of downstream effectors (Dai, Aye Thu, Liu, Xi, and Cheung, 2012; Kishimoto, Matsumoto, and Ninomiya-Tsuji, 2000). Based on our coimmunoprecipitation data, we supposed that the p.(Thr467Tyrfs*6) TAB2 variant affects TAK1 autophosphorylation and activation by impairing the formation of the TAK1-TAB2 complex. In order to explore the functional impact of this variant, we assessed the level of TAK1 in cultured skin fibroblasts from affected individuals by western blot analysis. We found reduced amounts of TAK1 levels in the fibroblasts from patients, as compared to endogenous TAK1 amounts in control cells (Figure 2b). To analyze the activity level of TAK1, we used an anti-phospho-TAK1 Ab in order to test its autoactivation. Western blot analysis revealed the reduction of TAK1 autophosphorylation in both affected subjects compared to control (Figure 2b). Reduced autophosphorylation of TAK1 in the presence of TAB2 substitution suggested that signaling pathways downstream of this complex should exhibit altered activity. Multiple signaling pathways are activated downstream of TAK1, including NF-κB and MAPK, under transforming growth factor-β (TGFβ) stimulation. To test the functional impact of the TAB2 variant on this signaling pathway, we evaluated the level of NF-κB and p-MAPK in patient fibroblast lysates treated with TGFβ. We observed a decreased level of NF-κB and p-MAPK in the patient's cells compared to control (Figure 2b,c).
3.5 The TAB2 c.1398dup variant affects cell proliferation
TAK1-TABs complex is a key player in many death-related programs like apoptosis and cell survival dynamics (Aashaq, Batool, and Andrabi, 2019). In order to better define the biological relevance of mutated TAB2, we assessed the proliferative capacity of patient skin fibroblasts. We found that after 10 days of in vitro growth, both patients’ fibroblasts tended to cycle less actively than wild-type cells as measured by the DNA content (Figure 3a). Patients’ cells were indeed significantly enriched in G0/G1 phases with respect to the wild-type fibroblasts, more frequent in the S+G2/M phases (Figure 3b).
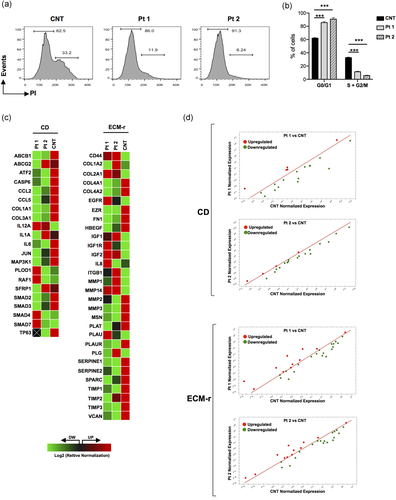
Patient-derived fibroblasts divide less than control cells and show an altered expression of extracellular matrix (ECM) components. (a) Cell-cycle distribution of dermal fibroblasts with wild-type (CNT) and heterozygous (Pt 1 and Pt 2) mutant genotypes was evaluated by flow cytometry and after staining with propidium iodide (PI). In the plots, the cells in G0/G1 and S+G2/M phases are depicted. Results are representative of multiple replicates. (b) Bar graph reports the percent of cells in G0/G1 and S+G2/M phases for the wild-type (CNT) and heterozygous (Pt 1 and Pt 2) fibroblasts, as measured by the reported cell-cycle analysis (c). Values are expressed as mean ± SEM (***p < .001, n = 3). (c) Heat map of a PCR array-based collagen diseases (CD) and ECM remodeling (ECM-r) gene expression. Activated transcripts are colored in red, while inhibited processes are colored in green. (d) Graph plots the log 2 of normalized expression levels of every gene on the array in a control individual (x-axis) versus patients (y-axis). Central line indicates unchanged gene expression. Symbols outside the delimited area indicate fold-differences larger than a threshold that we set to 1. The red symbol in the upper-left corner readily identifies upregulated genes, and the green symbols in the lower right corner readily identify downregulated genes. Values were derived by taking the means of fold changes of three biological replicates per time point
3.6 The TAB2 c.1398dup variant affects ECM organization in patients’ fibroblasts
In line with the consolidated evidence that TAK1-TAB signaling regulates the expression of collagens and FN (Hocevar et al., 2005; Kim et al., 2007; Ono et al., 2003), we hypothesized that the TAB2 c.1398dup variant may cause a disarray of different ECM-related proteins. In this regard, we analyzed the expression profile of gene coding for various ECM components and proteins and enzymes involved in collagen biogenesis in patient fibroblasts by using two different predesigned PCR arrays. We observed a global dysregulation of the collagen disease pathway in patients’ fibroblasts compared to control cells (Figure 3c,d). For instance, ATF2, CASP6, CCL2, CCL5, COL1A1, COL3A1, IL1A, IL6, JUN, MAP3K1, SMAD2, and SMAD3 were found significantly downregulated, while IL12A was found upregulated in both patients’ cells compared to control cells. Regarding to the ECM remodeling array, we found 22 differentially expressed transcripts out of 30 analyzed genes, which are consistently perturbed in the same direction in both patients’ fibroblasts (Figure 3c,d). All differed significantly of at least 1.5-fold (16 upregulated and six downregulated transcripts out of 22 differentially expressed genes).
To confirm the aberrant effect of the TAB2 c.1398dup variant on the ECM homeostasis, we analyzed the organization of COLLIII, COLLV, and FN as well as the expression of their canonical integrin receptors, that is, α2β1 and α5β1, respectively, by immunofluorescence staining. COLLIII and COLLV were organized in fibrillar networks in control fibroblasts (i.e., a healthy unrelated individual and cells derived from the unaffected mother of Pt 2; Figure 4). Contrariwise, fibroblasts derived from Pt 1 and Pt 2 displayed a disarray of both the COLLIII- and COLLV-ECMs. These collagens were not assembled into ECMs overlaying the cells but were only detected in the cytoplasm. FN was organized in an abundant fibrillar ECM covering control fibroblasts, whereas it was strongly reduced in patients’ cells. Consistent with the COLLIII and COLLV disorganization, patient fibroblasts showed a slightly reduced expression of the α2β1 integrin receptor, whereas, despite an altered FN-ECM organization in patient cells, controls and patient's fibroblasts showed a similar expression of the α5β1 integrin.
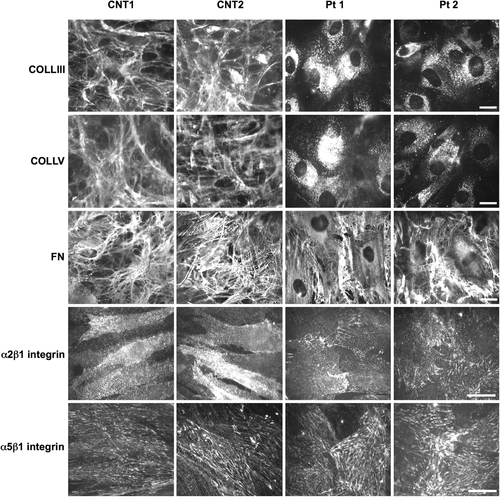
TAB2 c.1398dup variant induces disorganization of ECM in patient's fibroblasts. Immunofluorescence analysis of COLLIII-, COLLV-, and FN-ECM, and α2β1 and α5β1 integrin receptors in fibroblasts from an unrelated healthy individual (CNT1), proband's unaffected mother (CNT2), and patients (Pt 1 and Pt 2) carrying the c.1398dup TAB2 variant. Scale bar: 10 μm. ECM, extracellular matrix
4 DISCUSSION
Here, we report the molecular proof of the pathogenic considerations, which emerged by the previous description of a novel disorder resembling the 6q25.1 microdeletion syndrome and additional connective tissue features (Ritelli et al., 2018). In our previous work, we emphasized the pleiotropic nature of the disorder due to the heterozygous TAB2 c.1398dup variant. At that time, we were not able to demonstrate such an assumption and the extreme rarity of the phenotype hampers its confirmation by multiple observations. Therefore, we decided to explore the multifaceted pathogenesis of the identified TAB2 c.1398dup variant with focus on its potential roles in the ECM organization/homeostasis, in order to verify the hypothesis that the 6q25.1 microdeletion syndrome is indeed a hereditary soft connective tissue disorder caused by TAB2 haploinsufficiency. In fact, we previously highlighted the clinical similarities between our family with the TAB2 c.1398dup variant and the previously reported patients with microdeletions encompassing TAB2 (Ritelli et al., 2018). Additional subjects with 6q25.1 microdeletion syndrome confirming its pleiotropic nature with predominant involvement of the cardiovascular system have been previously described (Cheng et al., 2017). Therefore, we think that the family with TAB2 c.1398dup variant by Ritelli et al. (2018) recapitulates the key findings of 6q25.1 microdeletion syndrome and offers the opportunity to investigate its molecular pathogenesis, as an emerging hereditary soft connective tissue disorder.
A survey of the literature of cell culture and animal model experiments reveals an interesting role of TAB2 as an adapter protein linking TRAF6 toTAK1 (Walsh et al., 2008). TRAF6 is an E3 ligase involved in the synthesis of lysine-63-linked polyubiquitin chains (Walsh et al., 2008). In particular, upon TGFβ1 stimulation, TAB2 binds the lysine-63-linked polyubiquitin chains of TRAF6 and transfers them to TAK1, which autophosphorylates for the activation of downstream signaling pathways (Figure 5; Kim et al., 2012; Takaesu et al., 2000). TAK1 posttranslational modification (PTM), including site-specific lysine-63-linked polyubiquitination, appears to fine-tune and coordinate TAK1 activities by conferring specificity for upstream activators as well as downstream substrates (Adhikari, Xu, and Chen, 2007). In this context, TAB2 plays a critical role in modulating TAK1 activation through the regulation of TAK1 polyubiquitination. TAB2 functions through two conserved domains: an N-terminal coupling of ubiquitin conjugation to endoplasmic reticulum degradation (CUE) domain and a C-terminal zinc finger (ZnF) domain, both present in a variety of proteins that bind ubiquitin (Schnell & Hicke, 2003). While deletion of the CUE domain of TAB2 partially impairs its ability to activate TAK1 and its downstream signaling pathways, deletion of the ZnF domain dramatically blocks them. Of note, the C-terminal zinc finger domain of TAB2 is essential for binding both TAK1 and polyubiquitin chains that link TRAF6 to TAK1. Engineered mutations of this zinc finger domain abolish the ability of TAB2 to bind polyubiquitin chains as well as its capability to link TAK1 to TRAF6 mediating TAK1 activation (Kanayama et al., 2004). At the same time, replacement of the ZnF domain with a heterologous ubiquitin binding domain derived from unrelated proteins restores the ability of TAB2 to activate TAK1 (Kanayama et al., 2004).
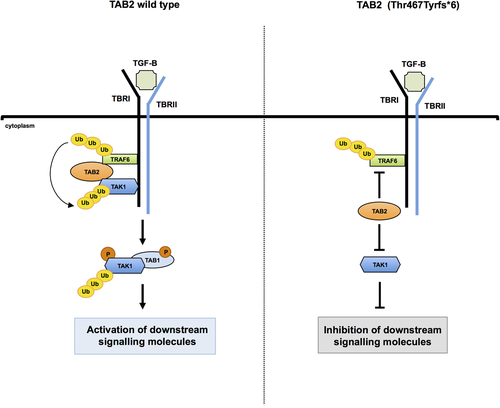
Schematic model proposed for the TAB2 c.1398dup molecular effect. In the absence of TGFβ1 stimulation, TAK1 associates with TGFβ receptor type I (TβRI) through complex formation with TAB2 and TRAF6. Upon TGFβ1 stimulation and formation of TβRI–TβRII heterotetrameric complexes, autopolyubiqitination of TRAF6 leads to polyubiquitination (Ub) of TAK1 through TAB2. Subsequently, TAK1 interacts with TAB1, which, in turn, induces autophosphorylation of TAK1 for activation. Activated TAK1 leads to the activation of downstream signaling molecules (Kim et al., 2012; Takaesu et al., 2000; Walsh et al., 2008). We hypothesize that TAB2-truncated protein impairs its ability to physically link TRAF6 to TAK1. This results in an altered activity of multiple signaling pathways regulated by the TAK1 kinase complex. TGFβ, transforming growth factor-β
In silico findings predicted that the TAB2 c.1398dup variant produces loss of a C-terminal coiled-coil domain (532–619), TAK1 binding domain (586–663), zinc finger domain, and polyubiquitin-interacting region (663–693). Here, we molecularly explored the pathological effect of TAB2 variant. Our analysis showed that TAB2 c.1398dup transcripts undergo NMD and this suggests that TAB2 haploinsufficiency could be a key molecular mechanism of disease. However, we also found that patient's fibroblasts express an amount of TAB2 shortened protein and this might suggest that a proportion of the mutated transcripts escapes NMD. Therefore, both NMD and the synthesis of a truncated protein may result from the identified causative variant.
In vitro studies revealed that the TAB2-truncated protein impairs its ability to physically bind TAK1, destabilizes TAK1, decreases TAK1 autophosphorylation, and alters the activity of more than one downstream signaling pathway, including NF-κB and MAPK, and cell proliferation. Notably, flow cytometric analysis showed that patient's skin fibroblasts exhibit a reduced proliferative capacity, according to the role of TAK1-TAB2 complex in establishing cellular commitments for death and survival (Aashaq et al., 2019). These findings suggest that the loss of the C-terminal domain of TAB2 could prevent the binding of polyubiquitinated chains of TRAF6 to TAK1. This, in turn, may lead to a dysregulation of TAK1 PTM, which contributes to reduced TAK1 protein levels and impaired TAK1 complex.
As TAK1-TAB2 complex orchestrates a wide array of cellular functions, it is reasonable to hypothesize that the constellation of clinical features displayed by our family is influenced by a deregulation of the TAK1 complex-mediated signaling. Specifically, the reduced proliferative ability observed in patients’ fibroblasts could explain some observed clinical features, such as postnatal growth retardation, skeletal underdevelopment and facial features. Accordingly, homozygous tak1 and tak2-deficient mice show embryonic lethality due to various cellular defects, such as abnormal cell differentiation, increased cell death, and decreased inflammatory responses (Gunnell et al., 2010; Sakurai, 2012; Sanjo et al., 2003). Moreover, knockout of tak1 in chondrocytes results in skeletal defects, including impairment of chondrocyte proliferation and maturation (Gao et al., 2013) and targeted ablation of tak1 in mice osteoblasts generates craniofacial defects (Liu et al., 2007).
Involvement of TAK1-regulated downstream pathways in the molecular pathogenesis beneath the TAB2 c.1398dup variant is also suggested by the similarities between the observed phenotype (Ritelli et al., 2018) and cardiospondylocarpofacial syndrome (CFCS), the latter recently associated with heterozygous TAK1 variants (Le Goff et al., 2016). CFCS is an ultrarare, autosomal dominant disorder featuring postnatal growth delay, facial dysmorphism, vertebral and carpal anomalies, and congenital heart defect (Le Goff et al., 2016). More recently, the concurrence of disabling functional gastrointestinal disorder, joint hypermobility, cardiac valve disease, and minor skin features pointed out the possibility that also CFCS might be considered a hereditary soft connective tissue disorder, at least in specific cases (Morlino et al., 2018). The apparent overlap between TAB2 haploinsufficiency and CFCS due to TAK1 abnormalities can also be inferred by the allelic nature of selected TAB2 and TAK1 (MAP3K7) variants in the etiology of frontometaphyseal dysplasia (FMD), a skeletal dysplasia mostly characterized by hyperostosis of the torus frontalis, metaphyseal flaring, and joint contractures. In fact, FMD is a genetic heterogeneous disorder caused by heterozygous variants in FLNA, TAK1, or TAB2 genes (Robertson et al., 2003; Wade et al., 2017). Although the molecular pathogenesis explaining FMD is presumably different from that underlying TAB2 haploinsufficiency and CFCS, the documented locus heterogeneity for FMD suggests close relationships between TAB2 and TAK1 in health and disease.
In order to formally demonstrate primary disarray of the ECM as the intermediate phenotype bridging the molecular finding to the clinical phenotype, we analyzed the effect of TAB2 loss-of-function on ECM organization and component expression in skin fibroblasts. Abnormal ECM is a well-known hallmark of hereditary disorders affecting the soft connective tissues (Zoppi, Chiarelli, Binetti, et al., 2018, Zoppi, Chiarelli, Ritelli, et al., 2018). TAK1-TABs complex has been shown to play a crucial role in ECM remodeling and in fibrotic response. In particular, TAK1 mediates TGFβ-induced expression of ECM proteins, including type I and IV collagens and FN (Kim et al., 2007; Ono et al., 2003). Indeed, conditional Tak1 mice demonstrated that TAK1 modulates collagen deposition and expression of profibrotic genes in the kidney and myocardium (Ma et al., 2011; Zhang et al., 2000). In our disease model, we demonstrated that TAB2 loss-of-function strongly influences the expression of ECM structural proteins and ECM composition and this formally indicates that TAB2 loss-of-function is capable to generate a clinical phenotype with multiple soft connective tissue anomalies. In turn, our findings might indicate the opportunity to consider TAB2 haploinsufficiency in patients presenting with multiple soft connective tissue features and resembling cognate disorders such as Ehlers-Danlos syndromes, but resulted negative to the corresponding molecular screening. The wide range of molecular ECM disarray pointed out in this study suggests more complex pathogenesis, whose understanding could assist in explaining the clinical uniqueness of TAB2 loss-of-function and in helping its prompt recognition.
In conclusion, we deeply dissected the molecular pathogenesis of the TAB2 c.1398dup variant and formally demonstrated that the resulting phenotype is well explained by TAB2 loss-of-function. These findings and the previously outlined overlap between this phenotype and the 6q25.1 microdeletion syndrome support the concept that TAB2 is the major gene of the 6q25.1 microdeletion syndrome. Further reports are expected to confirm whether the recognizable phenotype associated with 6q25.1 microdeletion syndrome may be occasionally caused by TAB2 null variants. Our data also offer initial insights on the ECM homeostasis impairment as a molecular mechanism probably underlying a connective tissue disease linked to TAB2. Inherited abnormalities of components of the TAK1-TABs complex could be increasingly considered in the expanding nosology of hereditary connective tissue disorders.
ACKNOWLEDGMENTS
The authors thank the family for their kind availability in sharing the findings within the scientific community. The authors acknowledge Prof. Stephen P. Robertson (University of Otago, Dunedine, New Zealand) for providing pCMV-HA-TAB2 and pCMV-FLAG-TAK1 plasmids. The authors are also grateful to the Genomic and Genetic Disorder Biobank, member of the Telethon Network of Genetic Biobanks (Telethon Italy, grant: GTB12001) and the EuroBioBank for providing the specimens. This work was supported by the Ricerca Corrente 2018–2020 Program from the Italian Ministry of Health to L. M., G. M., and M. C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTION
L. M., M. C., and S. M. designed the study and wrote the manuscript. A. N. generated primary skin fibroblasts. L. M., C. F., G. N., and A. C. carried out the functional assays. L. M. and M. C. interpreted functional data. V. G. and P. P. performed cytofluorimetric analyses, N. Z. performed immunofluorescence analyses. M. C. and S. M. provided a clinical evaluation of the patients. All authors contributed to writing and reviewing and approved the main manuscript text.