Three new cases of ataxia-telangiectasia-like disorder: No impairment of the ATM pathway, but S-phase checkpoint defect
Abstract
Ataxia-telangiectasia-like disorder (ATLD) is a rare genomic instability syndrome caused by biallelic variants of MRE11 (meiotic recombination 11) characterized by progressive cerebellar ataxia and typical karyotype abnormalities. These symptoms are common to those of ataxia-telangiectasia, which is consistent with the key role of MRE11 in ataxia-telangiectasia mutated (ATM) activation after DNA double-strand breaks. Three unrelated French patients were referred with ataxia. Only one had typical karyotype abnormalities. Unreported biallelic MRE11 variants were found in these three cases. Interestingly, one variant (c.424G>A) was present in two cases and haplotype analysis strongly suggested a French founder variant. Variants c.544G>A and c.314+4_314+7del lead to splice defects. The level of MRE11 in lymphoblastoid cell lines was consistently and dramatically reduced. Functional consequences were evaluated on activation of the ATM pathway via phosphorylation of ATM targets (KAP1 and CHK2), but no consistent defect was observed. However, an S-phase checkpoint activation defect after camptothecin was observed in these patients with ATLD. In conclusion, we report the first three French ATLD patients and a French founder variant, and propose an S-phase checkpoint activation study to evaluate the pathogenicity of MRE11 variants.
1 INTRODUCTION
Ataxia-telangiectasia-like disorder (ATLD; MIM# 604391) is a rare autosomal disorder caused by biallelic variants of MRE11 (meiotic recombination 11; Stewart et al., 1999). ATLD belongs to the group of genomic instability syndromes, which also includes ataxia-telangiectasia (A-T; MIM# 208900), Nijmegen breakage syndrome (NBS; MIM# 251260) and Nijmegen breakage syndrome-like disorder (NBSLD; MIM# 613078). The main symptoms of ATLD are neurologic and begin during childhood (Taylor, Groom, & Byrd, 2004). Patients develop progressive cerebellar ataxia with progressive cerebellar atrophy. They typically present with dysarthria, writing dystonia, choreiform movements, and walking difficulties (Palmeri et al., 2013). They also experience oculomotor apraxia, occurring after the first signs of ataxia, with saccadic dysfunction and convergence abnormality. They subsequently also experience nystagmus with abnormalities in smooth pursuit and vestibular ocular reflex (Khan, Oystreck, Koenig, & Salih, 2008). These symptoms are common to those of A-T, caused by ATM (ataxia-telangiectasia mutated) inactivating variants, but the age of onset is usually later and the progression is slower and less severe in ATLD. Other typical features of A-T, never reported in ATLD, are telangiectasia, high serum alpha-fetoprotein (AFP) levels, immunodeficiency, and proneness to lymphoid tumors. NBS is another rare pediatric disease, caused by NBN biallelic variants, which also shares some of the features of A-T (Varon et al., 1998). The main symptoms are severe microcephaly with inconstant mental retardation, growth defects, characteristic dysmorphic features (bird face), and, as in A-T, immunodeficiency, and proneness to malignancies. NBSLD was described for the first time in a patient with RAD50 (radiation repair 50) deficiency, who presented with microcephaly, NBS dysmorphic features, short stature, mental retardation, normal AFP level, and no telangiectasia (Waltes et al., 2009). Two other patients with NBSLD with similar features associated with MRE11 variants were reported (Matsumoto et al., 2011). None of these three patients presented progressive cerebellar ataxia or oculomotor apraxia, unlike patients with ATLD or A-T, and none had immunodeficiency or proneness to cancer unlike patients with NBS or A-T. Importantly, these four genomic instability syndromes share common and pathognomonic lymphocyte cytogenetic features, consisting of an increased level of chromosome translocations involving chromosomes 7 and 14 at T-cell receptor and immunoglobulin loci, which guides the diagnosis of these diseases. These diseases also share variable degrees of hypersensitivity to ionizing radiation (IR).
MRE11 is a protein of 708 amino acids (aa) with endonuclease and exonuclease activity. The MRE11 nuclease domain is located at the N-terminal with five phosphoesterase motifs (aa 1–246), two DNA binding sites (aa 407–421, aa 643–692), and a glycine-arginine-rich domain (aa 568–595; Figure 1a; Stracker & Petrini, 2011). MRE11 is associated with RAD50 and NBN to form the MRN complex. MRN plays a major role in the DNA damage response (DDR) and is thought to be the earliest DNA double-strand breaks (DSBs) sensor, which relays the signal by activating ATM. During replication, ATM then regulates the S-phase checkpoint by two different pathways involving CHK2 and the MRN complex, respectively (Falck, Petrini, Williams, Lukas, & Bartek, 2002). MRE11-deficient cells have been reported with a less severe S-phase checkpoint defect than ATM-deficient cells (Taylor et al., 2004). The MRN complex is also involved in DSBs repair by homology-directed repair and nonhomologous end-joining pathways. The clinical features of MRN/ATM-deficient patients can be explained by the circumstances predisposing to DSBs. The unrepaired DSBs caused by IR or clastogenic agents explain the radiosensitivity of MRN/ATM-deficient patients. Infertility and immunodeficiency are secondary to the DSBs repair defect during meiotic and V(D)J recombination, respectively. Proteins of the MRN complex are phylogenetically highly conserved and the absence of any of these proteins is embryonically lethal in animal models (Xiao & Weaver, 1997; Zhu, Petersen, Tessarollo, & Nussenzweig, 2001). ATLD, NBS, and NBSLD are associated with biallelic hypomorphic variants, with poorly characterized but likely residual activities.
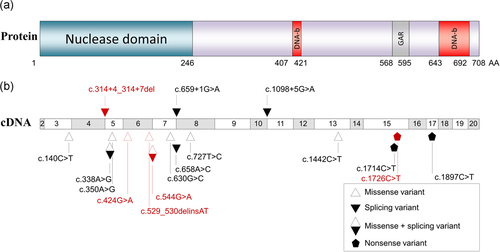
MRE11 protein and ATLD variants. (a) Schematic representation of the MRE11 protein. DNA-b, DNA binding site; GAR, glycine-arginine rich domain; AA, amino acid. (b) Schematic representation of the cDNA of the MRE11 transcript. The 11 known variants of MRE11 are indicated in black, the five new variants are indicated in red. ATLD: ataxia-telangiectasia-like disorder; MRE11: meiotic recombination 11
In this study, we describe the first three French patients with progressive cerebellar ataxia diagnosed with ATLD, associated with compound heterozygosity for unreported MRE11 variants. One of these variants was common in two unrelated patients with ATLD. Transcript and protein analyses confirmed the deleterious consequences of the variants. Surprisingly, contrary to previously reported MRE11 or NBN variants, ATM activation pathway analysis revealed no ATM pathway defect. However, S-phase checkpoint was consistently altered in the cell lines of all three patients.
2 MATERIALS AND METHODS
2.1 Patient diagnostic pipeline, variant detection, and cell lines
Patient 1 was referred for MRE11 sequencing after normal ATM sequencing. The 20 exons of MRE11 and flanking nucleotides were analyzed by direct sequencing of genomic DNA (RefSeq NM_005591.3). MRE11 variants in patients 2 and 3 were identified by clinical exome sequencing, using TruSight One Sequencing Panel, (Illumina, San Diego, CA), which targets 4,813 genes associated with known clinical phenotypes, and the identified MRE11 variants were confirmed by Sanger sequencing. Primer sequences and PCR amplification conditions are available upon request. Blood samples obtained with informed consent were used for genetic analysis and to establish Epstein-Barr virus-immortalized lymphoblastoid cell lines (LCLs). The reference genome is hg38.
2.2 Cell culture and treatment
LCLs from the three patients with ATLD (P1, P2, P3), A-T patients (AT13, AT14, AT22), and healthy individuals (HC2, HC3, HC4, HC5) were established by Genethon (www.genethon.fr) or the Biological Resource Center of Cochin Hospital. Briefly, Epstein-Barr virus (EBV) was produced with the marmoset cell line B95-8. Peripheral blood mononuclear cells (3 to 5 × 106) were exposed to 1 ml of B95-8 cells supernatant containing EBV and were treated with cyclosporine A (Novartis Pharmaceuticals UK Ltd, Frimley, UK) 20 µg/ml during 15 days. Multiple clusters of cells were visible within 15 days after infection. The LCL derived from an NBS patient with the NBN (NM_002485.4:c.657_661del) variant (GM15808) was obtained from the NIGMS Human Genetic Mutant Cell Repository (Camden, NJ). LCLs were grown in RPMI 1640 (Invitrogen, Oxon, UK) supplemented with 15% of heat-inactivated fetal calf serum (FCS; Invitrogen) at 37°C in a humidified incubator with 5% CO2. Cell viability threshold for ATM signaling experiments was 90% and was determined using the trypan blue exclusion assay. Treatment with camptothecin (CPT; Sigma-Aldrich, St Louis, MO), bleomycin (Sigma-Aldrich), ATM inhibitor KU60019 and DNA-PK inhibitor NU7441 (Tocris Bioscience, Bristol, UK) were performed at indicated doses and times.
2.3 Haplotype analysis
Blood samples were used for genetic analysis with informed consent. DNA was genotyped at the Center National de Génotypage (Evry, France) using an Infinium Global Screening Array (GSA, ~640,000 single nucleotide polymorphism [SNPs]; Illumina). Genotypes were called using default parameters in GenomeStudio (Illumina). All SNPs were filtered, mapped and synchronized with respect to strand. dbSNP150 on human genome build 37.1 (GRCh37) was used as the reference map. Only SNPs mapping to a single unique location on GRCh37 with only two alleles were included in analyses. Using Plink v1.07 (Purcell et al., 2007), SNPs or individuals yielding a genotype completion <95% were filtered out from analyses. SNPs were then filtered for departure from Hardy-Weinberg equilibrium at p < 0.001 and SNPs with a minor allele frequency <0.01 were filtered out. Individuals were tested for cryptic relatedness (Identity By State [IBS] < 30%). SNPs located on chromosome 11 were finally extracted using Plink v1.07 and local IBS was used to detect common haploblocks as described in Popova et al. (2013).
2.4 Transcriptional analysis
Total RNA was extracted from patient- and control-derived LCLs cultured with or without puromycin that inhibits nonsense-mediated mRNA decay (NMD). mRNA was reverse-transcribed using the GeneAmp RNA PCR Core kit according to the manufacturer's instructions (Applied Biosystems, Waltham, MA). PCR primers were designed in exon 3 and exon 8 of the MRE11 gene to detect alternative transcripts between these exons. Primer sequences and PCR amplification conditions are available upon request. RT-PCR products of patient P1 were cloned with TA-Cloning Kit into pCR®2.1 vector (Invitrogen) according to the manufacturer's instructions, and were transformed into DH5α competent cells. Full-length inserts were sequenced with ABI PRISM Big Dye Terminator v1.1 Ready Reaction Cycle sequencing kit (Applied Biosystems).
2.5 Western blot analysis
Cells were collected by centrifugation at 1,400 rpm and washed in cold phosphate-buffered saline. Cells were lysed in radioimmunoprecipitation assay buffer (100 mM Tris-HCL, 0.1 M NaCl, 1 mM EDTA, 1% Triton, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS]), supplemented with a cocktail of protease and phosphatase inhibitors (cOmplete©; PhosSTOP, Sigma-Aldrich). Protein supernatants were collected and quantified (Pierce BCA Protein Assay Kit; ThermoFisher Scientific, Waltham, MA). Equal amounts of protein were separated by SDS-polyacrylamide gel electrophoresis (4–15% polyacrylamide gel; Bio-Rad, Hercules, CA) and electrotransferred to a nitrocellulose membrane (Thermo Fisher). Membranes were blocked with Odyssey blocking buffer (LI-COR #927–40000) for 1 hr at room temperature and incubated in Odyssey blocking supplemented with Tween-20 to a final concentration of 0.1% overnight at 4°C with primary antibodies at the indicated concentration: phospho-ATM Ser1981 (1/1,000, #4526; CST), ATM (1/1,000, NB110–55475; Novus Biologicals, Littleton, CO), MRE11 (1/1,000, NB100–142; Novus Biologicals), RAD50 (1/1,000, #3427; Cell Signaling Technology, Danvers, MA), NBN (1/1,000, 611871; BD Biosciences, San Jose, CA), phospho-KAP1 Ser824 (1/2,000, A300–767A; Bethyl Laboratories, Montgomery, TX), KAP1 (1/1,000, A300-275A; Bethyl Laboratories), phospho-CHK2 Thr68 (1/1,000, #2661; CST), CHK2 (1/1,000, #3440; CST), phospho-DNA-PKcs Ser2056 (1/1,000, ab18192; Abcam, Cambridge, UK), DNA-PKcs (1/1,000, MS-369-P1; Neomarkers, Fremont, CA), FUS (1/1,000, #B1141000; Santa Cruz Biotechnology, Dallas, TX). β-actin (1/10,000, A-5316; Sigma-Aldrich) was used as a control for protein loading. After incubation with appropriate fluorescent secondary antibody (1/10,000 mixed with 0.1% Tween-20), immunoreactive bands were visualized using LI-COR-Odyssey infrared scanner (LI-COR) and band intensities were quantified using the ImageJ software (imagej.nih.gov/ij/).
2.6 Cell cycle analysis by fluorescence-activated cell sorting
After 24 hr, cells were fixed with 4% paraformaldehyde for 15 min, washed in Tris buffer saline (TBS) with 1% bovine serum albumin (BSA), permeabilized with fresh TST solution (0.2% Triton X-100/0.2% FCS) for 10 min, washed in TBS with 1% BSA and nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) 4 ng/ml (#62248; Thermo Fisher Scientific). A fluorescence-activated cell sorting LSRII instrument (BD Biosciences) was used and the results were analyzed with FlowJo software (v. 7.6.5). Both debris and doublets were removed from the analysis. Cell cycle distribution was analyzed with the FlowJo cell cycle Watson Pragmatic model. Results are based on two separate experiments.
3 RESULTS
3.1 Patient characteristics
3.1.1 Patient 1
Patient P1 was a girl born to nonconsanguineous Caucasian parents at 37 weeks of gestation with neonatal measurements in the normal range (birth weight: 2630 g, birth length: 46 cm, and occipitofrontal head circumference: 32 cm). She had no siblings and no family history. At the age of 3 months, she developed unilateral amblyopia, which was diagnosed as being due to unilateral optic nerve hypoplasia. She also presented progressive cerebellar ataxia with the onset of the first symptoms at the age of 13 months, when she showed difficulties learning to walk. Imprecise saccadic eye movements, with a normal ocular pursuit and no nystagmus, were noticed at the age of 2.5 years. At the age of 4 years, she was ataxic with symptoms accentuated by tiredness, sometimes resulting in falls. At the age of 8 years, her cerebellar syndrome became stable with dysmetria and writing difficulties. However, walking was sub-normal, although she was able to walk on heels and tiptoes. At the age of 9 years, she presented with oculomotor apraxia and slightly increased instability. At last news, at the age of 10 years, she presented action tremor, with more disabling ataxia, but was still able to walk. MRI, performed at the age of 9 years, showed cerebellar hypoplasia, while no cerebellar abnormalities were observed at the age of 2 years (Figure 2). She did not present any signs of an intellectual deficit, microcephaly (head circumference at the age of 4 years: 50 cm), growth retardation (length: 1.44 m, weight: 32 kg at the age of 10 years), telangiectasia, recurrent infection or cancer. She had normal AFP levels (AFP: 10 ng/ml at the age of 3 years) and normal immunoglobulin levels (IgA: 0.61 g/L, IgG: 5 g/L, and IgM: 1.26 g/L at the age of 4 years). As her karyotype showed a translocation between chromosomes 7 and 14, genomic analysis of the ATM gene was performed but did not reveal any variants or large rearrangements. A diagnosis of ATLD was suspected and MRE11 analyses were performed.
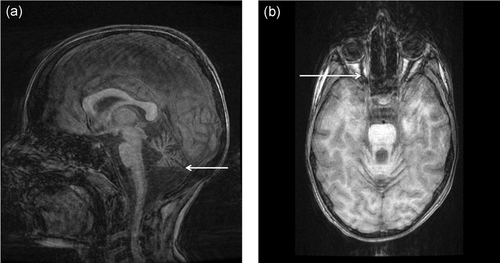
Cerebral MRI of the patient P1 at 9 years old. (a) Progressive cerebellar hypoplasia (3D sagittal T1 BRAVO). (b) Hypoplasia of the right optic nerve (Rec BRAVO Axial)
3.1.2 Patient 2
Patient P2 was a girl, the first child of nonconsanguineous Caucasian parents. She presented gait ataxia from the age of independent walking at 13 months. Examination at 3 years revealed cerebellar symptoms, dysarthria, and oculomotor apraxia. Cerebellar MRI was normal. At the age of 7 years, she presented mild learning difficulties with apparently stable disease. Subsequent follow-up showed the progression of the disease from the age of 8 years, with increased ataxia, falls, tremor and choreoathetotic movements. At the last assessment (9 years), the child was able to walk a few steps unaided but needed a wheelchair for everyday activities. Brain MRI performed at the age of 8 years showed progressive cerebellar hypoplasia. Karyotype on lymphocytes and AFP and immunoglobulin levels were normal. Clinical exome sequencing was performed to determine the etiology of this isolated ataxia.
3.1.3 Patient 3
Patient P3 was a girl born to nonconsanguineous parents. She presented early-onset ataxia, which was more severe at the age of 6 years. She also presented tremor and dystonia of the hands. Brain MRI showed cerebellar atrophy. Karyotype and AFP level were normal; immunoglobulin levels were not measured. Clinical exome sequencing was performed to determine the etiology of this isolated ataxia.
3.2 Variant analysis
Genomic sequencing of MRE11 in patient P1 found two unreported missense variants, both in exon 6 (g.94478855C>T, c.424G>A, p.(Asp142Asn) and g.94478735C>T, c.544G>A, p.(Gly182Arg); Figure 1b). Both variants were situated within the highly conserved nuclease domain coding region. The second variant c.544G>A was located on the last base of the exon and splicing defect prediction indicated a decrease of the splice donor site strength in the presence of the variant (−13%, −43%, −35% using SpliceSiteFinder-like (Shapiro & Senapathy, 1987; Zhang, 1998), MaxEntScan (Yeo & Burge, 2004), and NNSPLICE 0.9 (Reese, Eeckman, Kulp, & Haussler, 1997) tools, respectively).
Two variants were identified in MRE11 in patient P2. The first was the same missense variant c.424G>A reported in patient P1; the second was an unreported nonsense variant in exon 15 (g.94447276G>A, c.1726C>T, p.(Arg576*); Figure 1b).
Two novel variants were identified in MRE11 in patient P3. The first was a missense variant in exon 6 (g.94478749_94478750delinsAT, c.529_530delinsAT, p.(Ala177Met)). The second variant was a deletion of 4 nucleotides in intron 4 with in silico tools predicting loss of the canonical splice site donor of exon 4 (g.94485917_94485920del, c.314+4_314+7del; Figure 1b). An alternative splice donor site located four nucleotides upstream from the canonical site was predicted, possibly promoted by the presence of this variant.
The three patients harbored one variant inherited from their mother, and one variant inherited from their father, indicating that the variants were located in trans.
The c.424G>A variant was common to patients P1 and P2, belonging to unrelated families originating from different areas of France. Germline DNAs from these patients and their parents were genotyped on the whole genome. IBS was negative between patients P1 and P2, excluding an unknown familial relationship. A unique haploblock was found to be shared between patients P1 and P2 on chromosome 11, between the single nucleotide polymorphisms (SNPs) rs1456243 and rs620767, a 1.6 Mb region that contains MRE11. Furthermore, this haplotype was not found to be shared with more than 800 French controls. These new variants were added to the LOVD database (https://databases.lovd.nl/shared/variants/MRE11A).
3.3 Splicing analysis
- 1.
Patient P1: Two supplementary bands were demonstrated compared with WT, which corresponded to exon 5 and 6 skipping, leading to a premature termination codon (r.315_544del/p.(Phe106Ilefs*5)) and exon 5 to 7 skipping, predicting a loss of 115 amino acids (r.315_659del/p.(Phe106_Arg220del); Figure S1A). Cloning and sequencing of the full-length RT-PCR products revealed different transcripts, 16/18 carried the c.424G>A, whereas 2/18 carried the c.544G>A. This distribution revealed a significant decrease of full-length transcript carrying the c.544G>A variant compared with the 50% theoretical distribution (p = 0.03, Fisher's exact test) demonstrating the causality of this variant in the observed splicing defect and the leakiness of this defect (Figure S1B). The c.544G>A variant is therefore both a missense variant and a splice variant.
- 2.
Patient P3: As expected, sequencing of cDNA showed deletion of the last four nucleotides of exon 4, leading to a premature termination codon (r.311_314del/p.(Lys105Phefs*4)); expression of this deletion was increased when NMD was inhibited by puromycin treatment. This transcript was barely detected in healthy controls but in the presence of puromycin treatment (Figure S1C).
Transcript analyses, therefore, demonstrated the pathogenicity of these two variants.
3.4 MRN complex
To investigate the consequences induced by MRE11 variants, ATM, MRE11, RAD50, and NBN protein levels were analyzed by immunoblotting using patients’ LCLs, as compared with three healthy controls, three A-T and one NBS LCLs (Figure 3a). As expected, ATM was normal in the three MRE11 mutated patients, while MRE11, RAD50, and NBN were reduced to around 17%, 28%, and 20% of healthy control levels, respectively (Figure 3b). This decrease of the three MRN compounds, presumably by destabilization of the complex, confirmed the deleterious nature of MRE11 variants (Stewart et al., 1999). Interestingly, patient P1 with the c.544G>A-mediated leaky splice defect had slightly more MRE11 and RAD50 proteins than the other patients. This variant, therefore, probably still leads to small amounts of MRE11 protein with the amino acid 177 change, without a splice defect.
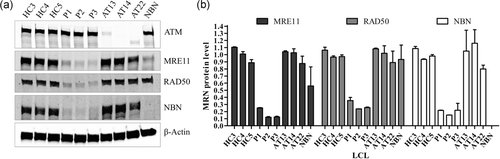
MRN complex proteins analysis. (a) Western blot of ATM and proteins of the MRN complex. (b) Quantification of MRN protein levels based on western blot values of two independent protein extractions. β-Actin was used as loading control. ATM: ataxia-telangiectasia mutated
3.5 Functional analysis
The ATM autophosphorylation and the ATM-dependent phosphorylation in response to 1 µM camptothecin (CPT) of two well-described targets, KAP1 and CHK2, were analyzed to evaluate the DDR defect caused by these variants (Figure 4a). As expected, ATM, KAP1, and CHK2 phosphorylation was decreased in the NBS and A-T LCLs. Surprisingly, ATM, KAP1, and CHK2 phosphorylation in the three patients with ATLD was similar to that of healthy controls, suggesting a functional ATM pathway in response to DSBs in these ATLD patients (Figure 4b).
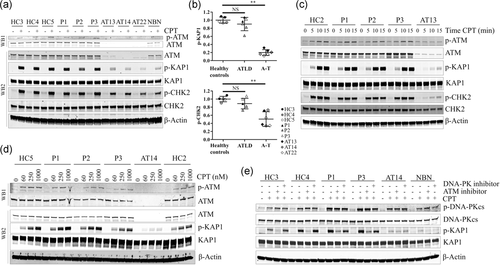
ATM pathway activation analyses. (a) Western blot of ATM autophosphorylation and phosphorylation of target proteins with and without high-dose CPT (1 µM camptothecin for 1 hr). β-Actin was used as a loading control. This experiment was analyzed with two different western-blots to allowed adapted migration times (WB1, prolonged migration; WB2, standard migration). (b) Quantification of KAP1 and CHK2 phosphorylation based on two independent experiments. Mean values obtained from healthy controls cell lines were normalized at 1. Error bars represent standard deviation (SD). Mann–Whitney test. NS, no significance difference; **p < 0.01. (c) Time points of the phosphorylation of ATM, KAP1, and CHK2 under high dose of CPT (1 µM). (d) Phosphorylation of ATM and KAP1 under different doses of CPT (60, 250, or 1 µM) for 1 hr. This experiment was analyzed with two different western-blots to allowed adapted migration times (WB1, prolonged migration; WB2, standard migration). (e) Assessment of the kinase dependency of the phosphorylation of KAP1. Cell lines were treated for 90 min with ATM inhibitor (KU60019, 1 µM) or DNA-PK inhibitor (NU7441, 5 µM) prior addition of 1 µM of CPT for 1 hr. ATM: ataxia-telangiectasia mutated; CPT: camptothecin
We further evaluated the phosphorylation of ATM itself as well as that of KAP1 and CHK2 at early time points (Figure 4c; Figure S2). The phosphorylation of ATM and its targets was observed in the 3 ATLD LCLs after 5, 10, and 15 min of 1 µM CPT exposure, with similar intensities than that observed in the healthy control LCL. We then looked at the ATM pathway activation in response to lower doses of CPT (Figure 4d). No difference of phosphorylation of ATM or KAP1 was observed in ATLD LCLs as compared with healthy controls with low doses of CPT (60 and 250 nM). These results were consistent with normal ATM pathway activation under DNA double-strand breaks in these patients with ATLD.
Cross-talk between ATM and DNA-PK pathways has been reported, especially in Mre11-deficient cells (Hartlerode, Morgan, Wu, Buis, & Ferguson, 2015). Thus, we verified the ATM dependency of KAP1 phosphorylation in our experimental conditions. The highly selective ATM-inhibitor KU60019 strongly reduced the level of p-KAP1 in healthy controls as well as in ATLD LCLs, whereas it had no impact on the residual p-KAP1 level in the ATM-deficient LCL. Interestingly, the low level of p-KAP1 in a NBN-deficient LCL was slightly reduced. The highly selective DNA-PK inhibitor NU7441 had no impact on the phosphorylation of KAP1 in all LCLs (Figure 4e). Bleomycin, which induced DSBs in all phases of cell cycle, induced similar levels of KAP1 phosphorylation in ATLD LCLs than in healthy controls LCLs (Figure S3). Interestingly, bleomycin-induced KAP1 and DNA-PKcs phosphorylation were inhibited by NU7441 DNA-PK inhibitor in all LCLs. In conclusion, our findings do not support a significant compensating role of the DNA-PK pathway in ATLD LCLs under CPT exposure.
ATM and MRN play a well-known role in checkpoint control in response to DSBs. The S-phase checkpoint in response to 24-hr exposure to low-dose CPT (4 nM) was therefore tested. As expected, the three healthy controls presented normal accumulation of cells in the S-phase after CPT compared with untreated conditions (range: [58.1–62.4%] and [45.5–47.7%], respectively). The G2-M phase was stable with or without treatment ([11.1–12.5%] and [10.3–11.7%], respectively). The three A-T and the NBS LCLs had fewer cells in S-Phase after CPT than in the absence of CPT ([28.1–33.3%] and [40.5–47.3%], respectively) and accumulation in the G2-M phase ([43.4–51.7%] vs. [10.8–12.6%], with and without CPT, respectively). Interestingly, the three ATLD LCLs presented an intermediate cell cycle distribution between healthy controls and A-T and NBS LCLs, as the S-phase was stable with or without CPT ([36.9–53.0%] vs. [37.3–47.4%], respectively) and the G2-M phase showed cell accumulation, but to a lesser extent than in A-T LCLs ([15.4–32.4%] vs. [10.1–19.2%], respectively; Figure 5; Figure S4A). The level of p-KAP1 was assessed at 4 nM during 48 hr to explore potential ATM activation defect under long term exposure to low CPT level. The level of p-KAP1 was very low compared with the usual treatment of 1 hr of 1 µM of CPT, but no difference was observed between ATLD and healthy controls LCLs (Figure S4B). Therefore, patients with ATLD had mild ATM-independent S-phase checkpoint defects following exposure to CPT.
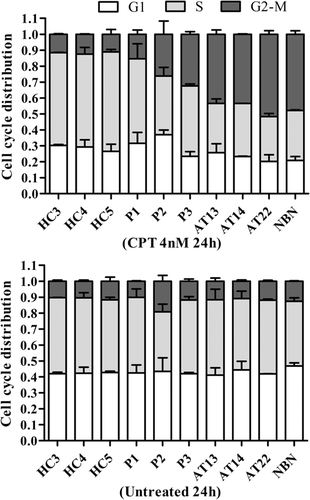
Cell cycle analysis. Quantification of the cell cycle phase distribution with (upper panel) and without (lower panel) low-dose CPT (4 nM camptothecin for 24 hr), based on values of 2 independent experiments. Error bars represent standard deviation
4 DISCUSSION
We report three new cases of patients with ATLD harboring unreported MRE11 variants, and associated with a proficient ATM activation pathway and mild S-phase checkpoint defects. These patients were investigated in a context of slowly progressive cerebellar ataxia. Only one case was associated with pathognomonic karyotype abnormalities, which were not detected in the other two cases, despite repeated karyotypes, as already described in four previously reported cases (Delia et al., 2004; Fernet et al., 2005). The intensity and clinical course of the disease were similar to those previously reported in ATLD. However, ATLD phenotype is variable and difficult to define because of the rarity of this disease. Moreover, MRE11-related disorders are diagnosed as ATLD in only a fraction of cases (20 of 30 reported cases), NBSLD (two patients), ataxia with oculomotor apraxia (five patients), progressive myoclonic ataxia (one patient) or nephronophthisis-related ciliopathy (two patients), highlighting the variability of phenotypes and severity (Bohlega et al., 2011; Chaki et al., 2012; Delia et al., 2004; Fernet et al., 2005; Matsumoto et al., 2011; Miyamoto et al., 2014; Pitts et al., 2001; Stewart et al., 1999; Uchisaka et al., 2009; Yoshida et al., 2014). It is noteworthy that one of the patients reported here (patient P1) also presented optic nerve hypoplasia, which was never been previously reported in MRE11-related diseases and which may, therefore, be unrelated to the MRE11 variants.
These three French patients were compound heterozygous for one missense variant in exon 6 and one splicing or nonsense variant. Interestingly, the c.424G>A/p.(Asp142Asn) missense variant was found in patients P1 and P2. These patients were not known to be related and originated from different areas of France. SNP analysis showed that these two patients shared a 1.6 Mb haplotype encompassing MRE11, which supported c.424G>A as a French founder mutation. Interestingly, the second variant, c.544G>A, found in patient P1 was associated with two deleterious effects: a change of highly conserved amino acid and a leaky splice defect. The second variant, c.1726C>T, found in patient P2 was a nonsense variant in exon 15. No truncated MRE11 protein was visible on Western blot, indicating that mRNA may be degraded by NMD, as previously described for the close c.1714C>T nonsense variant (Delia et al., 2004). The second variant of patient P3 was a splice variant located in intron 4, leading to a frameshift deletion of four nucleotides in the transcript, which is degraded by NMD.
Consequently, MRE11, together with RAD50 and NBN, was dramatically reduced in the three patients, as usually observed in patients with ATLD. Including the five new variants described in the present report, only 16 germline MRE11 variants have been reported to date, in English, Pakistani, Italian, Japanese and Saudi ATLD patients, who were either compound heterozygous or more often homozygous carriers (Figure 1b; Bohlega et al., 2011; Chaki et al., 2012; Delia et al., 2004; Fernet et al., 2005; Matsumoto et al., 2011; Miyamoto et al., 2014; Pitts et al., 2001; Stewart et al., 1999; Uchisaka et al., 2009; Yoshida et al., 2014). These variants are located from exon 3 to exon 17. A founder variant c.630G>C/p.W210C was found in 16 patients from Saudi families (Alsbeih, Al-Hadyan, & Al-Harbi, 2008; Bohlega et al., 2011; Fernet et al., 2005). The variable phenotype among reported patients is probably at least partially related to the location and consequences of these various MRE11 variants, but no obvious genotype-phenotype correlation has yet been defined.
MRN complex facilitates ATM phosphorylation and activation after DSBs (J. H. Lee & Paull, 2004). ATM phosphorylation after IR has been reported to be decreased in cell lines harboring MRE11 variants, with variable intensities (Delia et al., 2004). ATM kinase activity has also been evaluated via the phosphorylation of several targets, including p53 and CHK2 in cell lines harboring MRE11 variants, but with inconsistent results (Delia et al., 2004; Fernet et al., 2005; Matsumoto et al., 2011; Stewart et al., 1999), or even no impact on this pathway (Falck et al., 2002). Hartlerode et al. highlighted that Mre11 null cells had ATM pathway activation defects partially substituted by DNA-PKcs, while Mre11 nuclease deficient cells, more closely resembling human ATLD MRE11 mutants, were not associated with such Atm pathway defect (Hartlerode et al., 2015). The results of our analysis of ATM autophosphorylation and ATM-dependent phosphorylation of KAP1 and CHK2 are consistent with the results reported by Falck et al. (2002), showing no impairment of ATM pathway activation upon DSBs in these three new patients. ATM activation defects in ATLD, therefore, appear to be highly variable, and potentially depending on the impact of the MRE11 variant on the MRN complex, and especially on NBN which directly interacts with ATM (You, Chahwan, Bailis, Hunter, & Russell, 2005).
The common neurologic features of ATLD and A-T were suspected to be the common consequence of a defective ATM pathway (Shull et al., 2009; Uziel et al., 2003). It has been suggested that defective ATM activation and p53 phosphorylation would prevent apoptosis in response to DNA damage, leading to persistent cells harboring DNA damage in the mature nervous system, which would promote neurodegeneration. Interestingly, the patients with NBSLD described by Matsumoto et al. (2011), presenting the most severe clinical features, had the less affected ATM pathway. It was therefore suggested that microcephaly in NBSLD or NBS was the consequence of p53-mediated apoptosis in response to endogenous DNA damage, facilitated by a more functional ATM pathway than in ATLD and AT patients (Frappart et al., 2005; Matsumoto et al., 2011). However, activation of the ATM pathway was dramatically more effective in our patient cell lines than in the NBS cell line, contrasting with their typical ATLD clinical features and the absence of microcephaly. All these findings are not in favor of a strong correlation of ATM activation and the neurologic phenotype of ATLD patients, and, at most, the ATM activation defect probably only partially explains the ATLD phenotype.
MRN-deficient cells have been reported to be associated with radioresistant DNA synthesis with a lesser degree of G1-S checkpoint defects than in ATM-deficient cells (Delia et al., 2004; Fernet et al., 2005; Stewart et al., 1999; Taylor et al., 2004). We have fully confirmed these results and showed that the intra-S checkpoint of MRE11-deficient cells from the three patients was consistently less severely altered than in ATM-deficient cell lines. This milder defect has been suggested to be the result of the combination of an efficient ATM-CHK2-CDC25A-CDK2 S-phase checkpoint pathway and a deficient ATM-NBN-MRE11 pathway (Falck et al., 2002). Efficient CHK2 phosphorylation in response to DSBs in the three ATLD appears to corroborate this hypothesis. Intra-S checkpoint is also mediated by ATR/CHK1 pathway, activated by MRN-dependent resection (Bartek & Lukas, 2007). It is, therefore, possible that this intra-S checkpoint defect results from the defective exonuclease activity of MRE11.
Unlike other genetic stress such as IR, the CPT treatment leads to protein-DNA adducts which leads to replication fork collapses and single-end DNA DSB (Chanut, Britton, Coates, Jackson, & Calsou, 2016). MRE11 nucleases activities are involved in the cleavage of the DNA end bearing adducts and in the process of the single-end DNA to allow homologous recombination (Chanut et al., 2016; K. C. Lee et al., 2012). Therefore, highlighted functional defect under CPT exposure could result from the inability to remove protein-DNA adducts.
In conclusion, we have identified three new typical patients with ATLD and characterized five novel pathogenic variants. Functional analyses revealed no defect of the ATM pathway, but an intra-S checkpoint defect. These findings suggest that ATLD features could be largely independent of the ATM pathway defect and that other functions of the MRN complex could, therefore, be determinant in the ATLD phenotype.
ACKNOWLEDGMENTS
The authors would like to thank the patients and their families, the physicians for referring their patients, Genethon and the Biological Resource Center of Cochin Hospital, APHP - Paris for EBV cell lines. This work was supported the Institut Curie, INSERM and by a grant of the Fondation at Europe.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.