Mutations in the gene PDE6C encoding the catalytic subunit of the cone photoreceptor phosphodiesterase in patients with achromatopsia
Funding information: Fondation Voir et Entendre, Grant/Award Number: ANR-10-LABX-65; Agence Nationale de la Recherche, Grant/Award Number: ANR-11-IDEX-0004-0; Foundation Fighting Blindness Center Grant, Grant/Award Numbers: C- C-CL-0912-0600-INSERM01 and GE-0912-0601-INSERM02; Research Foundation Flanders, Grant/Award Number: FWO 3G079711; Ghent University Special Research Fund, Grant/Award Number: BOF15/GOA/011
Communicating Editor: Dr Arupa Ganguly
Abstract
Biallelic PDE6C mutations are a known cause for rod monochromacy, better known as autosomal recessive achromatopsia (ACHM), and early-onset cone photoreceptor dysfunction. PDE6C encodes the catalytic α′-subunit of the cone photoreceptor phosphodiesterase, thereby constituting an essential part of the phototransduction cascade. Here, we present the results of a study comprising 176 genetically preselected patients who remained unsolved after Sanger sequencing of the most frequent genes accounting for ACHM, and were subsequently screened for exonic and splice site variants in PDE6C applying a targeted next generation sequencing approach. We were able to identify potentially pathogenic biallelic variants in 15 index cases. The mutation spectrum comprises 18 different alleles, 15 of which are novel. Our study significantly contributes to the mutation spectrum of PDE6C and allows for a realistic estimate of the prevalence of PDE6C mutations in ACHM since our entire ACHM cohort comprises 1,074 independent families.
Achromatopsia (ACHM; ACHM2 MIM# 216900, ACHM3 MIM# 262300, ACHM4 MIM# 613856, ACHM5 MIM# 613093, ACHM6 MIM# 610024, and ACHM7 MIM# 616517) is a rare autosomal recessive cone disorder characterized by color vision defects, photophobia, nystagmus, and severely reduced visual acuity. To date, six genes have been linked to ACHM. In the Western population, approximately 80% of the patients carry mutations in the genes CNGA3 (MIM# 600053; Kohl et al., 1998) and CNGB3 (MIM# 605080; Kohl et al., 2000; Sundin et al., 2000) encoding the two subunits of the cone photoreceptor cyclic nucleotide-gated channel. Much less frequently, causative mutations have been found in genes encoding other crucial components of the cone phototransduction cascade, namely GNAT2 (MIM# 139340; Aligianis et al., 2002; Kohl et al., 2002), PDE6C (MIM# 600827; Chang et al., 2009; Thiadens et al., 2009), and PDE6H (MIM# 601190; Kohl et al., 2012), or in ATF6 (MIM# 605537; Kohl et al., 2015), which is not involved in the phototransduction cascade, but in the unfolded protein response pathway. Larger case series or genetic screens of ACHM-associated genes are sparse (Kohl et al., 2005; Mayer et al., 2017; Nishiguchi, Sandberg, Gorji, Berson, & Dryja, 2005; Wissinger et al., 2001; Zelinger et al., 2015), therefore we lack a comprehensive view of the prevalence especially of the minor disease genes in ACHM.
In the present study, 176 patients diagnosed with ACHM who remained unsolved after Sanger sequencing of the most frequent genes accounting for ACHM, namely CNGB3, CNGA3, and GNAT2, were screened for exonic and splice site variants in the PDE6C gene. Samples from all patients and family members were recruited in accordance with the principles of the Declaration of Helsinki and were obtained with written informed consent accompanying the patients' samples. The study was approved by the institutional review board of the Ethics Committee of the University Hospital of Tuebingen.
While two patients were screened using a custom capture panel targeting 105 retinal disease genes including PDE6C (Glöckle et al., 2014), the others were analyzed by means of an amplicon-based next generation sequencing approach. Briefly, target enrichment of coding sequences including exon–intron boundaries of PDE6C (Supporting Information, Table S1) was performed with Fluidigm, CA, USA 48.48 Access Arrays. Library capture was completed using the Nextera XT DNA Library Prep Kit and sequencing was performed on a MiSeq instrument at a core facility (c.ATG, Tübingen, Germany, CA, USA). Sequence data were aligned using the Burrows–Wheeler Aligner (Li & Durbin, 2009), and variants were called using an in-house bioinformatic pipeline. Only nonsynonymous single nucleotide variants, nonsense variants, splice site (±10 bps) variants, insertions, duplications, and deletions represented by more than 20 sequence reads were considered for further analysis. In addition, variants with a minor allele frequency (MAF) >1% in the Genome Aggregation Database (gnomAD) Version r2.0.2 were excluded from further investigation. The potential pathogenicity of missense changes was assessed using five online prediction software tools, namely SIFT (https://sift.jcvi.org/; Kumar, Henikoff, & Ng, 2009), PolyPhen-2 (https://genetics.bwh.harvard.edu/pph2/; Adzhubei et al., 2010), Mutation Taster (https://www.mutationtaster.org/; Schwarz, Cooper, Schuelke, & Seelow, 2014), Mutation Assessor (https://mutationassessor.org/r3/; Reva, Antipin, & Sander, 2011), and Provean (https://provean.jcvi.org; Choi & Chan, 2015). Prediction outcomes are given in Supporting Information, Table S2. The variant designation is based on the NCBI reference sequence for PDE6C (NC_000010.11, NM_006204.3; GRCh38) comprising 22 coding exons. We were able to identify potentially pathogenic biallelic variants in 15 index cases, thereby achieving a detection rate of 8.5%. All putative disease-associated variants in the PDE6C gene were validated and tested for segregation with the phenotype in available family members by conventional Sanger sequencing. The variants were seen in true homozygous state in two patients and in apparent homozygous state in seven patients, respectively (Supporting Information, Table S3). Compound heterozygosity was observed for two patients based on the analysis of paternal alleles. In one patient, trans configuration of variants was established by allelic cloning. Compound heterozygosity could not be demonstrated for three patients because DNA of family members was not available and the respective variants were located too far apart for allelic cloning. The mutation spectrum comprises 18 different alleles, 15 of which are novel (Table 1). All novel variants were deposited to the ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/; Landrum et al., 2014) with accession codes provided in Table 1. The location of the variants identified in this study with respect to the PDE6C protein is depicted in Figure 1a.
| Nucleotide (NM_006204.3) | PDE6C protein (NP_006195.3) | Reference(s) | ClinVar accession no. | Protein domain | Consensus prediction for missense variantsa | ACMGb | ACMG predictionc | gnomAD MAF |
|---|---|---|---|---|---|---|---|---|
| c.939+5G>A | n.a.d (p.F290_E314del) | Abouelhoda et al., 2016 | RCV000171185.1 | n.a. | PM2;PM4;PP3 | VUS | None | |
| c.1004+1G>A | n.a.d (p.E314Dfs*11) | Huang et al., 2013 | n.a. | n.a. | PM2;PVS1;PP3 | Pathogenic (Ic) | 0.00003228 | |
| c.1269+1G>A | n.a.d (p.K374_E423del) | This study | SCV000678420 | n.a. | PM2;PVS1;PM4 | Pathogenic (Ib) | None | |
| c.211G>T | p.E71* | This study | SCV000678421 | n.a. | PM2;PVS1 | Likely pathogenic (I) | 0.00000813 | |
| c.775C>T | p.R259* | This study | SCV000678422 | n.a. | PM2;PVS1 | Likely pathogenic (I) | 0.00000813 | |
| c.1579C>T | p.R527* | This study | SCV000678423 | n.a. | PM2;PVS1 | Likely pathogenic (I) | 0.0000447 | |
| c.78del | p.K27Sfs*27 | This study | SCV000678424 | n.a. | PM2;PVS1 | Likely pathogenic (I) | None | |
| c.497del | p.D166Afs*28 | This study | SCV000678425 | n.a. | PM2;PVS1 | Likely pathogenic (I) | None | |
| c.857del | p.K286Rfs*16 | This study | SCV000678426 | n.a. | PM2;PVS1 | Likely pathogenic (I) | None | |
| c.85C>T | p.R29W | Grau et al., 2011; Thiadens et al., 2009 | SCV000086939.1 | N terminal domain | Damaging | PM2;PM1;PP3;PS3 | Pathogenic (II) | 0.00002164 |
| c.304C>T | p.R102W | This study | SCV000678427 | GAF domain | Damaging | PM2;PM1;PP3 | VUS | None |
| c.836T>C | p.I279T | This study | SCV000678428 | GAF domain | Damaging | PM2;PM1;PP3;PM3 | Likely pathogenic (IV) | 0.00001804 |
| c.1637C>A | p.T546N | This study | SCV000678429 | Catalytic domain | Damaging | PM2;PM1;PP3 | VUS | None |
| c.2141T>A | p.I714N | This study | SCV000678430 | Catalytic domain | Damaging | PM2;PM1;PP3 | VUS | 0.00000407 |
| c.2156T>C | p.M719T | This study | SCV000678431 | Catalytic domain | Damaging | PM2;PM1;PP3 | VUS | 0.00006457 |
| c.2246G>A | p.G749E | This study | SCV000678432 | Catalytic domain | Damaging | PM2;PM1;PP3 | VUS | None |
| c.2288T>C | p.M763T | This study | SCV000678433 | Catalytic domain | Damaging | PM2;PM1;PP3 | VUS | None |
| c.2294A>C | p.D765G | This study | SCV000678434 | Catalytic domain | Damaging | PM2;PM1;PP3 | VUS | None |
- ACMG, American College of Medical Genetics and Genomics; n.a., not applicable; VUS, variant of uncertain significance; MAF, minor allele frequency.
- a Consensus determined as prediction of a damaging effect by at least four of five free web-based applications (SIFT, PROVEAN, PolyPhen-2, Mutation Taster, and Mutation Assessor).
- b Categories published in the ACMG guidelines.
- c Combination of scores leading to final classification in one of five pathogenicity categories.
- d In silico assessment using five algorithms embedded in the Alamut software (https://www.interactive-biosoftware.com) predicted that skipping of the respective exon is likely. The consequence of the putative exon skipping on protein level is given in brackets.
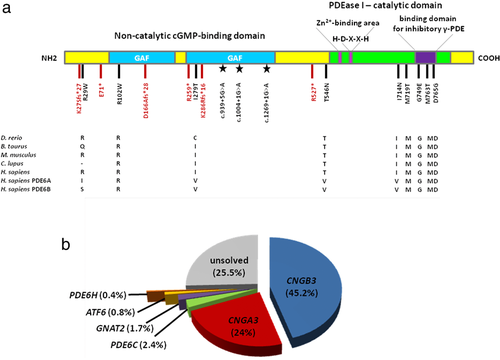
All index patients harbored unique PDE6C genotypes with the exception of the missense variant p.I714N, which was found in homozygous state in three independent patients. In gnomAD, this variant is present in heterozygous state in only one single subject (1/245,508 alleles). The fact that we saw it in three independent patients was therefore indicative of a founder effect. Genotyping of microsatellite and single-nucleotide polymorphism markers indeed revealed a common haplotype for the three patients (see Supporting Information, Figure S1).
Potential pathogenicity of variants was determined on the basis of: (1) representing ultrarare alleles observed only in single cases or being absent in 277,264 general population alleles in gnomAD; (2) in the case of missense variants being predicted to be damaging by at least four out of five effect prediction programs listed above; (3) representing likely null alleles (nonsense, canonical splice site and frameshift variants); (4) having already been described to be pathogenic; and (5) analyzed functionally (p.R29W; Grau et al., 2011). All variants were classified according to their pathogenicity based on the American College of Medical Genetics and Genomics (ACMG) guidelines (Richards et al., 2015; see Table 1). Because nonsense, canonical splice site and frame-shifting variants have a strong weight in the ACMG scoring system, this class of variants are consequently classified either as likely pathogenic or pathogenic. An exception is the known noncanonical splice site variant c.939+5G>A (Abouelhoda et al., 2016), which is classified as a variant of unknown significance (VUS) because the +5 position is not invariable.
All missense variants we identified have an extremely low MAF or are even absent in gnomAD. In addition, their evolutionary conservation and localization as well as the type of the respective amino acid substitution are strong indicators of pathogenicity. However, following the ACMG guidelines, seven of the eight missense variants we identified are classified as VUS. This classification would only change to pathogenic if functional data (e.g., of an enzymatic activity assay) were supportive of a damaging effect as is the case for the p.R29W variant (Grau et al., 2011).
A summary of clinical findings is shown in Supporting Information, Table S3 including all index patients and two affected siblings (patients 1–1 and 1–2). All patients were diagnosed in early childhood (ranging from birth to 5 years) and displayed characteristics of ACHM like photophobia, nystagmus, and impaired color vision. Electroretinography (ERG) results were not available from every patient but generally revealed normal rod responses and either extinguished or severely reduced cone responses with the exception of patient 6 in whom ERG recordings also showed reduced b-waves in the scotopic ERG and an electronegative standard flash. We therefore reclassified his diagnosis from ACHM to cone-rod dystrophy. Optical coherence tomography images were only available from six patients and revealed the typical disappearance of P2 (photoreceptor reflectivity) in patients with ACHM (Barthelmes et al., 2006).
The Human Gene Mutation Database currently lists 38 variants in PDE6C that explain the disease phenotype in the respective patients. Our study significantly contributes to the mutation spectrum of PDE6C and allows for a realistic estimate of the prevalence of PDE6C mutations in ACHM in the European population because our entire cohort comprises 1,074 independent families mainly originating from Europe or being of European descent (USA, Canada, Australia, and New Zealand). Considering an estimated prevalence of 1:30,000 to 1:50,000 for ACHM in Europe, this number is certainly high enough to give a comprehensive view on the spectrum and prevalence not only of the more common CNGB3 and CNGA3 mutations, but also on the four minor, non-CNG channel encoding ACHM genes. Taking together the results of the present study and a previous screening approach (Grau et al., 2011), we calculate a prevalence of 2.4% for PDE6C mutations in our cohort, which is most probably representative for ACHM in the Western population (Figure 1b). As ACHM is in the focus of retinal gene therapy with four clinical trials ongoing, our study provides a valuable resource for putative gene therapy trials targeting PDE6C.
ACKNOWLEDGMENTS
The authors would like to acknowledge the contribution of Francoise Meire (Brussels, Belgium) and the late Christian Hamel (Montpellier, France). Several DNA samples incorporated in this study were obtained from the NeuroSensCol DNA bank, for research in neuroscience (PI: JA Sahel, co-PI I Audo, partner with CHNO des Quinze-Vingts, Inserm and CNRS). Part of this study was supported by Fondation Voir et Entendre, LABEX LIFESENSES [reference ANR-10-LABX-65] supported by French state funds managed by the Agence Nationale de la Recherche within the Investissements d'Avenir program [ANR-11-IDEX-0004-0], Foundation Fighting Blindness center grant [C- C-CL-0912-0600-INSERM01 and GE-0912-0601-INSERM02], Prix de la Fondation de l’Œil (IA). This work was further supported by grants from the Research Foundation Flanders (FWO) (FWO 3G079711 to E.D.B.), by Ghent University Special Research Fund (BOF15/GOA/011); E.D.B. and B.P.L. are Senior Clinical Investigators of the FWO. We further acknowledge support by the Deutsche Forschungsgemeinschaft and the Open Access Publishing Fund of the University of Tuebingen.