Mutations in TIMM50 cause severe mitochondrial dysfunction by targeting key aspects of mitochondrial physiology
Abstract
3-Methylglutaconic aciduria (3-MGA-uria) syndromes comprise a heterogeneous group of diseases associated with mitochondrial membrane defects. Whole-exome sequencing identified compound heterozygous mutations in TIMM50 (c.[341 G>A];[805 G>A]) in a boy with West syndrome, optic atrophy, neutropenia, cardiomyopathy, Leigh syndrome, and persistent 3-MGA-uria. A comprehensive analysis of the mitochondrial function was performed in fibroblasts of the patient to elucidate the molecular basis of the disease. TIMM50 protein was severely reduced in the patient fibroblasts, regardless of the normal mRNA levels, suggesting that the mutated residues might be important for TIMM50 protein stability. Severe morphological defects and ultrastructural abnormalities with aberrant mitochondrial cristae organization in muscle and fibroblasts were found. The levels of fully assembled OXPHOS complexes and supercomplexes were strongly reduced in fibroblasts from this patient. High-resolution respirometry demonstrated a significant reduction of the maximum respiratory capacity. A TIMM50-deficient HEK293T cell line that we generated using CRISPR/Cas9 mimicked the respiratory defect observed in the patient fibroblasts; notably, this defect was rescued by transfection with a plasmid encoding the TIMM50 wild-type protein. In summary, we demonstrated that TIMM50 deficiency causes a severe mitochondrial dysfunction by targeting key aspects of mitochondrial physiology, such as the maintenance of proper mitochondrial morphology, OXPHOS assembly, and mitochondrial respiratory capacity.
1 INTRODUCTION
Mitochondrial energy metabolism disorders are a large and heterogeneous group of diseases presenting with a wide variety of clinical symptoms (Gorman et al., 2016). Although the diagnosis of these diseases is still challenging, the implementation of next-generation sequencing approaches in the last decade has identified disease-causing mutations in an increasing number of genes involved in the mitochondrial function (Carroll, Brilhante, & Suomalainen, 2014). Nevertheless, the physiopathological basis underlying these disorders is still not well understood. Accurate characterization of these processes at the molecular, cellular, and biochemical levels will provide new insight into these diseases that will be essential for the future development of potential therapeutic strategies.
Deficient enzymatic activities or altered levels of metabolites in body fluids facilitate the interpretation of the genomic data. Of these, increased urinary excretion of 3-methylglutaconic acid (3-MGA) has become a well-established and informative biomarker for metabolic disorders, particularly for those involving mitochondrial dysfunction (Wortmann et al., 2013). The persistently high excretion of this metabolite has been linked to a group of disorders characterized by defects located at the mitochondrial membrane. An increasing number of disease-causing mutations in genes encoding for proteins related to the mitochondrial membrane have been reported in patients with 3-methylglutaconic aciduria (3-MGA-uria), including: TAZ (MIM# 300394; Barth et al., 1983; Bione et al., 1996), OPA3 (MIM# 606580; Anikster, Kleta, Shaag, Gahl, & Elpeleg, 2001), DNAJC19 (MIM# 608977; Davey et al., 2006), ATPAF2 (MIM# 608918; De Meirleir et al., 2004), TMEM70 (MIM# 612418; Cízková et al., 2008), ATP5F1E (MIM# 606153; Mayr et al., 2010), SERAC1 (MIM# 614725; Wortmann et al., 2012), AGK (MIM# 610345; Aldahmesh, Khan, Mohamed, Alghamdi, & Alkuraya, 2012; Mayr et al., 2012), CLPB (MIM# 616254; Capo-Chichi et al., 2015; Kanabus et al., 2015; Saunders et al., 2015; Wortmann et al., 2015), HTRA2 (MIM# 606441; Mandel et al., 2016), QIL1 (MIM# 616658; Guarani et al., 2016; Zeharia et al., 2016), TIMM50 (MIM# 607381; Shahrour et al., 2017), and ATP5F1D (MIM# 603150; Oláhová et al., 2018). Disorders associated to 3-MGA-uria are usually linked to variable mitochondrial respiratory chain defects and abnormal mitochondrial morphology. In addition, alterations in the metabolism of cardiolipin (CL), a phospholipid that plays a crucial role in the maintenance of the inner mitochondrial membrane structure, have been reported in individuals carrying mutations in TAZ (Barth syndrome, BS); (Houtkooper et al., 2009; Schlame & Ren, 2009) and SERAC1 (Wortmann et al., 2012).
Two recent studies reported TIMM50 mutations in individuals with epileptic encephalopathy and 3-MGA-uria (Shahrour et al., 2017; Reyes et al., 2018). TIMM50 is a subunit of the TIM23 complex, located in the inner mitochondrial membrane, that plays a major role in protein import into the mitochondria (Geissler et al., 2002; Mokranjac et al., 2003; Mokranjac et al., 2009; Tamura et al., 2009). Although the molecular function of TIMM50 has been well studied in different organisms and cellular models, the pathophysiological mechanisms underlying TIMM50 deficiency in human disease still remain incompletely understood (Reyes et al., 2018).
Here, we present a comprehensive study that provides further insight into elucidating the potential pathomechanisms underlying TIMM50 deficiency. We found severe morphological defects and ultrastructural abnormalities with aberrant mitochondrial cristae organization in muscle and fibroblasts from a boy with 3-MGA-uria. Respiratory chain activities in muscle biopsy were normal. However, when normalized to citrate synthetase (CS) activity, indicative of the mitochondrial mass, a general decrease of the complexes I–IV activity was detected. In addition, the levels of fully assembled OXPHOS complexes and supercomplexes were strongly reduced, and high-resolution respirometry demonstrated a significant reduction in the maximum respiratory capacity. Using CRISPR/Cas9, we generated a HEK293T TIMM50-deficient cell line and found that it mimicked the respiratory defect observed in the patient fibroblasts; notably, transfection with a plasmid encoding for the TIMM50 wild-type protein could rescue this defect. Our results demonstrated that TIMM50 mutations lead to a generalized defect of the mitochondrial function by targeting several key aspects of it physiology, including maintenance of proper mitochondrial morphology, OXPHOS assembly, and mitochondrial respiratory capacity.
2 MATERIAL AND METHODS
2.1 Case report
We report a case study of a 17-year-old boy who was the second son of non-consanguineous, healthy Spanish parents. At the age of 2.5 months, the patient presented with abnormal ocular movements. At 3.5 months, a diagnosis of West syndrome was made, and he showed a good response to ACTH and antiepileptic treatment. Nevertheless, neurological regression was detected, and brain MRI showed bilateral symmetric lesions in the globus pallidus and brain stem, suggesting Leigh syndrome. Visual evoked potential and electroretinogram were abnormal, and the patient developed optic atrophy and strabismus. After 6 months, antiepepileptic drugs were stopped. Further, the patient presented with dilated cardiomyopathy, which evolved favorably under digoxin treatment.
During childhood, the boy showed spastic tetraparesia with dystonia and severe visual impairment. He presented two episodes of diarrhea associated with neutropenia. At the present time, he shows evidence of failure to thrive, severe encephalopathy, dystonia, and piramidalism. In addition, he has scoliosis and osteoarticular problems and is wheelchair-dependent.
Biochemical investigations revealed elevated levels of blood lactate (3 mmol/L; control levels, 0.63–2.49 mmol/L) and CSF lactate (5 mmol/L; control levels, < 2.2 mmol/L). Levels of plasma amino acids and acyl carnitines were normal, and the most relevant biochemical finding in urine was a persistent increase of 3-MGA (range: 53–308 mmol/mol creatinine, control levels: < 20) and 3-methylglutaric acid (range: 20–220 mmol/mol creatinine, control levels: <15), setting the suspicion of a mitochondrial disorder (Figure S1).
Pyruvate dehydrogenase activity, 14C-pyruvate oxidation rate, and levels of coenzyme Q10 were measured in fibroblasts of the patient and found to be within the control range. Muscle biopsy investigations revealed normal activities of the mitochondrial respiratory chain enzymes with an elevated activity of CS. Thus, when normalized to CS, a generalized decrease of complexes I-IV activities was detected (Table 1).
| Enzyme activities in muscle | Patient1 | Control values1, ‡ | Patient2 | Control values2, ‡ |
|---|---|---|---|---|
| CI (NADH-CoQ1 oxidoreductase) | 26 | 24–49 | 9 | 15–30 |
| CII (Succinate dehydrogenase) | 12 | 10–29 | 4 | 6–17 |
| CIII (Decyl-ubiquinol Cytochrome c oxidoreductase) | 100 | 62–161 | 36 | 38–98 |
| CIV (cytochrome c oxidase) | 55 | 36–131 | 20 | 22–80 |
| Citrate synthase (CS) | 280 | 127–222 | - | - |
- 1 nmol min−1 mg−1 protein.
- 2 nmol min−1 mg−1 protein enzyme activity expressed in percentage respect to the CS activity.
- ‡ control range.
- Altered values are indicated in bold.
Histopathology studies showed normal muscle architecture with no evidence of glycogen accumulation or ragged red fibbers, as confirmed by negative PAS and Masson's trichrome staining. SDH and sequential COX-SDH staining showed no abnormalities (Figure 1a). However, oil-red staining showed an accumulation of lipids in muscle fibbers. Ultrastructural analysis of muscle by electronic microscopy confirmed that aggregation of lipidic material was associated with the mitochondria (Figure 1a).
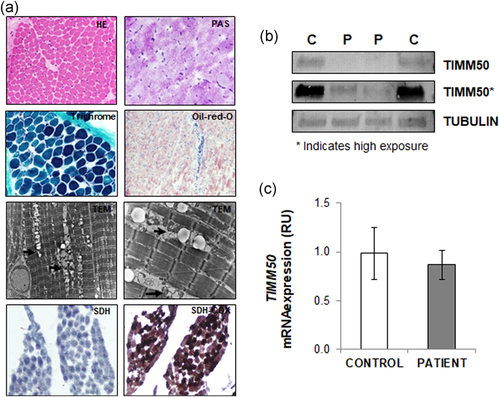
Histopathology studies and TIMM50 expression. (a) Histopathology studies showed normal muscle architecture (HE, hematoxylin-eosin staining) with no evidence of glycogen accumulation (PAS, periodic acid schiff) or ragged red fibers (trichrome). Oil-red staining showed accumulation of lipids in muscle fibers. Electronic microscopy (TEM) showed aggregation of lipid material with mitochondria (indicated by arrows). SDH and sequential COX-SDH staining showed no abnormalities in muscle fibers. (b) Western blot showed strongly reduced levels of TIMM50 protein in patient fibroblasts as compared with those from control individuals. Two different extracts of the patient fibroblasts and from two different controls are shown. Asterisk indicates higher exposure. GAPDH was used as a loading control. (c) RT-qPCR showed similar TIMM50 mRNA expression in patient fibroblasts and control fibroblasts. RU: relative units
2.2 Whole exome sequencing
Informed consent for exome sequencing was obtained from the parents. A Trio-exome analysis was performed in the Centre Nacional d'Anàlisi Genòmica (CNAG). Exome enrichment was performed using the Agilent SureSelect Human All Exon 50 Mb kit followed by sequencing using the Illumina HiSeq. 2000 genome analyzer platform. The analysis of the primary data (FASTQ files) was done using the pipeline developed by CNAG. Base calling and quality control were performed on the Illumina RTA sequence analysis pipeline. Sequence reads were mapped to Human genome build hg19 (GRCh37) using GEM mapper (Marco-Sola, Sammeth, Guigó, & Ribeca, 2012) and BFAST (Homer, Merriman, & Nelson, 2009). SAM tools suite version 0.1.18 (Li et al., 2009) with default settings was used for calling single-nucleotide variants and short indels on uniquely-mapping, non-duplicate read pairs. Variants on regions with low mappability (Derrien et al., 2012); read depth <10; strand-bias p value < 0.001; or tail distance–bias p value < 0.05 were filtered out. Annotation was performed with Annovar (Wang, Li, & Hakonarson, 2010) and snpEff (Cingolani et al., 2012), and population frequencies from the 1000 Genomes Project (1000 Genomes Project Consortium et al., 2010), NHLBI Exome Sequencing Project (http://evs.gs.washington.edu/EVS/), and an internal database were included.
2.3 Cell culture and generation of a TIMM50-deficient cell line
Human skin fibroblasts and HEK293T were maintained in DMEM (4.5 g/L glucose, 10% fetal calf serum, 1 mM glutamine, and 1% penicillin-streptomycin).
HEK293T TIMM50-deficient cells were generated by CRISPR/Cas9 genome-editing technologies. Briefly, three gRNAs sequences targeting the TIMM50 exon 2 were designed using the CRISPR design tool from the Feng Zhang Lab (http://crispr.mit.edu/). Guide RNAs were synthesized (Table S1) and cloned into the plentiCRISPRv2 vector following the lentiviral CRISPR Toolbox instructions from Zhang Lab available from Addgene. HEK293T cells were cotransfected with plentiCRISPRv2 containing TIMM50 sg1, sg2 and/or sg3 guide RNAs by CalPhos mammalian transfection kit. The cells were then exposed to 8 μg/ml puromycin for 1 week. Limiting dilution assays were carried out to generate individual clones. After 3 weeks, several clones were analyzed for DNA mutation and TIMM50 expression (Figure S2).
Wild type or TIMM50-deficient HEK293T cells were transfected using a pcDNA3.1 plasmid (Thermo Fisher Scientific, Waltham, MA) containing either the TIMM50 wild-type coding region or an empty vector. Transfection was performed using Lipofectamine 2000 Reagent (Thermo Fisher Scientific) following the manufacturer's protocol.
2.4 mRNA expression analysis
Total RNA was extracted from patient and control fibroblasts using QIAshredder and RNeasy mini kit (QIAGEN, Hilden, Germany). Single-strand cDNA was obtained using oligo-dT primers and M-MLV Reverse Transcriptase, RNase H Minus Point Mutant (Promega, Madison, WI), following the manufacturer's protocol.
TIMM50 mRNA expression was analyzed by quantitative polymerase chain reaction (qPCR) using SYBR Select Master Mix (Applied Biosystems, Foster City, CA) in a Step One Plus Real-Time PCR thermocycler (Applied Biosystems). Specific oligonucleotides were used to detect mRNA expression from TIMM50 or GAPDH (which was used as an internal control). Oligonucleotides are listed in Table S1.
2.5 Protein expression analysis
Fibroblasts were homogenized in SETH buffer (10 mM Tris-HCl pH 7.4, 0.25 M sucrose, 2 mM EDTA, 5 × 104 U/l heparin). Muscle extracts were prepared in tissue extraction buffer (250 mM mannitol, 75 mM sucrose, 10 nM Tris-HCl, 0.1 mM EDTA). Cleared lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted, and proteins were visualized by immunostaining with specific antibodies followed by colorimetric detection (using the Opti-4CNTM Substrate Kit; Bio-Rad, Hercules, CA). The ImageJ software was used for the densitometry analysis of protein expression levels. For subcellular fractionation experiments, fibroblasts were permeabilized with 0.01% digitonin, and the cell lysate was centrifuged for 10 min at 15,000×g. Pellets (containing mitochondria), supernatant fractions (containing cytosol), and total fractions were analyzed by Western blot using the indicated antibodies (listed in Table S2; Navarro-Sastre et al., 2011).
2.6 Blue native PAGE analysis of mitochondrial respiratory chain complexes and supercomplexes
Mitochondrial enriched pellets from fibroblasts and muscle tissue were obtained as described (Wittig, Braun, & Schägger, 2006). To analyze the mitochondrial respiratory chain complexes, the mitochondrial pellets were solubilized in 1% n-dodecyl β-D-maltoside. Supercomplexes extraction was performed using digitonin (1.2 g digitonin/g protein), a weaker detergent that maintains the interactions between complexes. Mitochondrial respiratory complexes and supercomplexes were analyzed by blue native PAGE (BN-PAGE) with 4–15% polyacrylamide gradient gels, followed by inmunoblotting with specific antibodies. Colorimetric detection (Opti-4CN™Substrate Kit, Bio-Rad) allowed visualization of complexes and supercomplexes. Antibodies are listed in Table S2.
2.7 Mitochondrial network and mitochondria morphology analysis
Mitochondrial network and mitochondria morphology were analyzed by immunofluorescence using confocal microscopy. Briefly, the cells were grown on glass coverslips, rinsed in PBS, and fixed for 10 min with 4% paraformaldehyde. The reaction was stopped with NH4Cl, and samples were permeabilized with 0.1% TritonX-100. The cells were stained with anti-TOMM20 (sc-11415) antibody, and coverslips were mounted with Mowiol 4–88 Mounting Medium (Sigma-Aldrich, Sant Luis, MI).
Images were obtained using a Leica TCS SL laser scanning confocal spectral microscope (Leica Microsystems GmbH, Wetzlar, Germany) and analyzed using the ImageJ software. The ratios between mitochondria length and width (aspect ratio, AR) and the degree of mitochondrial network branching (form factor, FF) were calculated.
2.8 Transmission electron microscopy
Fibroblasts were trypsinized and pelleted by centrifugation. Samples were fixed with 2.5% glutaraldehyde and 2% paraformaldehyde in PB (0.1 M phosphate buffer, pH 7.4). Pellets were washed five times with PB and post-fixed in 1% osmium tetroxide with 0.8% potassium hexacyanoferrate(III)-trihydrate in PB for 90 min. Samples were washed six times with milliQ water and dehydrated in ethanol with an increasing amount of acetone (from 50% to 100%). Samples were embedded in Spurr resin and polymerized at 60ºC for 72 hr. Semithin sections were stained with toluidine blue, and selected ultrathin sections, with uranyl acetate and lead citrate. Sections were analyzed using a JEOL (JEM 2011) electron microscope.
2.9 High-resolution respirometry
High-resolution respirometry was performed at 37°C using polarographic oxygen sensors in a two-chamber Oxygraph-2k system according to manufacturer's instructions (OROBOROS Instruments, Innsbruck, Austria). Manual titration of OXPHOS inhibitors (Oligomycin, Antimycin) and uncouplers (CCCP) was performed using Hamilton syringes (Hamilton Company, Reno, NV) as previously described (Pesta & Gnaiger, 2012). The data were recorded using the DatLab software v5.1.1.9 (Oroboros Instruments).
2.10 Statistics
In all cases, statistical analyses were performed using the two-tailed Student's t test to compare the means of two independent groups of normally distributed data. The data were reported as the mean ± S.E.M. Values of p < 0.05 were considered statistically significant.
3 RESULTS
3.1 Identification of mutations in TIMM50
The clinical and biochemical phenotype of the patient reported here were suggestive of a mitochondrial energy metabolism disorder. Mutations in the mtDNA previously associated to Leigh disease and to the genes involved in 3-MGA-uria were ruled out. To determine the genetic cause of the disease, whole exome sequencing of the affected patient and his healthy parents was performed. Data interpretation and filtering steps for gene prioritization are summarized in Figure S3. Two heterozygous mutations in TIMM50 (NM_001001563.5), which have not been previously reported, were identified. These mutations (c.341 G>A and c.805 G>A) are predicted to change arginine 114 to glutamine (p.Arg114Gln) and glycine 269 to serine (p.Gly269Ser), respectively. The mutations were confirmed by Sanger sequencing, and compound heterozygosity was corroborated by the carrier status of the mother (c.[341 G>A];[=]) and the father (c.[805 G>A];[=]) (Figure S4a). Both mutations were located in highly evolutionary conserved residues, and in silico analysis (PolyPhen-2 and SIFT) predicted a damaging effect on the protein (Figure S4b). The identified variants have been submitted to LOVD database (http://www.lovd.nl/TIMM50). TIMM50 encodes for a translocase localized in the inner mitochondrial membrane and is a subunit of the TIM23 complex (Geissler et al., 2002; Mokranjac et al., 2003; Mokranjac et al., 2009; Tamura et al., 2009). This multiprotein complex is involved in the recognition and import of mitochondria-targeted proteins and in the maintenance of the mitochondrial membrane.
3.2 Levels of TIMM50 protein, but not of TIMM50 mRNA, were strongly reduced in the patient fibroblasts
Notably, the TIMM50 protein levels were strongly reduced (albeit not completely absent) in patient fibroblast cells, as shown by western blot analysis (Figure 1b). In stark contrast, mRNA expression levels were not significantly different than those in control fibroblasts (Figure 1c). Altogether, these results suggest that the TIMM50 mutations reported here affect protein stability rather than the gene expression.
3.3 Expression and sublocalization of mitochondria-targeted proteins are not affected in TIMM50 patient fibroblasts
As TIMM50 is a component of the TIM23 complex, we next studied the expression and sublocalization of a subset of nuclear-encoded mitochondrial proteins. In particular, we analyzed the steady state levels of 10 subunits of the mitochondrial complexes I–V in fibroblasts. No significant differences of expression between TIMM50 and control fibroblasts for any of the mitochondrial proteins tested were observed by immunostaining followed by quantitative densitometry analysis (Figure 2a). To determine whether TIMM50 mutations could compromise the subcellular localization of mitochondrial proteins, we analyzed several subunits of the complexes I–V that are in the inner mitochondrial membrane (NDUFS3, SDHA, SDHB, UQCRFS1, COX5A, and ATP5A), as well as the mitochondrial matrix protein ECHS1. We used extracts from patient fibroblasts containing total, cytosolic, or mitochondria-enriched fractions. Western blot analysis of these extracts showed no differences between patient fibroblasts and fibroblasts from healthy controls. Therefore, we found no evidence for mislocalization because of the TIMM50 mutations (Figure S5).
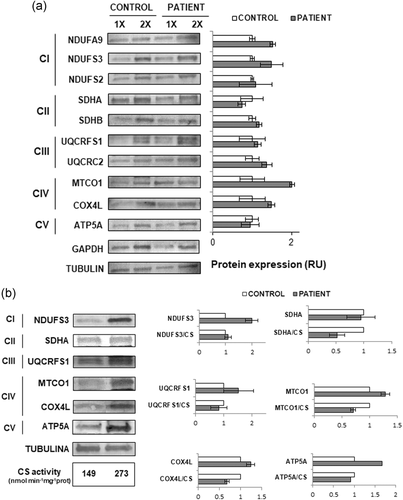
The expression of OXPHOS subunits in TIMM50 fibroblasts and muscle. (a) Immunostaining analysis showed no differences in the expression of the indicated proteins in TIMM50 patient fibroblasts as compared with control cells. 1× and 2× indicate 15 µg and 30 µg of loaded protein, respectively. Tubulin was used as a loading control. (b) Western blot and densitometry analysis showed reduced levels of SDHA, COX4L, and MTCO1 in patient muscle compared with control muscle after normalization for the citrate synthase activity. Tubulin was used as the loading control. C: control; P: patient; RU: relative units; CS: citrate synthase activity (nmol min−1 mg−1)
3.4 Expression of mitochondria-targeted proteins is altered in TIMM50 patient muscle biopsy
The steady state levels of a subset of OXPHOS subunits were also analyzed in extracts from muscle biopsy (Figure 2b), but the analysis was restricted to one or two proteins of each complex because of the limited amount of available tissue. Similar to what was observed for the activities of the mitochondrial respiratory chain (Table 1), after normalization to the CS activity, results showed reduced protein levels of SDHA, COX4L, and MTCO1, whereas the levels of NDUFS3, UQCRFS1, and ATP5A were similar to those seen in controls (Figure 2b).
3.5 Mitochondrial network and mitochondria morphology are altered in TIMM50 fibroblasts
We next calculated the mitochondrial network parameters AR (indicative of morphology) and FF (indicative of network branching), and the number of mitochondria per cell (Figure 3a). Inactivation of TMEM70, a mitochondrial membrane protein that also has been associated with 3-MGA-uria, has been shown to lead to fragmentation of the mitochondrial network and to an increased number of mitochondria per cell (Jonckheere et al., 2011). Therefore, fibroblasts from a patient with mutations in TMEM70 were included in this study and used as a positive control. As expected, an important reduction of AR and FF, together with a high number of mitochondria per cell, were observed in TMEM70-mutated fibroblasts. Strikingly, TIMM50-mutated fibroblasts also showed a significant reduction of AR and FF as compared with control cells (p < 0.001). However, the decrease of the mitochondrial network parameters in TIMM50 cells was less prominent than that of TMEM70 cells. On the other hand, the number of mitochondria per cell in TIMM50 fibroblasts was similar to control individuals, in contrast to that observed in TMEM70 fibroblasts, which were significantly higher (p < 0.001). Our results thus provide evidence of rounder and shorter mitochondria, as well as reduced mitochondrial network branching degree in the individual with TIMM50 mutations, suggesting that TIMM50 has a role in the maintenance of mitochondria architecture.
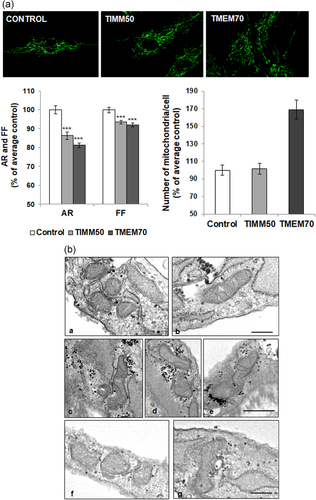
Mitochondria morphology and network are altered in TIMM50 patient fibroblasts. (a) Immunofluorescence analysis using TOMM20 as a mitochondrial marker showed a significant reduction in the aspect ratio (AR) and form factor (FF), indicating more rounded mitochondria and a reduced mitochondrial network branching degree in TIMM50 patient. A TMEM70 patient was used as a positive control for mitochondrial morphology abnormalities. ***p < .001. (b) Electron microscopy in fibroblasts of the patient carrying TIMM50 mutations compared with TMEM70 and control cells; a,b: control fibroblasts; c–e: fibroblasts from a patient with TMEM70 mutations; f,g: fibroblasts from the patient with TIMM50 mutations. Marked reduction in the number of mitochondrial cristae is seen in TMEM70 and TIMM50 fibroblasts. Scale bar = 0.2 μm
We next analyzed the protein expression levels of DRP1 and MFN2, which are involved in mitochondrial fission and fusion, respectively. Results showed no significant alterations in the levels of these proteins or in their ratio (DRP1/MFN2; Figure S6). This suggests that the alterations of the mitochondria morphology were not caused by a general imbalance of mitochondrial dynamics.
3.6 Aberrant mitochondrial ultrastructure in TIMM50 fibroblasts
To determine whether the TIMM50 mutations affect the mitochondria ultrastructure, we studied TIMM50 fibroblasts by Transmission electron microscopy (TEM). Fibroblasts from an individual with mutations in TMEM70 were used as a positive control. As expected, a severe defect in mitochondria cristae characterized by a reduction in number was observed in the TMEM70 patient compared with controls. Similarly, TIMM50 fibroblasts revealed a marked reduction in the number of mitochondrial cristae. Other organelles were not altered (Figure 3b).
3.7 The assembly of OXPHOS complexes and supercomplexes is altered in TIMM50 patient
The previous alterations seen in TIMM50 fibroblasts raised the question whether the stability of OXPHOS complexes and supercomplexes could also be compromised as a consequence of TIMM50 mutations. Therefore, we analyzed the mitochondrial respiratory chain complexes and supercomplexes from the patient fibroblasts by BN-PAGE followed by western blot, using specific antibodies against several subunits of the OXPHOS system (Figure 4). Interestingly, the levels of fully assembled respiratory chain complexes I, II, IV, and V were reduced (Figure 4a). In addition, when the analysis was performed using digitonin (which is a milder detergent that preserve the interactions with lipids and among complexes), we could observe that the levels of respiratory supercomplexes were also reduced (Figure 4b).
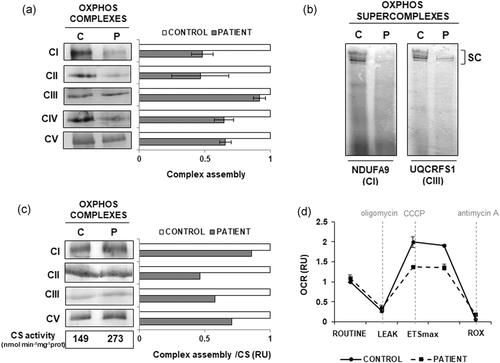
OXPHOS assembly and high-resolution respirometry. (a) BN-PAGE showed a reduction in the assembly of individual OXPHOS complexes I, II, IV, and V in TIMM50 patient cells. (b) BN-PAGE showed decreased amounts of high molecular weight OXPHOS supercomplexes in TIMM50 patient. (c) BN-PAGE showed reduced levels of assembled of complexes I, II, III, and V in patient's muscle upon normalization against citrate synthetase activity. (d) High-resolution respirometry analysis in TIMM50 patient cells and control individuals showed that the routinary oxygen consumption rate (OCR) was similar in both of them. After treatment with the mitochondrial uncoupler CCCP a reduction of Electron Transport System maximal respiratory capacity (ETSmax) was observed in patient cells compared with control. ETSmax is experimentally induced by titration with CCCP. ROUTINE, routinary oxygen consumption rate in untreated cells; LEAK, residual oxygen consumption after oligomycin treatment; ROX, residual oxygen consumption after treatment with antimycin A. OCR is expressed as relative units (RU) of control cells. C: control; P: patient; CS: citrate synthase activity (nmol min−1 mg−1)
We next analyzed the assembly of OXPHOS complexes in muscle extracts. Although the results showed apparently normal levels of fully assembled complexes I, II, III, and V, a general reduction was observed upon normalization to the CS activity. The decrease was more prominent for complexes II, III, and V (Figure 4c). Unfortunately, because of the limited amounts of available tissue, the assembly of complex IV could not be evaluated.
3.8 Altered respiratory capacity in TIMM50 fibroblasts
To determine whether the alterations observed in TIMM50 patient fibroblasts could affect the mitochondrial respiratory capacity, we next analyzed the oxygen consumption rate (OCR) by high-resolution respirometry (Figure 4d). While the basal respiratory rate was similar for patient and control cells, a pronounced difference in OCR was detected when the cells were treated with a mitochondrial uncoupler (CCCP) showing a marked reduction of the maximal respiratory capacity of the TIMM50 fibroblasts.
3.9 A TIMM50-deficient cell line mimics the mitochondrial respiratory defect observed in patient fibroblasts
We generated a TIMM50 knock-out cell line in HEK293T cells (TIMM50-KO) using CRISPR/Cas9 genome-editing technology. Immunostaining analysis showed no TIMM50 protein expression in the TIMM50-KO cells (Figure 5a, upper panel). Similarly to the patient fibroblasts, CCCP treatment resulted in a marked reduction of the maximal respiratory capacity in TIMM50-KO cells (Figure 5a, lower panel). Importantly, TIMM50-KO cells showed a rescue of the maximal respiratory capacity following transient transfection with a plasmid encoding for TIMM50 wild-type protein (Figure 5b).
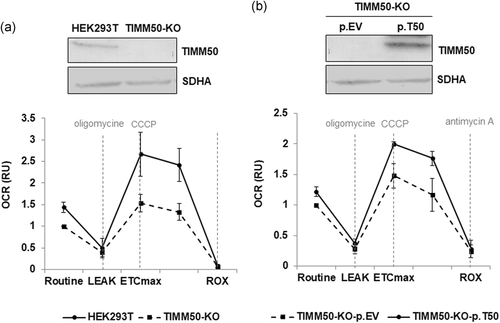
Functional complementation of the HEK293T TIMM50-deficient cells respiratory defect. (a) Western blot analysis demonstrated an absence of TIMM50 protein expression in a TIMM50 deficient cell line generated in HEK293T (TIMM50-KO; upper panel). High-resolution respirometry showed reduced Electron Transport System maximal respiratory capacity (ETSmax) after treatment with the mitochondrial uncoupler CCCP in HEK293T TIMM50-deficient cells (TIMM50-KO) compared with the parental cell line (HEK293T). (b) Western blot showed a complete absence of TIMM50 protein expression in HEK293T-KO cells transfected with empty vector (p.EV) and the recovery of protein expression upon transfection with a plasmid encoding for TIMM50 wild type protein (p.T50; upper panel). TIMM50-KO cells showed a recovery of the maximal respiratory capacity upon transient transfection with p.T50 compared with cells transfected with p.EV. ETSmax is experimentally induced by titration with CCCP. ROUTINE: basal oxygen consumption rate in untreated cells; LEAK: residual oxygen consumption after oligomycin treatment; ROX: residual oxygen consumption after treatment with antimycin A. Oxygen consumption rate (OCR) is expressed as relative units (RU) of control cells
4 DISCUSSION
3-MGA-uria comprises a heterogeneous group of disorders of the mitochondrial energy metabolism, usually associated to mitochondrial membrane defects (Wortmann et al., 2013). Here, we report on the identification of a patient with 3-MGA-uria, lactic acidosis, Leigh syndrome and mutations in TIMM50, which encodes for an essential component of the TIM23 multiprotein complex, that is involved in the import of mitochondria-targeted polypeptides into the mitochondria (Geissler et al., 2002; Mokranjac et al., 2003; Mokranjac et al., 2009; Tamura et al., 2009).
The identified mutations (c.341 G>A and c.805 G>A) affect two amino acids that are evolutionarily highly conserved, suggesting that each substitution could have a strong impact on the protein. Notably, TIMM50 protein levels were strongly reduced but not completely absent, regardless of the normal mRNA expression levels. These observations suggested that the mutated residues are important for TIMM50 protein stability.
Mutations in TIMM50 have been recently identified in three unrelated families (Reyes et al., 2018; Shahrour et al., 2017). The clinical and biochemical phenotypes of the previously described individuals were similar to those of the patient in this study, except that 3-MGA-uria was not observed in one of them (Reyes et al., 2018), and that cardiac involvement and neutropenia were not reported in any of the previous cases. However, cardiac involvement is a characteristic finding of BS (Barth et al., 1983; Bione et al., 1996) and of patients with mutations in DNAJC19 (Davey et al., 2006). Several studies in animal models have proposed a role for TIMM50 in the pathogenesis of heart dysfunction and have shown that TIMM50-deficient mice have cardiac hypertrophy (Tang et al., 2017). On the other hand, inactivation of the TIMM50 protein ortholog in zebrafish results in brain malformations and heart abnormalities (Guo et al., 2004). Altogether, these observations are in agreement with the clinical symptoms observed in our patient and point to an important role of TIMM50 in the brain and cardiac physiology. In addition, the patient in this study had two episodes of neutropenia, which is also a characteristic finding of BS (Aprikyan & Khuchua, 2013; Finsterer & Frank, 2013) and of patients with mutations in CLPB (Saunders et al., 2015; Wortmann et al., 2015) and HTRA2 (Kovacs-Nagy et al., 2018; Mandel et al., 2016; Oláhová et al., 2017) who also present with 3-MGA-uria.
The function of TIMM50 has been studied in different organisms and cellular models (Geissler et al., 2002; Mokranjac et al., 2003; Mokranjac et al., 2009; Tamura et al., 2009), but the pathophysiological mechanisms underlying TIMM50 deficiency as a cause of human disease has not yet been completely understood. Interestingly, while preparing this manuscript, Reyes et al. (2018) provided the first mechanistic insight that delineates some of the pathophysiological consequences of TIMM50 impairment in human disease. Specifically, they demonstrated that TIMM50 mutations impair mitochondrial protein import through the TIM23 complex, resulting in decreased levels of several components of the mitochondrial respiratory chain and in reduced mitochondrial respiration. Our results are complementary to these observations and provide new aspects underlying the mitochondrial dysfunction caused by TIMM50 mutations.
As TIMM50 is a component of the inner mitochondrial membrane translocase, we wondered whether the sublocalization of mitochondria-targeted proteins could be affected in our patient. However, no mislocalization in any of the nuclear-encoded mitochondrial proteins, included in our study, was detected in the patient fibroblasts. Accordingly, a study performed in mitochondria from Timm50-deficient yeast cells demonstrated that inactivation of TIMM50 had a variable effect on the import of different mitochondria-targeted proteins (Schendzielorz et al., 2017), which could explain the normal localization of the subset of proteins we observed in the patient fibroblasts.
On the other hand, Reyes et al. (2018) demonstrated that TIM23-dependent protein import was severely affected in mitochondria isolated from TIMM50 patient fibroblasts. In addition, these authors also showed that the steady state levels of particular components of complexes I, II, and IV were significantly reduced in these cells; in contrast, we did not observe any reduction in the steady state levels with the ten OXPHOS subunits tested, including those found to be reduced in the previously reported patient (Reyes et al., 2018). Interestingly, the analysis of OXPHOS components in the muscle tissue from our patient showed reduced levels of SDHA (complex II), MTCO1, and COX4L (complex IV) when normalized to the CS activity. It is remarkable that these three proteins were also reduced in fibroblasts of the patient reported by Reyes et al. (2018) although they did not determine its expression in muscle. The differences observed between our patient and the patient reported by Reyes et al. (2018) could be because of the different TIMM50 mutations identified. The patient reported here is compound heterozygous for two missense mutations affecting amino acids localized in the intermembrane domain of the protein (p.Arg114Gln and p.Gly269Ser). In contrast, the patient reported by Reyes et al. (2018) harbors a nonsense mutation (p.Ser122Ter) in one allele, encoding for a potentially truncated protein that is likely not expressed under physiological conditions, together with a missense mutation (p.Gly190Ala) on the other allele that maps to the conserved transmembrane domain of the protein. The clinical progression of our patient was also different and was similar to the initially reported TIMM50 patients, who also had missense mutations affecting amino acids of the intermembrane portion of the protein (Shahrour et al., 2017). In contrast, the patient of Reyes et al. (2018) showed a severe and rapid progression of the disease, leading to death at 2 years of age. Therefore, we speculate that the TIMM50 mutations described herein may have a milder impact on the TIMM50 protein function.
A key aspect of the mitochondrial function is to maintain its morphology. This highly regulated process relies on the adequate structural organization of the inner mitochondrial membrane as well as regulation of fusion/fission cycles (Kasahara & Scorrano, 2014). In fact, mitochondria morphological abnormalities have been observed in patients with 3-MGA-uria mutations in QIL1 (Guarani et al., 2016; Zeharia et al., 2016), TMEM70 (Cameron et al., 2011; Jonckheere et al., 2011), and OPA3 (Grau et al., 2013; Powell, Davies, Taylor, Wride, & Votruba, 2011). In addition, cells depleted for DNAJC19 expression also shown aberrant cristae structure (Richter-Dennerlein et al., 2014). These findings prompted us to analyze mitochondrial network and morphology in the TIMM50 patient fibroblasts in comparison with fibroblasts from controls or to those from a patient with mutations in TMEM70. Interestingly, our results demonstrated that the TIMM50 fibroblasts had significantly shorter and rounder mitochondria, together with a reduction in the branching degree of the mitochondrial network. In addition, a detailed TEM analysis of mitochondria ultrastructure revealed abnormalities of cristae organization. These alterations were less pronounced than those seen in TMEM70 fibroblasts, probably because of the fact that TMEM70 is an assembly factor of complex V and is directly involved in their dimerization, a process required for the proper maintenance of cristae shape (Cogliati, Enriquez, & Scorrano, 2016; Jonckheere, Smeitink, & Rodenburg, 2012). In a physiological context, the mitochondrial network maintenance is regulated by the balance between mitochondrial fusion and fission cycles (Sesaki & Jensen, 1999). However, the morphological defects observed in TIMM50 fibroblasts are not likely to rely on mitochondrial fusion/fission imbalances, as these events were unaltered in patient-derived fibroblasts, as seen by the normal ratio between the proteins DRP1 (involved in fission) and MFN2 (involved in fusion). We speculate that the alterations of mitochondrial morphology observed in TIMM50 patient cells could be linked to structural changes that affect the inner mitochondrial membrane because of the reduction of the TIMM50 protein. In fact, the mitochondrial import machinery has been reported to be linked to the mitochondrial inner membrane organizing system, which is necessary for maintaining mitochondrial cristae structure (Becker, Böttinger, & Pfanner, 2012). In addition, alterations in TIM17, another component of TIM23 complex, have been recently found to be associated with abnormal mitochondrial morphology (Matta, Pareek, Bankapalli, Oblesha, & D'Silva, 2017).
Variable defects in the OXPHOS system have been reported in patients with 3-MGA-uria (Wortmann et al., 2013). Several pieces of evidence reported a physical connection between the TIM23 complex and the mitochondrial respiratory chain complexes and supercomplexes (Becker, Böttinger, & Pfanner, 2012). Here, the analysis of the mitochondrial respiratory chain complexes in patient muscle biopsy showed a generalized reduction in the activities of complexes I–IV when normalized to the CS activity, while the previously reported patients showed variable mitochondrial respiratory chain defects, ranging from a normal activity detected in one patient to an isolated defect in the activities of complex V (Shahour et al., 2017) or complex II (Reyes et al., 2018). However, the impact of TIMM50 mutations in the assembly of the OXPHOS system and in supercomplexes formation has not been explored in any of the previously reported patients. We demonstrated for the first time a significant reduction in the levels of fully assembled OXPHOS complexes and supercomplexes in an individual with TIMM50 mutations. The effects of these alterations on the mitochondrial function were determined by high-resolution respirometry. We demonstrated that TIMM50-mutated fibroblasts had a significant reduction of the maximal respiratory capacity, suggesting that the optimal function of the electron transport chain might be compromised. These observations were further corroborated in a TIMM50-deficient model generated in HEK293T cells that mimicked the respiratory defect observed in the patient fibroblasts; in HEK293T cells, this defect could be rescued by transfection with a plasmid encoding for the TIMM50 wild-type protein.
In summary, our study provides new insight into the physiopathological mechanisms underlying mitochondrial energy metabolism associated with 3-MGA-uria. In particular, this study together with the recently reported observations of Reyes et al. (2018) represents the first comprehensive characterization of the molecular mechanisms underlying the pathogenesis of the mitochondrial disease caused by TIMM50 mutations. We demonstrated that TIMM50 deficiency leads to a severe mitochondrial dysfunction by targeting pivotal aspects of mitochondrial physiology, such as assembly of OXPHOS complexes and supercomplexes, and maintenance of mitochondria morphology. Moreover, we have demonstrated that these alterations have a remarkable impact on mitochondrial functionality, leading to a defect characterized by an important reduction of the maximal electron transport chain respiratory capacity.
ACKNOWLEDGMENTS
We would like to thank the advanced optic microscopy and the electron microscopy facilities of the CCiT-UB. We also thank Dr Miguel Angel Martín from Hospital 12 de Octubre, Madrid for providing the muscle biopsy. We are grateful to the family involved in this study. This work was performed in the context of the Biomedicine PhD Program of the University of Barcelona (UB).
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICS
All procedures were approved by the ethics committee of the Hospital Clínic, Barcelona.