Functional classification of ATM variants in ataxia-telangiectasia patients
Present Address: Alice Fievet, Gustave Roussy, Service Génétique des Tumeurs, Villejuif, France.
Abstract
Ataxia-telangiectasia (A-T) is a recessive disorder caused by biallelic pathogenic variants of ataxia-telangiectasia mutated (ATM). This disease is characterized by progressive ataxia, telangiectasia, immune deficiency, predisposition to malignancies, and radiosensitivity. However, hypomorphic variants may be discovered associated with very atypical phenotypes, raising the importance of evaluating their pathogenic effects. In this study, multiple functional analyses were performed on lymphoblastoid cell lines from 36 patients, comprising 49 ATM variants, 24 being of uncertain significance. Thirteen patients with atypical phenotype and presumably hypomorphic variants were of particular interest to test strength of functional analyses and to highlight discrepancies with typical patients. Western-blot combined with transcript analyses allowed the identification of one missing variant, confirmed suspected splice defects and revealed unsuspected minor transcripts. Subcellular localization analyses confirmed the low level and abnormal cytoplasmic localization of ATM for most A-T cell lines. Interestingly, atypical patients had lower kinase defect and less altered cell-cycle distribution after genotoxic stress than typical patients. In conclusion, this study demonstrated the pathogenic effects of the 49 variants, highlighted the strength of KAP1 phosphorylation test for pathogenicity assessment and allowed the establishment of the Ataxia-TeLangiectasia Atypical Score to predict atypical phenotype. Altogether, we propose strategies for ATM variant detection and classification.
1 INTRODUCTION
Ataxia-telangiectasia (A-T; MIM# 208900) is a human recessive genetic disorder caused by biallelic inactivation of the ataxia-telangiectasia mutated gene (ATM; Savitsky et al., 1995). This pediatric syndrome is characterized by progressive cerebellar degeneration, telangiectasia, increased susceptibility to lymphoma and leukemia and later to solid tumors, radiosensitivity, immunodeficiency, and infertility (Perlman, Boder, Sedgewick, & Gatti, 2012; Shiloh & Ziv, 2013; Suarez et al., 2015). ATM is a phosphatidylinositol 3-kinase protein, involved in signaling and repair of double-strand breaks (DSB) and control of the cell cycle. DSB ATM activation leads to its phosphorylation and dissociation of inactive ATM dimers into active ATM monomers phosphorylated on Ser1981 (Bakkenist & Kastan, 2003). This activation allows many processes, including chromatin relaxation via KAP1 phosphorylation, DSB recognition, and signal spreading via H2AX phosphorylation. ATM indirectly activates cell cycle checkpoints after phosphorylation of CHK2 and initiates DNA repair (Perlman et al., 2012; Shiloh & Ziv, 2013).
Due to the dramatic increase of large scale genetic analyses in many clinical conditions, a number of pediatric and adult patients are found with biallelic ATM variants associated with atypical and/or delayed and/or mild clinic-biological features (Dork, Bendix-Waltes, Wegner, & Stumm, 2004; Keimling et al., 2011; Meissner et al., 2013; Meneret et al., 2014; Verhagen et al., 2012). Therefore, the pathogenicity of such ATM variants, especially in an unusual clinical context is a daily question in diagnostic laboratories.
To better define a strategy for evaluating the pathogenicity of ATM variants, we analyzed a large series of typical and atypical A-T patients by genomic and transcript analyses, protein expression, sub-cellular location analysis, kinase activity through ATM targets proteins phosphorylation, and cell cycle analysis. This strategy using biallelic ATM mutated cell lines, with a majority of homozygous or compound heterozygous with one known pathogenic variant, allowed us to demonstrate the pathogenicity of the 49 ATM variants of this series. Furthermore, we compared the functional consequences of variants found in typical and atypical A-T patients and found that the defect of KAP1 phosphorylation was a robust marker of variant pathogenicity, while a combined approach of functional tests lead us to establish a score predicted of the phenotype (Ataxia-TeLangiectasia Atypical Score [ATLAS]). Our approach constitutes an effective strategy for classification of ATM variants.
2 MATERIALS AND METHODS
2.1 Patient selection and ATM variant detection and classification
Among the 36 patients, 16 cases were already described in the literature (Dork et al., 2004; Jacquemin et al., 2012; Meissner et al., 2013; Micol et al., 2011; van Os et al., 2019), and 20 were unreported patients. Those unreported patients suggestive of A-T were referred to the Institut Curie genetics laboratory for ATM mutation screening. Patients, or their parents when they were underage, gave their informed consent for ATM gene analysis and subsequent researches. Germline DNA and lymphoblastoid cell lines (LCLs) were derived from blood samples. ATM variants were screened by Sanger sequencing associated with multiplex ligation-dependent probe amplification (MLPA) as previously described (Micol et al., 2011), or by Next-Generation Sequencing (NGS) with a home-made gene panel as previously described (Fievet et al., 2019). Depth of coverage was > 30X. Variant calling was performed using VarScan2, Pindel, MELT (Gardner et al., 2017), and DESeq. In silico prediction of splice defects was performed using the MaxEntScan tool (MaxEnt; Yeo & Burge, 2004). Variants associated with a predicted decreased score above 15% of acceptor or donor splice sites and/or a predicted cryptic splice site stronger than the canonical site were further explored. The reference gene sequence is NM_000051.3. Forty-nine different variants were identified in these patients, including 24 variants for which the pathogenic effect needed to be highlighted. These 24 variants were either unreported in ClinVar (15 variants), reported as “uncertain significance” (6) or “likely pathogenic” (3). The ClinVar classification dated September 14th, 2017 was used (www.ncbi.nlm.nih.gov/clinvar/?term=ATM%5Bgene%5D). These new variants were added to the LOVD database (https://databases.lovd.nl/shared/variants/ATM).
2.2 Cell culture and treatment
Epstein-Barr virus-immortalized LCLs were established by Genethon (http://www.genethon.fr/); five LCLs derived from healthy individuals were provided by the Hôpital Cochin biobank. The LCL derived from an NBS patient with the NBN (NM_002485.4:c.657_661del) variant (GM15808) was obtained from the NIGMS Human Genetic Mutant Cell Repository (Camden, NJ). LCLs were grown in RPMI 1640 (Invitrogen, Oxon, UK) supplemented with 15% of heat-inactivated fetal calf serum (FCS) (Invitrogen) at 37°C in a humidified incubator with 5% CO2. Cell viability threshold for ATM signaling experiments determined using the trypan blue exclusion assay was 90%. One-hour treatment with 1 µM camptothecin (CPT; Sigma-Aldrich) was performed for phospho-KAP1 (pKAP1) and phospho-CHK2 (pCHK2) activation by western blot. ATM inhibition was performed with 1 µM of KU60019 (Tocris), added 1 hr before CPT treatment. Cell cycle arrest was induced by 4 nM CPT for 24 and 48 hr.
2.3 cDNA sequencing
Total RNA was extracted from LCLs and reverse-transcribed using the GeneAmp RNA PCR Core kit according to the manufacturer's instructions (Applied Biosystems). cDNA was amplified and sequenced surrounding predicted splicing defect or tandem duplication breakpoints. Primer sequences and PCR amplification conditions are available upon request.
2.4 Western blot analysis
LCLs were collected by centrifugation at 1,400 rpm for 5 min and washed in phosphate-buffered saline. For the pKAP1 and pCHK2 studies, cell pellets were lysed for total extraction in radioimmunoprecipitation assay buffer (100 mM Tris-HCl/pH 7.5, 0.1 M NaCl, 1 mM EDTA, 1% Triton, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]), supplemented by a cocktail of protease and phosphatase inhibitors (cOmplete©, Roche; PhosSTOP, Merck).
Thirty to 40 µg of protein extracts containing 50 mM dithiothreitol and loading buffer were resolved by 4 to 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 4–15% Criterion™ TGX™ Precast Midi Protein Gel #5671085; Biorad) and transferred to 0.2 µm nitrocellulose membranes (ThermoFisher).
Membranes were blocked with Odyssey blocking buffer (LI-COR #927–40000) for 1 hr at room temperature (RT) and incubated in Odyssey blocking supplemented with Tween 20 to a final concentration of 0.1% overnight at 4°C with primary antibodies at the indicated concentration: ATM (1/1,000, NB110–55475; Novus Biologicals), phospho-KAP1 Ser824 (1/2,000, A300–767A; Bethyl Laboratories), KAP1 (1/1000, A300-275A; Bethyl Laboratories), phospho-CHK2 Thr68 (1/1,000, #2661; CST), CHK2 (1/1,000, #3440; CST). β-Actin (1/10,000, A-5316; Sigma-Aldrich) was used as a control for protein loading.
After incubation with appropriate fluorescent secondary antibody (1/10,000 mixed with 0.1% Tween-20), immunoreactive bands were visualized using LI-COR-Odyssey infrared scanner (LI-COR) and band intensities were quantified using the ImageJ software (imagej.nih.gov/ij/). The mean levels of ATM, p-KAP1 and p-CHK2 of healthy controls were normalized at 1. Eight independent western blots were performed with four healthy controls to define the mean standard deviation (SD) of ATM, p-KAP1 and p-CHK2 levels in healthy controls (0.143, 0.087, and 0.108, respectively).
For ATM subcellular localization, cell pellets were lysed with NE-PER™ nuclear and cytoplasmic extraction reagents (#78833; Pierce) according to the manufacturer's instructions. Corresponding fractions of cytoplasmic and nuclear extracts from equal cell numbers were separated in SDS-PAGE gel. The purity of each extract was monitored by GAPDH (1/1000, #9485; Abcam) and INI1 (1/1,000, BAF47-25, BD Pharmingen) as markers of the cytoplasmic and nuclear compartments, respectively. The healthy controls of the 9 western blots produced were used to determine the mean percentage and SD of ATM nuclear fraction.
2.5 Cycle analysis by fluorescence-activated cell sorting
After 24 or 48 hr, cells were fixed with 4% paraformaldehyde (PFA) for 15 min, washed in Tris buffer saline (TBS) with 1% bovine serum albumin (BSA), permeabilized with fresh TST solution (0.2% Triton X-100/0.2% FCS) for 10 min, washed in TBS with 1% BSA, and nuclei were stained with DAPI 4 ng/ml (#62248; Thermo Fisher Scientific). A FACS LSRII instrument (BD Biosciences) was used and the results were analyzed with the FlowJo software (v.7.6.5). Both debris and doublets were removed from the analysis. Cell cycle distribution was analyzed with the FlowJo cell cycle Watson (Pragmatic model). Results are based on a minimum of two separate experiments.
2.6 Construction of plasmids and transfection
Five ATM variants were constructed using oligonucleotide-mediated mutagenesis (QuickChange site-directed mutagenesis kit; Agilent Technologies, Santa Clara, CA; sequences available on request) from a wild-type (WT) pcDNA3.1(+) Flag-His-ATM plasmid (a kind gift of M. Kastan; Figure S5). One ATM construct with deletion of exon 31 was performed by long-range RT-PCR from RNA extracted from AT26 LCL, and cloned into pcDNA3.1 ATM WT plasmid. After transformation and extraction, the DNA sequence of each mutant was confirmed by DNA sequencing. Transient transfections of GM09607 fibroblasts (ATM−/−) were performed using Lipofectamine® LTX Reagent with PLUS™ Reagent (Invitrogen) for plasmid transfections.
2.7 Immunofluorescence
GM09607 fibroblasts were plated in four-well Lab-Tek plates 24 hr before the experiment and fixed for 10 min in TBS, 4% PFA, washed in TBS, permeabilized for 5 min in TBS 0.1% SDS. Cells were washed in TBS, blocked for 1 hr in TBS 10% BSA, washed in TBS and incubated for 1 hr at RT with 1/1000 of FLAG (Sigma) primary antibody. Cells were washed in TBS, incubated with secondary antibody Alexa Fluor 488 (1/400; Molecular Probes) for 1 hr at RT in the dark. Slides were air-dried and mounted in VectaShield (H1200; Vector Laboratories) with DAPI. Image acquisitions were performed using an inverted laser scanning confocal LSM 700 microscope UV Zeiss equipped with x63 and x40 oil objectives. Images were processed with the Zeiss software (Zen).
3 RESULTS
3.1 Clinico-biological features of the series of A-T patients
Patients referred for ATM analysis mostly presented typical clinical features, such as ataxia with onset in early childhood and immunodeficiency. However, some patients had delayed and attenuated/absent clinical features. These atypical patients are often diagnosed in adulthood, on the basis of laboratory test results suggestive of A-T, such as increased serum level of alpha-fetoprotein or the presence of at least 5% of mitoses of a lymphocyte karyotype harboring translocations between chromosomes 7 and 14 (Table S1). We arbitrarily selected six typical A-T clinical features to grade the phenotype: (a) onset of ataxia before 8 years old (y-o), (b) loss of walking ability before 15 y-o, (c) oculomotor apraxia before 15 y-o, (d) ocular telangiectasia before 15 y-o, (e) clinical immunodeficiency and (f) profound IgA deficiency (Table 1). We then classified the patient's phenotype as typical when more than 50% of positive criteria or atypical when a maximum of 50% of positive criteria, with a minimum of two available criteria. Twenty-two patients were classified as typical and 13 as atypical using the criteria described above. One patient (AT36) could not be classified because of her young age.
| Patients | Ataxia <8 year | Loss of walking ability <15 year | Oculomotor apraxia <15 year | Ocular telangiectasia <15 year | IgA deficiency | Clinical ID | % criteria | Phenotype classification |
|---|---|---|---|---|---|---|---|---|
| AT01† | Yes | NA | Yes | Yes | Yes | No | 80 | Typical |
| AT02†, ‡ | Yes | Yes | Yes | Yes | Yes | Yes | 100 | Typical |
| AT03‡ | Yes | No | Yes | NA | Yes | NA | 75 | Typical |
| AT04‡, § | Yes | No | Yes | Yes | No | Yes | 67 | Typical |
| AT05‡ | Yes | NA | Yes | Yes | No | No | 60 | Typical |
| AT06‡ | Yes | NA | Yes | Yes | Yes | Yes | 100 | Typical |
| AT07‡ | Yes | No | NA | Yes | Yes | NA | 75 | Typical |
| AT08‡ | Yes | NA | NA | Yes | Yes | Yes | 100 | Typical |
| AT09‡ | Yes | NA | Yes | Yes | Yes | No | 80 | Typical |
| AT10† | Yes | NA | NA | Yes | Yes | Yes | 100 | Typical |
| AT11† | Yes | NA | NA | Yes | Yes | NA | 100 | Typical |
| AT12 | Yes | NA | Yes | Yes | No | No | 60 | Typical |
| AT13 | Yes | Yes | Yes | Yes | No | No | 67 | Typical |
| AT14 | Yes | Yes | NA | Yes | Yes | Yes | 100 | Typical |
| AT15 | Yes | NA | NA | Yes | Yes | No | 75 | Typical |
| AT16 | Yes | NA | Yes | Yes | Yes | Yes | 100 | Typical |
| AT17 | Yes | NA | NA | NA | Yes | NA | 100 | Typical |
| AT18 | Yes | Yes | Yes | Yes | NA | No | 80 | Typical |
| AT19 | Yes | NA | Yes | NA | Yes | Yes | 100 | Typical |
| AT20 | NA | Yes | Yes | NA | NA | NA | 100 | Typical |
| AT21 | Yes | NA | NA | NA | Yes | NA | 100 | Typical |
| AT22 | Yes | NA | NA | NA | Yes | No | 67 | Typical |
| AT23¶ | No | No | No | No | Yes | No | 17 | Atypical |
| AT24‡, § | Yes | No | NA | NA | No | No | 25 | Atypical |
| AT25† | No | No | NA | No | NA | No | 0 | Atypical |
| AT26 | No | NA | NA | No | Yes | NA | 33 | Atypical |
| AT27 | NA | No | NA | No | Yes | No | 25 | Atypical |
| AT28 | Yes | No | Yes | Yes | No | No | 50 | Atypical |
| AT29 | Yes | No | No | No | Yes | No | 33 | Atypical |
| AT30 | Yes | No | No | No | No | No | 17 | Atypical |
| AT31 | No | No | NA | NA | No | No | 0 | Atypical |
| AT32 | No | No | No | Yes | No | No | 17 | Atypical |
| AT33 | No | No | No | NA | No | No | 00 | Atypical |
| AT34 | No | No | No | No | No | No | 00 | Atypical |
| AT35⊣ | Yes | No | NA | NA | NA | NA | 50 | Atypical |
| AT36§ | NA | NA | NA | NA | NA | No | NA | NA |
Two criteria appeared to better define typical or atypical A-T phenotypes: the loss of walking ability before 15 y-o and clinical immunodeficiency were only observed in typical A-T patients; the absence of ataxia at 8 y-o and ocular telangiectasia or oculomotor apraxia at 15 y-o were only observed in atypical A-T patients.
3.2 Detection and classification of ATM variants
In this series of 36 A-T patients, ATM characterization was performed by Sanger sequencing associated with MLPA in 30 cases. Six patients were directly sequenced by NGS. Four of the Sanger-sequenced patients were secondly sequenced by NGS to identify the second missing ATM variant, successfully in two cases. The second missing variant was identified by transcript analysis in one additional case (Figure S1; Table S2). Therefore, biallelic ATM variants were found in all A-T patients, with the exception of a typical A-T case (AT09), in whom only one monoallelic variant was identified. Among the 49 different variants, 17 were classified as “pathogenic” in ClinVar (one start codon loss, one intronic, five missense, six frameshift, and four nonsense variants), eight were other truncated variants, reported as “likely pathogenic” in ClinVar (2) or unreported (6; one splice donor site, four frameshift, and three nonsense variants). The remaining 24 variants not leading to obvious truncated protein were either reported in ClinVar and classified as “likely pathogenic” (3/24), “uncertain significance” (6/24), or never reported (15/24). These variants needed to be evaluated for pathogenic effects (6 intronic and 16 missense variants, 2 large duplications; Table S2). Importantly, nine of these 24 variants were at homozygous state, which was ideal condition to transcript and protein analyses. Eight other unknown significance variants were in trans with variants leading to absent or different sized ATM protein products, which allowed to attribute the status of each variant ATM protein product.
3.3 Characterization of ATM transcripts
-
The cDNA sequence of AT17 LCL revealed an out-of-frame retention of 58 bases from intron 18, due to a de novo donor site created by the c.2639–384A>G variant (Figure 1a).
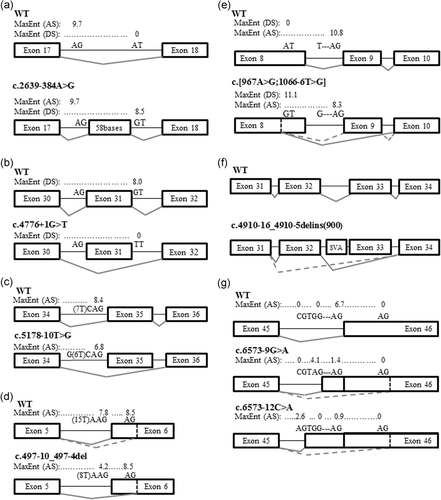 Figure 1
Figure 1ATM transcript analyses. (a) AT17 transcript analysis. (b) AT26 transcript analysis. (c) AT30 transcript analysis. (d) AT15 transcript analysis. (e) AT16 and AT20 transcript analysis. (f) AT18 transcript analysis. (g) AT03 transcript analysis. AS, acceptor site score; DS, donor site score; dashed line, minor transcript; MaxEnt, MaxEntScan in silico prediction
-
The c.4776+1G>T variant of AT26 LCL was predicted to abolish the donor site of exon 31, and in-frame exon 31 skipping was observed (Figure 1b).
-
The c.5178–10T>G variant of AT30 LCL was predicted to weaken the acceptor site of exon 35 (MaxEnt: −19.4%), and out-of-frame exon 35 skipping was observed (Figure 1c).
-
The c.497–10_497-4del variant of AT15 LCL was predicted to weaken the acceptor site of exon 6 (MaxEnt: −46.1%). The use of a cryptic acceptor site in exon 6 and an out-of-frame deletion of 22 bases were observed (Figure 1d).
-
The c.967A>G variant in cis of the c.1066–6T>G relatively common variant (rs201686625, allele frequency of 0.23% in the non-Finnish European population of Exac) of AT18 LCL was predicted to create a de novo donor site in exon 8. Two transcripts resulting from this complex c.[967A>G;1066-6T>G] variant were observed: the major one with an out-of-frame deletion including 99 bases of exon 8 and the entire exon 9, and the minor transcript with only in-frame deletion of 99 bases of exon 8 (Figure 1e).
-
The c.4910–16_4910-5delins(900) variant of AT03 LCL was detected by MELT analysis of the NGS sequencing (Gardner et al., 2017). This insertion in intron 32 was a SVA sequence (SINE-VNTR-Alu: nonautonomous retrotransposon; Shen et al., 1994). In-frame exon 33 skipping and out-of-frame exon 32–33 skipping were observed (Figure 1f).
-
The c.6573–9G>A and c.6573–12C>A variants of patient AT16 and AT20 LCLs were both predicted to weaken the acceptor site of exon 46 (MaxEnt: −78.8% and −86.3%, respectively), and to create intronic de novo acceptor sites. Major transcripts were observed with out-of-frame retentions of 7 bases and 10 bases of the intron 45, respectively. Minor transcripts using a cryptic acceptor site in exon 46 leading to the in-frame deletion of 81 bases were also observed in both LCLs (Figure 1g).
-
The cDNA of the AT17 and AT33 LCLs with duplications of the exons 18 to 33 and 17 to 61, respectively, were analyzed and the tandem positions were confirmed. These c.(2638+1_2639-1)_(5005+1_5006-1)dup and c.(2466+1_2467-1)_(8850+1_8851-1) dup variants lead to in-frame duplication of 789 and 2,128 amino acids, respectively.
-
An apparently full-length ATM protein was detected in patient AT13 LCL harboring a homozygous c.491_492insT variant in exon 5 (see below). Transcript analysis highlighted the use of the same cryptic acceptor site in exon 6 as in case AT15, located 22 bases downstream from the canonic acceptor site. Consequently, the insertion of one base restored the correct reading frame in a transcript with a deletion of 22 bases, explaining the detectable full-length ATM.
| Variants | Patients | Status | RNA sequencing | Protein prediction | Detected ATM proteins with western-blot |
|---|---|---|---|---|---|
| c.4910–16_4910–5delins(900) | AT03 | HTZ | r.[4910_5005del,4777_5005del] | p.[(Asp1637_Leu1668del,Glu1593Argfs*13)] |
|
| c.491_492insT | AT13 | HMZ | r.[491_492insT,492_516delinsTGTT] | p.[(Trp164Cysfs*21),(Trp164_Phe172delinsCysVal)] |
|
| c.497–10_497–4del | AT15 | HTZ | r.[497_518del,?] | p.[(Glu166Glyfs*4),?] |
|
| c.6573–9G>A | AT16 | HMZ | r.[6572_6573ins6573–7_6573–1,6573_6653del] | p.[(Arg2191Serfs*8,Arg2191_Phe2217del)]. |
|
| c.(2638+1_2639–1)_(5005+1_5006–1)dup | AT17 | HTZ | r.2639_5005dup | p.(Gly880_1668Leudup) |
|
| c.2639–384A>G | AT17 | HTZ | r.2638_2639ins[2639–442_2639–385] | p.(Gly880Glufs*15) | |
| c.[967A>G;1066–6T>G] | AT18 | HMZ | r.[967_1235del,967_1065del] | p.[(p.Ile323Alafs*17,Ile323_Gln355del)] |
|
| c.6573–12C>A | AT20 | HMZ | r.[6572_6573ins6573–10_6573–1,6573_6653del] | p.[(Arg2191Serfs*9,Arg2191_phe2217del)] |
|
| c.4776+1G>T | AT26 | HTZ | r.4612_4776del | p.(Val1538_Glu1592del) |
|
| c.5178–10T>G | AT30 | HTZ | r.5178_5319del | p.(Asp1726Glufs*8) |
|
| c.(2466+1_2467–1)_(8850+1_8851–1)dup | AT33 | HTZ | r.2467_8850dup | p.(Ala823_Glu2950dup) |
|
- Abbreviations: aa, amino acid; ATM, ataxia-telangiectasia mutated; HMZ, homozygous; HTZ, compound heterozygous; wt, wild-type.
ATM transcript in vitro analyses were informative in all cases, confirming the predicted in silico splicing defects and further revealing more complex abnormal splicing events.
3.4 Characterization of ATM protein products
ATM protein expression was evaluated by western blot analyses: the level of ATM was considered to be decreased when less than 3 SD of the mean (<−3SD) and intermediary when [−3SD;−2SD[ as compared with five control LCLs. The quantity of ATM protein was decreased for most A-T LCLs. However, three patients (AT25, AT28, AT34) had a normal level of ATM protein, and one patient (AT31) had an intermediary level (Figure S2). Considering the 91 independent values of the 36 A-T LCLs, the sensitivity of the ATM level <−3SD as biomarker to predict pathogenicity would be 0.81. Truncated ATM protein products were detected in the seven cases with nonsense or frameshift variants located 3′ of the motif recognition of the ATM antibody (surrounding serine 1981). Noteworthy, a truncated approximately 300 kDa product was observed for AT09, for which the causal variant has yet to be identified. ATM products compatible with the in-frame duplications of exons 18 to 33 and exons 17 to 61 were demonstrated in the two LCLs with large duplications (AT17, AT33, respectively). Apparently full-length ATM products were detected for all missense variants and predicted small in-frame exon skipping. Interestingly, apparently full-length ATM product was observed in the AT15 LCL with c.497-10_497-4del variant (in trans with a truncated variant), suggesting leaky or complex effects of this variant on splicing (Figure 2; Figure S3).
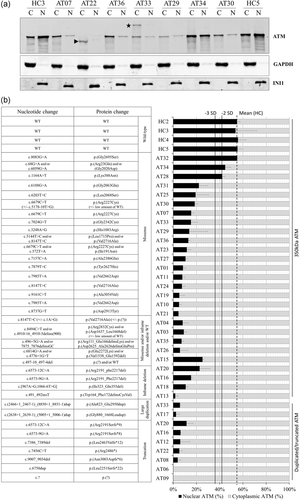
Localization and quantification of ATM. (a) Nuclear and cytoplasmic fractions from A-T and healthy controls lymphoblastoid cell lines. GAPDH was used as a cytoplasmic fraction (C) control. INI1 was used as nuclear fraction (N) control. Arrows: ATM truncated forms resulting from nonsense or frameshift variants. Stars: ATM with large tandem duplication. (b) The histogram shows the distribution of nuclear and cytoplasmic forms of ATM. Apparently full length 350 kDa protein were either WT or containing missense or inframe deletion (exon skipping). Truncated forms resulted from nonsense or frameshift mutation and higher forms resulted from large tandem duplication. Error bar represents one standard deviation (SD). HC: healthy control. Number of independent experiments: 2 to 9. A-T, ataxia-telangiectasia; ATM, ataxia-telangiectasia mutated; WT, wild type
We have previously shown that missense variants can lead to abnormal localization of ATM in the cytoplasm (Jacquemin et al., 2012). Localization of ATM was therefore investigated by western blot of nuclear and cytoplasmic protein extracts (Figure 2; Figure S3). The 18 healthy controls of the 9 western blots had an average of 55 ± 6.4% of ATM in the nucleus; ATM was considered to be delocalized in the cytoplasm when the percentage of nuclear ATM was decreased to below 3 SD in the nucleus, i.e. ≤36%, and ATM was considered not to be delocalized when the percentage of nuclear ATM was not less than 2 SD (−2 SD), i.e. ≤42%. Three LCLs (AT34, AT32, and AT28) from atypical A-T patients harboring at least one missense variant showed more than 42% of nuclear ATM, not supporting delocalization of ATM in the cytoplasm for these missense variants. To be noticed, AT34 carried two missense variants, preventing to attribute the normal localization to a given variant. Considering the 66 independent values of the 33 A-T LCLs with detectable ATM, the sensitivity of the percentage of nuclear ATM <−3 SD as a biomarker to predict pathogenicity was 0.91.
ATM was delocalized in the cytoplasm, with less than 36% in the nucleus in the other 30 A-T LCLs with detectable ATM. Furthermore, the absolute quantity of nuclear ATM was significantly decreased in all A-T LCLs except for AT34 (Figure S4). ATM proteins with large duplication or truncated forms were almost purely cytoplasmic, with less than 12% of nuclear ATM. For ATM proteins resulting from in-frame exon skipping or missense variants, delocalization was more variable, but always with prominent cytoplasmic ATM.
As a proof of concept, and to establish a direct link between certain variants and ATM delocalization in the cytoplasm, six variants (five missense and one splice variants) were modeled and prominent cytoplasmic localization of ATM was confirmed after transient transfection by immunofluorescence (Figure S5).
3.5 Functional characterization of ATM variants
ATM phosphorylates many target proteins after DNA damage (Matsuoka et al., 2007). The phosphorylation of two of these target proteins, CHK2 and KAP1, was analyzed after treatment of cell lines for 1 hr with the Topoisomerase 1 inhibitor camptothecin (CPT), which induced DSB during S-phase (Figure 3; Figure S6). Phosphorylation of CHK2 was globally decreased in A-T cell lines with a mean of 0.59 (range 0.35–0.89). However, only 26 LCLs had reduced phosphorylation to less than 0.68 (−3 SD), five LCLs (AT04, AT23, AT26, AT27, and AT28) had intermediary levels [0.68;0.78[ and the AT01, AT24, AT34, AT35, and AT36 LCLs had a similar level of p-CHK2 to that of healthy controls, i.e. more than 0.78 (−2 SD). Interestingly, the 36 cell lines tested showed reduced phosphorylation of KAP1 (<−3 SD) with a mean of 0.27 (range 0.13–0.45). Considering the 91 independent values of the 36 A-T LCLs, the sensitivity of the p-CHK2 and p-KAP1 levels <−3 SD as biomarkers to predict pathogenicity was 0.69 and 1, respectively. Altogether, the pathogenic effect of the 49 ATM variants was confirmed by their association with major defects of KAP1 phosphorylation, a highly ATM-dependent process.
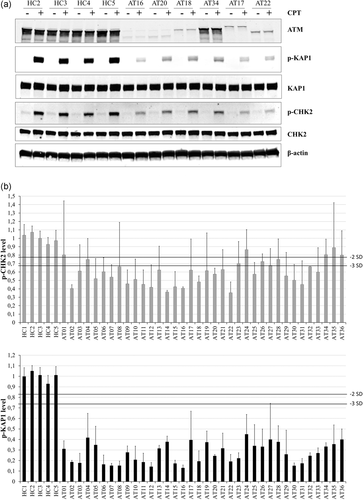
Phosphorylation of ATM target proteins. (a) Example of one western-blot of ATM and the phosphorylation of its target proteins KAP1 and CHK2 after CPT treatment (CPT: 1 µM for 1 hr). β-Actin was used as a loading control. (b) Histograms represent the quantity of p-CHK2 and p-KAP1 after CPT (1 µM for 1 hr) compared with the mean of healthy controls (HC). The p-KAP1 and p-CHK2 quantities were normalized on β-actin. Mean of ATM level of healthy controls was normalized at 1 for each western-blot. Error bar represents one standard deviation (SD). The healthy controls’ SD were calculated from experiments with four different HC. The A-Ts’ SD was calculated from experiments with at least three different HC. Number of independent experiments: 2 to 9. ATM, ataxia-telangiectasia mutated; CPT, camptothecin
It was noteworthy that all A-T cell lines had a low amount of phosphorylation of KAP1. Residual ATM kinase activity was expected for atypical patients with hypomorphic variants, but not for patients without detectable ATM. Therefore, to distinguish residual ATM kinase activity due to alternative activation pathways such as ATR, we first tested lower doses of CPT (80 nM–0,5 µM) and shorter exposure (10 and 30 min) on typical and atypical patients. The phosphorylation of KAP1 appeared similarly in both groups, suggesting that an alternative activation pathway mostly explained the apparent residual ATM activity (Figure 4a,b). We then tested the highly selective ATM-inhibitor KU60019 (1 µM) on LCLs to test the ATM-dependency of the low amount of p-KAP1 of the A-T LCLs (Figure 4c). Healthy controls pretreated with KU60019 had a drastic reduction of p-KAP1 after CPT exposure compared with healthy controls without ATM-inhibitor: only approximately 30% of expected p-KAP1 was present, suggesting that 70% of the p-KAP1 was ATM-dependent. We also confirmed this effect on the GM15808 cell line (NBN deficient) with residual ATM kinase activity. Indeed, this NBN cell line exposed to CPT treatment had reduced p-KAP1 (23% of healthy controls), further decreased by 47% with KU60019 pretreatment, suggesting that half of the observed p-KAP1 was ATM-dependent in this context (Figure 4c,d). We defined the ATM-dependent p-KAP1 as the level of p-KAP1 that disappears with pretreatment by the ATM-inhibitor. Analyzing seven typical A-T LCLs, eight atypical A-T LCLs, and the unclassified AT36, we showed that residual p-KAP1 was mostly ATM-independent in all A-T LCLs (Figure 4c,d). This was further confirmed in 20 additional A-T LCLs (Figure S7; Table S3).
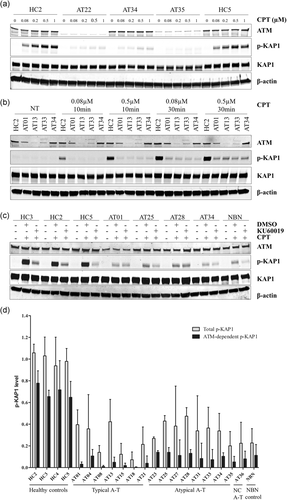
Detailed analysis of p-KAP1. (a) Western-blot of p-KAP1 with a range of concentrations of CPT (0.08, 0.2, 0.5, and 1 µM for 1 hr) on healthy controls, typical and atypical patients. β-Actin was used as a loading control. (b) Western-blot of p-KAP1 with two short exposure times (10 and 30 min) of two low concentrations of CPT (0.08 and 0.5 µM) on healthy control, typical and atypical patients. NT: not treated. β-Actin was used as a loading control. (c) Western-blot of p-KAP1 after CPT treatment (1 µM for 1 hr), with ATM-Inhibitor (KU60019, 1 µM, started 1 hr before CPT treatment) or DMSO (at the DMSO concentration of the reconstituted KU60019), on healthy controls, NBN deficient control (GM15808), typical and atypical patients. β-Actin was used as a loading control. (d) Quantification of the Total p-KAP1 (after DMSO pretreatment and CPT treatment) and the ATM-dependent p-KAP1 (difference between Total p-KAP1 and p-KAP1 after ATM-inhibitor (KU60019) pretreatment and CPT treatment). The p-KAP1 quantities were normalized on β-actin. Mean of Total p-KAP1 level of healthy controls (HC) was normalized at 1 for each western-blot; 1 to 3 healthy controls were used per experiments. Error bar represents one standard deviation (SD). Number of independent experiments: 2 to 10. NC: not classified. ATM, ataxia-telangiectasia mutated; CPT, camptothecin; DMSO, dimethyl sulfoxide
ATM is also known to play a major role in checkpoint control in response to DSB. This role was tested by cell cycle analysis in response to 24-hr exposure to CPT. As expected, cell cycle arrest in the S-phase was clearly visible in the five healthy control LCLs, as the mean percentage of cells in S-phase was 47.7 ± 9.1% and 36.5 ± 3.6% with or without treatment, respectively, while the mean percentage of cells in G2-M phase remained stable: 14.2 ± 1.9% and 14.2 ± 1.6% with or without treatment, respectively. The A-T LCLs tested did not present arrest in S-phase (with CPT: 29.5 ± 6.7%, without CPT: 37.6 ± 4.1%), but variable accumulation of cells in the G2-M phase (with CPT: 37.1 ± 10.9%, without CPT: 15.9 ± 2.3%). G2-M phase was considered to be increased compared with healthy controls when greater than +3 SD, i.e. >19.8%, and intermediary when situated between +2 SD and +3 SD, i.e. [17.9–19.8[. According to these criteria, G2-M was increased in 34 A-T LCLs tested, intermediary in AT23 (19.8%) and normal in AT36 with only 17.3% of G2-M after CPT at 24 hr (Figure 5). Considering the 79 independent values of the 36 A-T LCLs, the sensitivity of the G2-M percentage (24 hr) >+3 SD as a biomarker to predict pathogenicity was 0.94.
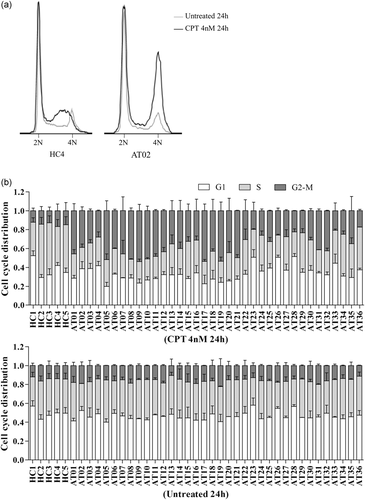
Cell cycle analysis. (a) Cell cycles with and without camptothecin (CPT) treatment (4 nM 24 hr) in representative healthy controls (HC4) and A-T (AT02) lymphoblastoid cell lines. (b) Cell distribution in G1, S, and G2-M phases with or without 24 hr of CPT treatment. Error bar represents one standard deviation (SD). Number of independent experiments: 2 to 6. A-T, ataxia-telangiectasia
Due to the variability of proliferation in LCLs, cell cycle analyses were then performed after 48 hr of CPT exposure. The 12 A-T cell lines with the lowest accumulation in G2-M were tested with four healthy controls and two A-T cell lines with high accumulation in G2-M. Healthy controls had 45.0 ± 10.2% of cells in the S-phase and 13.5 ± 1.9% of cells in the G2-M phase when exposed to CPT for 48 hr, while 33.1 ± 2.7% of cells were in S-phase and 13.6 ± 1.1% were in the G2-M phase when cells were not exposed to CPT. A-T LCLs had 22.7 ± 2.9% of cells in the S-phase and 29.8 ± 8.6% of cells in G2-M phase when exposed to CPT for 48 hr, while 30.6 ± 5.1% of cells were in S-phase and 14.9 ± 2.2% were in G2-M phase when cells were not exposed to CPT. The G2-M phase was considered to be increased compared with healthy controls when it was greater than +3 SD, that is >19.2%. Increased accumulation in G2-M at 48 hr was observed for 14 A-T LCLs. Nine A-T cell lines had higher accumulation in G2-M at 48 hr than at 24 hr, including AT23 and AT36 LCLs (Figure S8). Considering the 37 independent values of the 14 A-T LCLs, the sensitivity of the G2-M percentage (48 hr) >+3 SD as a biomarker to predict pathogenicity was 0.95.
Cell cycle analysis showed cell distribution differences, as healthy controls showed accumulation of cells in the S-phase after CPT exposure due to the presence of a functional S-phase checkpoint. A-T LCLs had no S-phase accumulation due to the presence of a defective S-phase checkpoint; as a result, cells with unrepaired damage accumulated in the G2-M phase. The pathogenic effect of the 49 ATM variants highlighted by weak phosphorylation of KAP1 was therefore confirmed by the defective S-phase checkpoint.
3.6 A-T patients’ comparison
Atypical patients’ results were confronted to those of typical patients to (a) determine the test the more reliable to predict variant pathogenicity, no matter the clinical phenotype (b) detect potential correlations between functional analyses and clinical phenotypes. The quantity of total ATM and nuclear ATM was significantly higher in the atypical A-T group than in the typical A-T group. However, no significant difference was observed when comparing only patients with at least one missense variant (Figure 6a,b). Noteworthy, three atypical A-T patients showed no delocalization of ATM in the cytoplasm (Figure 6c). In contrast with its poor sensitivity to highlight ATM-kinase activity deficiency, increased phosphorylation of CHK2 was observed in the atypical A-T group compared with the typical A-T group (p = .01; Figure 6d). The level of p-KAP1 was not significantly higher in the atypical A-T group as compared with typical A-T (Figure 6e). However, the analysis of the ATM-dependent p-KAP1 as previously defined, showed significant higher level in the atypical group (Figure 6f). Interestingly, LCLs from typical patients also had a significantly higher S-phase checkpoint defect after 24 hr of CPT as compared with atypical patients (Figure 6g).
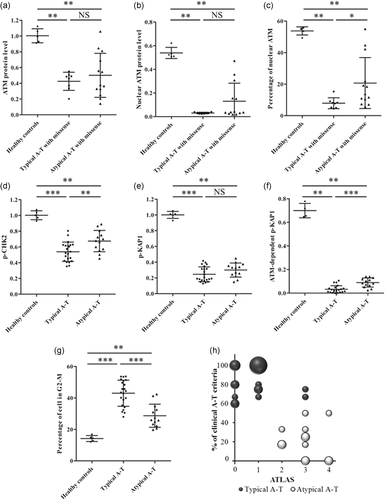
Comparison of functional tests in typical A-T and atypical A-T. (a) Comparison of total ATM quantity in A-T patients harboring a missense variant. (b) Comparison of nuclear ATM quantity in patients harboring a missense variant. (c) Comparison of nuclear ATM percentage in A-T patients harboring a missense variant. (d) Comparison of the level of phosphorylation of CHK2 after CPT treatment (1 µM, 1 hr). (e) Comparison of the level of phosphorylation of KAP1 after CPT treatment (1 µM, 1 hr). (f) Comparison of the level of ATM-dependent p-KAP1 after CPT treatment (1 µM, 1 hr). (g) Comparison of cell accumulation in phase G2-M after CPT treatment (4 nM, 24 hr). Mann–Whitney test. NS: no significant difference, *p < 0.05, **p < 0.01, ***p < 0.001. (h) Results of the Ataxia-TeLangiectasia Atypical Score (ATLAS) regarding the percentage of typical A-T clinical features (Table 1). The size of the bubbles is proportional to the number of patients. A-T, ataxia-telangiectasia; ATM, ataxia-telangiectasia mutated; CPT, camptothecin
-
Total ATM level >0.2 (1 being the mean of total ATM in healthy controls)
-
Nuclear ATM level >0.1 (1 being the mean of total ATM in healthy controls)
-
Percentage of cells in G2-M <35% (after 24 hr of CPT treatment at 4 nM)
-
ATM-dependent p-KAP1 level >0.05 (1 being the mean of total p-KAP1 in healthy controls)
Twenty typical patients had an ATLAS ≤ 1, while the 13 atypical patients had an ATLAS ≥ 2. Only two typical patients (AT03 and AT04) had an ATLAS ≥ 2 (3) and the unclassified patient also had an ATLAS = 3 (Figure 6h). Therefore, using a positive threshold at two, the sensitivity of the ATLAS is 1 and the specificity is 0.91.
4 DISCUSSION
A large study based on cell lines derived from A-T patients, including patients already described (Dork et al., 2004; Jacquemin et al., 2012; Meissner et al., 2013; Micol et al., 2011; van Os et al., 2019), was performed to assess the pathogenicity of unknown variants and to evaluate the consistency of our findings with previous analyses. Because of the highly variable presentation of the patients selected for this study, biological results were compared with the clinical phenotype to determine the best test including for hypomorphic variants and to assess a putative correlation.
A-T analyses included ATM transcript characterization, confirming the importance of this analysis for the diagnosis of A-T, as this analysis identified one deep intronic variant in one A-T patient, in whom standard exon and intronic junction sequencing had failed. The splicing consequences of this c.2639–384A>G variant, which creates a cryptic exon of 58 bases, were confirmed (Nakamura et al., 2012). Transcript analyses also showed two large rearrangements of ATM as being intragenic tandem duplications, allowing them to be classified as pathogenic. Finally, these analyses confirmed all in silico predictions of splice defects and revealed additional complexity of splice aberrations, such as minor transcripts. For example, ATM transcripts with a deletion from nucleotides 6,573 to 6,653 of the coding frame were identified in A-T cases harboring either c.6573–9G>A or c.6573–12C>A variants, which was not predicted by the bioinformatics tools or reported in the literature.
ATM protein quantification, qualification, and localization were performed at steady state. The total absence of ATM was observed in only three A-T patients harboring biallelic N-terminal truncating variants (variants located before the motif recognition of the ATM antibody). The nonsense mediated decay of transcripts harboring nonsense or frameshift variants may thus be mildly efficient for ATM. Abnormal ATM products predicted with large deletions or duplications were detected at expected sizes. In most cases, ATM was reduced and delocalized to the cytoplasm, confirming our previous observations (Jacquemin et al., 2012). Interestingly, despite the unequivocal diagnosis of A-T shown by a defective ATM kinase activity, no cytoplasmic delocalization of ATM was found in three cell lines derived from atypical A-T patients, two of which were also associated with a normal amount of ATM protein. No peculiar gene location or feature could be found for the missense variants harbored by these atypical A-T cases.
-
The c.967A>G variant has been previously reported to be a splicing variant, leading to no ATM protein (Lee et al., 2013). Analysis of an A-T LCL homozygous for this variant confirmed the predicted undetectable truncated ATM protein (r.967_1235del, p.(Ile323Alafs*17)). However, this c.967A>G variant was found in cis with the c.1066–6T>G variant in two unrelated families and it cannot be excluded that exon10 skipping is the consequence of the combination of the two variants. Minor transcripts were also detected in this cell line, leading to an in-frame deletion, with evidence of the predicted ATM product (r.967_1065del, p.(Ile323_Gln355del)) by western blot. Furthermore, no transcript with the (r.967A>G, p.(Ile323Val)) missense variant was detected.
-
ATM protein was detected in the A-T cell line homozygous for c.491_492insT. Transcript analysis was performed to determine the origin of this protein product and revealed an alternative transcript using an out-of-frame acceptor site in exon 6 in both healthy control and the A-T cell line. This alternative in-frame transcript could explain the ATM product detected in this patient. Interestingly, an apparently full-length ATM protein was also observed in the A-T cell line with c.497–10_497-4del variant and using the same out-frame acceptor site in exon 6. However, the predicted transcript was out-of-frame in this case, suggesting a leaky or more complex splice defect to explain the detectable ATM product.
Altogether, combined genomic, transcriptomic and protein analyses were instrumental to clarify the consequences of the ATM variants included in this study.
Total ATM protein was decreased in most A-T cases, even for missense variants. However, for missense variants, this decrease in ATM protein mainly concerned nuclear ATM, whereas the cytoplasmic fraction was less impacted, if at all. This cytoplasmic localization of apparently full-length ATM, such as in AT15 LCL, could be helpful for classifying this protein product as defective. This delocalization is not explained by variants in ATM nuclear localization signal (NLS, 385KRKK388; Young et al., 2005), as only one A-T cell line (AT28) had a missense p.(Lys388Asn) within the NLS, and paradoxically was one of few cell lines without delocalization of ATM (Figure S9). It has been previously shown that missense variants prevent nuclear localization of large proteins such as ATM or BRCA1 (Jacquemin et al., 2012; Thouvenot et al., 2016). Abnormal localization and stability of ATM could result from its incapacity to form stable dimers, as most missense or truncated forms studied had an altered or missing FATKIN domain (FRAP, ATM, TRRAP, and C-terminal phosphatidylinositol 3-kinase-like kinase domain; Figure S6), which mediates ATM dimerization (Baretic et al., 2017).
Biallelic truncating variants lead to absence or very small amounts of ATM and are correlated with the severe phenotype (Micol et al., 2011). However, the quantities of total and nuclear ATM among missense variants were not correlated with the phenotype. Nevertheless, the three outliers with normal ATM localization and the five patients with the highest nuclear ATM protein levels were all atypical patients. The effect of ATM variants on nuclear localization could, therefore, be essential for their pathogenic effects. ATM is required for signaling and repair of a subset of DSB, including those located in heterochromatin (Goodarzi et al., 2008). The phosphorylation of KAP1 by ATM leads to the opening of the heterochromatin structure, facilitating the DNA repair process (Ziv et al., 2006). KAP1 was selected as a marker of ATM kinase activity, because of its strong ATM dependency for phosphorylation. Defective ATM kinase activity was confirmed in the LCLs from all patients in response to the topoisomerase1 inhibitor camptothecin, including in challenging atypical A-T forms, confirming previous finding that phosphorylation of KAP1 is consistently decreased even in A-T patients with missense mutations and atypical forms of A-T (Roohi et al., 2017). However, KAP1 phosphorylation was variable among A-T LCLs.
Noteworthy, Keimling et al. (2011) measured the radiation-induced p-KAP1 level of two atypical A-T patients, including AT35, and seven typical A-T LCLs: the level was very low for everyone (<20% of healthy controls), however, the two atypical patients had higher level than typical patients. The second atypical patient harbored the c.8147T>C variant, repeatedly reported to have residual ATM kinase activity (Demuth et al., 2011; Verhagen et al., 2009) and to be associated with milder phenotype: longer survival, prolonged ability to walk, less immunodeficiency and less telangiectasia (van Os et al., 2019). Interestingly, AT04, AT24, and AT36 included in Van Os’ study also had the highest p-KAP1 level of our 36 A-T LCLs. Therefore, our findings regarding the phosphorylation of KAP1 are consistent with published data of independent laboratories.
Surprisingly, the previously reported correlation between ATM kinase activity and patient phenotype was not confirmed when measuring p-KAP1 after CPT (Verhagen et al., 2012). However, the more specific ATM-dependent p-KAP1, defined as the p-KAP1 that disappeared after ATM-inhibitor pretreatment, highlighted a significant difference between typical and atypical patients and confirmed the residual ATM-activity of atypical patients. The level of KAP1 phosphorylation in response to DNA damage, therefore, can be used as a robust marker of A-T diagnosis, whereas specific ATM-dependent p-KAP1 may be used as a potential marker to classify patients’ outcomes (Figure S10).
In response to DSB, ATM activates cell cycle checkpoints in G1/S, intra-S, and G2/M, preventing the dramatic consequences of unrepaired DSB during mitosis through cell division (Painter, 1981; Painter & Young, 1980; Shibata et al., 2010; Xu, Yang, Brugarolas, Jacks, & Baltimore, 1998). After CPT, DSB is generated during S-phase, which activate ATM intra-S checkpoint, ATM/MRN-dependent resection, and consequently the ATR-dependent intra-S checkpoint (Bartek & Lukas, 2007). The defective intra-S checkpoint in ATM-deficient cell lines allows cells to enter G2, where they accumulate. The level of G2-M accumulation was found to be consistently higher in A-T cell lines than in controls and, interestingly, this G2-M accumulation was significantly higher in typical A-T cases than in atypical A-T. Because this G2-M accumulation is not specific of ATM deficiency and can be observed in other pathologies, such as MRE11 deficiency responsible of ataxia-telangiectasia-like disorder (MIM# 604391; Falck, Petrini, Williams, Lukas, & Bartek, 2002), it should not be used as first-line functional test. However, it is a very good second-line test to confirm p-KAP1 results and can also be used as a potential marker to classify patients’ outcomes (Figure S10).
Altogether, we observed that the LCLs with the less altered functional tests were more likely to be derived from atypical patients. Because of the overlapping results in typical and atypical patients, none of the used tests could predict the phenotype on its own. However, a combined approach of four tests (total ATM, nuclear ATM, ATM-dependent p-KAP1 quantities, and G2-M accumulation) was used to establish the ATLAS. This score predicts an atypical phenotype if ≥2 (sensitivity: 1; specificity: 0.91). Interestingly, among the two typical patients with an ATLAS ≥ 2, one (AT04) harbored the same c.8147T>C variant than AT24, an atypical case, AT36, too young for phenotype classification, and atypical cases from the literature (van Os et al., 2019).
In conclusion, multiple approaches, including transcriptomic, proteomic and functional studies, were useful to identify, characterize and classify all ATM variants in a large series of patients. Refining the pathogenicity of ATM variants has consequences beyond neurodevelopmental genetics. Indeed, the importance of the classification of variant, even at heterozygous state, is increasing given the theranostic relevance of this gene and the increasing use of NGS especially in preconception genetic testing (Mateo et al., 2015; Punj et al., 2018). Some tests are more adapted to pathogenicity assessment in routine diagnosis, while such combined approach may be necessary for phenotype prediction. Therefore, we propose an ATM variant detection strategy (Figure S1), and an ATM variant classification strategy that includes a new phenotype prediction score (ATLAS; Figure S10).
ACKNOWLEDGMENTS
The authors would like to thank the patients and their families, the physicians for referring patients, Genethon and the Biobank of Institut Cochin for EBV cell lines, the CEREDIH (Center de Référence des Déficits Immunitaires Héréditaires) members for patients’ data, M. Kastan for kind gifts of ATM plasmids, the INSERM U900 for bioinformatics analysis of NGS and A. Houy for the ATM figure. This study was supported the Institut Curie, INSERM and by a grant of the Fondation AT EUROPE. G.R. was supported by grants from Ligue Nationale Contre le Cancer and Fondation pour la Recherche Médicale no. FDT20130928274.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.