Alu-Alu mediated intragenic duplications in IFT81 and MATN3 are associated with skeletal dysplasias
Stiftelsen Samariten, Promobilia, Stockholm County Council [ALF project 20150143], Sällskapet Barnavård, Stiftelsen Frimurare Barnhuset I Stockholm, Karolinska Institutet KID-funding, Science for Life Laboratory national sequencing projects grant, Swedish Research Council [2013-2603, 2017-02936], Swedish Society for Medical Research big grant, Marianne and Marcus Wallenberg foundation [2014.0084], Ulf Lundahl memory fund through the Swedish Brain Foundation and Erik Rönnberg Foundation.
Communicating Editor: Dr. Haig Kazazian
Abstract
Skeletal dysplasias are a diverse group of rare Mendelian disorders with clinical and genetic heterogeneity. Here, we used targeted copy number variant (CNV) screening and identified intragenic exonic duplications, formed through Alu-Alu fusion events, in two individuals with skeletal dysplasia and negative exome sequencing results. First, we detected a homozygous tandem duplication of exon 9 and 10 in IFT81 in a boy with Jeune syndrome, or short-rib thoracic dysplasia (SRTD) (MIM# 208500). Western blot analysis did not detect any wild-type IFT81 protein in fibroblasts from the patient with the IFT81 duplication, but only a shorter isoform of IFT81 that was also present in the normal control samples. Complementary zebrafish studies suggested that loss of full-length IFT81 protein but expression of a shorter form of IFT81 protein affects the phenotype while being compatible with life. Second, a de novo tandem duplication of exons 2 to 5 in MATN3 was identified in a girl with multiple epiphyseal dysplasia (MED) type 5 (MIM# 607078). Our data highlights the importance of detection and careful characterization of intragenic duplication CNVs, presenting them as a novel and very rare genetic mechanism in IFT81-related Jeune syndrome and MATN3-related MED.
1 INTRODUCTION
Abnormal development of the skeleton is the hallmark feature of a group of human genetic disorders, collectively termed skeletal dysplasia. Each individual disorder is distinguished by characteristic radiographic findings and more than 300 different skeletal dysplasia-associated genes have been reported to date (Bonafe et al., 2015; Krakow, 2015). Genes involved in skeletal dysplasia encode protein components or regulatory molecules important for skeletal morphogenesis. Even after extensive genetic tests such as whole exome sequencing (WES), a significant proportion of patients lack a molecular diagnosis. This could be due to (i) pathogenic variants in yet unknown genes, (ii) novel types of variants with unknown functional effects in already reported skeletal dysplasia genes, or (iii) due to the presence of mutations challenging to be detected by the technique applied in a given study.
Copy number variants (CNVs) are unbalanced structural genomic variants and involve deletions, insertions, and duplications. Their net effect on the genome in terms of affected nucleotides is higher than that of single nucleotide variations and the correlation between CNV burden and human disease has been an increasingly interesting and expanding field of research (Lieden, Kvarnung, Nilssson, Sahlin, & Lundberg, 2014; Lindstrand, Davis et al., 2014; Lindstrand et al., 2016; Pettersson et al., 2017). CNVs are associated with many human diseases and their clinical presentation depends on the type and location of the CNV. Duplications are in general more difficult to interpret than deletions, and can either be in tandem, potentially disrupting an open reading frame (ORF), or inserted elsewhere in the genome (Stankiewicz, Pursley, & Cheung, 2010). Whole genome sequencing (WGS) has enabled the identification of both location and orientation of duplications of unknown significance (Newman, Hermetz, Weckselblatt, & Rudd, 2015), and increased our understanding of intragenic duplications in human disease (Gilissen et al., 2014; Hjeij et al., 2013; Lieden, et al., 2014; Lindstrand, Davis et al., 2014; Lindstrand, Grigelioniene et al., 2014; Okamoto et al., 2014).
Here, we investigated rare CNVs in two individuals with distinct skeletal dysplasias, Jeune syndrome, and multiple epiphyseal dysplasia (MED) type 5, and a negative clinical exome. Short-rib thoracic dysplasia, also known as asphyxiating thoracic dysplasia (ATD) or Jeune syndrome (SRTD (MIM# 208500)), is an autosomal recessive skeletal ciliopathy characterized by narrow thoracic cage, short ribs, shortened tubular bones, and a trident acetabular roof, with or without polydactyly and in some individuals renal, liver, and retinal involvement (Schmidts et al., 2013). Many genes have been associated with SRTD (Huber & Cormier-Daire, 2012; Zhang et al., 2018) but to date only two individuals are reported with skeletal ciliopathies and pathogenic variants in IFT81 (Duran et al., 2016). MED (MIM# 132400) is a heterogeneous disorder of the epiphyses in the long bones, due to autosomal dominant heterozygous mutations in COMP, COL9A1, COL9A2, COL9A3, or MATN3 (Jackson et al., 2012) or autosomal recessive homozygous mutations in SLC26A2 (Makitie et al., 2003) and CANT1 (Balasubramanian et al., 2017).
In the present study, we hypothesized that small CNVs in known skeletal dysplasia genes, not detected using routine clinical genetic tests, may be involved in the pathogenesis of skeletal dysplasias. Using a targeted array and WGS approach we identified two individuals with intragenic tandem duplications predicted to disrupt IFT81 and MATN3, respectively. Clinical exome sequencing was negative for any disease causing variants in those individuals. The IFT81 intragenic homozygous duplication was inherited from heterozygous, unaffected parents, while the MATN3 duplication was heterozygous and de novo in the proband, findings that are consistent with the mode of inheritance for both disorders. Further studies in primary cells and zebrafish showed that the IFT81 duplication results in the loss of a specific splice isoform (NM_014055.3), further highlighting the importance of transcript-specific mutations in the pathogenesis of rare diseases.
2 MATERIALS AND METHODS
2.1 Clinical synopsis
2.1.1 Patient 1
Patient 1 (P1) is an eight-year-old boy born to healthy, nonconsanguineous parents of Caucasian origin. The boy had respiratory distress syndrome and patent foramen ovale until 6 months of age. He was diagnosed with tracheomalacia during his first years of life and suffered from significant respiratory problems due to increased bronchial mucus, and relatively narrow thorax. He had multiple acute hospital admissions due to hyperactive bronchi with obstruction, especially during common colds. At 3 years of age, the boy was referred to Department of Clinical Genetics at Karolinska University Hospital, for investigation of the genetic background to the suspected diagnosis of Jeune syndrome. He had narrow thorax, short arms, brachydactyly (Figure 1A–F) and short stature (88.5 cm, z-score –2.0). At age 8, he was 112 cm (z-score –2.5). Routine clinical genetic investigation included clinical trio exome sequencing, which did not reveal any pathogenic variants. Z-scores were calculated according to 2007 WHO Reference height-for-age chart 5–19 years for boys (https://www.who.int/growthref/who2007_height_for_age/en/).
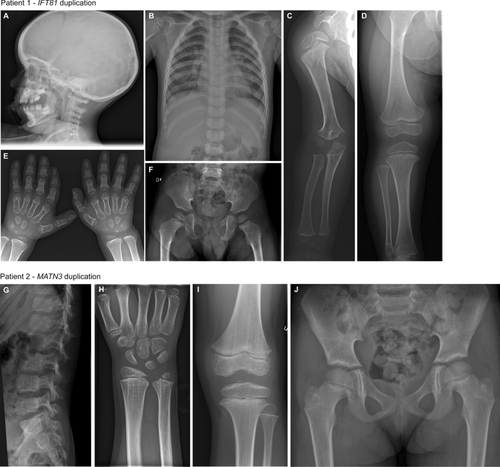
2.1.2 Patient 2
Patient 2 (P2) is a 17-year-old girl born to healthy, nonconsanguineous parents of Caucasian origin. At 7 years of age the girl had a wrist trauma and clinical examination revealed that she had irregular epiphyses of the ulna. From 9 years of age, she had pain in her knees and later developed difficulties running and a change of her walking pattern. Radiology demonstrated diffuse signs but no clinical diagnosis could be set then, while basic endocrine evaluation, karyotype, and MLPA and sequencing of SHOX were all normal. Her height at 14 years was 157 cm (z-score –0.6). She had continued joint pains in her knees and radiograms showed epiphyseal and metaphyseal changes (Figure 1G–J). She was diagnosed with MED and referred to Clinical Genetics, Karolinska University Hospital, but her clinical routine trio exome sequencing did not reveal any pathogenic variants. Z-scores were calculated according to 2007 WHO Reference height-for-age chart 5–19 years for girls (https://www.who.int/growthref/who2007_height_for_age/en/).
2.2 Array comparative genomic hybridization
We used a customized 2 × 400 K comparative genomic hybridization array targeting 1989 genes, including genes involved in skeletal dysplasias, ciliopathies, intellectual disability, and malformation syndromes (Hofmeister et al., 2018; Pettersson et al., 2017; Tham et al., 2016). The array also included all known genes in the cilia proteome and was ordered from OGT (Oxford Gene Technologies, Oxfordshire, UK). The resolution of the array within the targeted genes is one probe per 100 bp in exons, and one probe per 500 bp in introns. Overall resolution over the genome is 25–50 kb. Genomic DNA from both individuals was extracted from whole blood and hybridized with a pooled genomic DNA control from Promega (Madison, WI, USA) matched for sex. All samples were analyzed using Cytosure Interpret Software v4.6 (OGT, Oxfordshire, UK). Design of the array and wet lab was performed as described previously (Pettersson et al., 2017), and all CNVs were classified according to the ACMG guidelines (Richards et al., 2015). All CNVs presented here have been submitted to the ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/).
2.3 Whole genome sequencing
Genomic DNA from both patients was sequenced at National Genomics Infrastructure (NGI), Stockholm, Sweden. The DNA of P1, harboring a IFT81 duplication, was sequenced on an Illumina Hiseq 2500 machine, using a 30X PCR-free paired-end library (2 × 125 bp) and DNA from P2, harboring the MATN3 duplication, was sequenced on an Illumina Hiseq X Ten platform, using a 30X PCR free paired-end library (2 × 150 bp). The median insert size of both libraries was 400 bp. The sequencing data was preprocessed using Piper (v.2.0-beta25 and v1.2.0-beta29 for P1 and P2, respectively), the NGI Stockholm in-house pipeline (https://github.com/NationalGenomicsInfrastructure/piper), a pipeline that performs read alignment to the hg19 reference genome using BWA (version 0.7.5a for P1 and version 0.7.12 for P2) (Li & Durbin, 2009), resulting in 36X mapped coverage for P1 and 29X mapped coverage for P2. The BAM files were analyzed using FindSVCore (https://github.com/J35P312/FindSV_core), a pipeline that combines CNVnator (Abyzov, Urban, Snyder, & Gerstein, 2011) v0.3.2 and TIDDIT (Eisfeldt, Vezzi, Olason, Nilsson, & Lindstrand, 2017) to detect structural variants. The output of these callers was combined into one single variant calling file (VCF) per patient. The structural variants within these VCF files were annotated using VEP (McLaren et al., 2010) and sorted based on the variant frequencies of a local frequency database consisting of 160 patient samples. Lastly, the variants of interest were visualized using Integrative Genomics Viewer (IGV) (www.broadinstitute.org/igv) (Robinson et al., 2011), and the copy number variants of interest were estimated using AMYCNE (https://github.com/J35P312/AMYCNE).
The SNVs were detected using the PileupPipe pipeline (https://github.com/J35P312/PileupPipe). PileupPipe pipeline performs SNV calling using three callers: the GATK haplotype caller (McKenna et al., 2010), Bcftools (Li et al., 2009), and Freebayes (https://github.com/ekg/freebayes). The SNVs detected by these three callers were merged using the GATK-CombineVariants tool, and annotated using VEP and the SweFreq frequency database (Ameur et al., 2017).
2.4 Follow-up studies of IFT81 homozygous duplication
2.4.1 Statistical analysis of loss of heterozygosity
The VCF produced by the PileupPipe pipeline was filtered based on the Swefreq allele frequencies: SNVs having an allele frequency within the interval 0.01–0.2 were kept, and the other SNVs were discarded. Additionally, SNVs located on the sex chromosomes and SNVs not detected by all three callers were removed.
The regions of homozygosity (ROH) were detected using the rhocall software (https://github.com/dnil/rhocall). The rhocall software was run using the filtered PileupPipe VCF files.
Using K-means clustering (k = 2), P1 was compared to 13 reference individuals, where four were born to consanguineous parents, and the rest born to unrelated parents. The 14 individuals were clustered based on the ratio of homozygous SNVs, as well as the total amount of ROH. Both parameters were standardized to normal variety prior to clustering. The Mann–Whitney–Wilcoxon test was used to assess the significance of ROH found in P1.
2.4.2 IFT81 cloning and mRNA synthesis
Human wildtype (wt) and truncated (trunc) IFT81 complementary DNA (cDNA) were obtained by reverse-transcription (New England Biolabs, Ipswich, MA, USA) of RNA samples from P1 and a clinically unaffected, age, and sex-matched control. Amplification of wt- and truncIFT81 for cloning into pCS2+ plasmid was performed to include the BamHI restriction enzyme target sequence followed by the kozak sequence (GCCACCAGGG) on the 5′ end of IFT81 coding sequence, and the NotI restriction enzyme target sequence on the 3′ of IFT81. Primers used were: IFT81-F 5′ GGATCCGCCACCAGGGATGAGTGATCAAATTAAATTC 3′; wtIFT81-R 5′ GCGGCCGCTCACAGTATTAGCCGGTCCTCC 3′; truncIFT81-R 5′ GCGGCCGCTTAAACCTGTTGCCTATACAG 3′.
Cloning into pCS2+ plasmid was performed using the T4 DNA ligase after BamHI and NotI digestion. Sanger sequencing was performed between each step. In vitro mRNA transcription was performed by digesting the PCS2+-wt/truncIFT81 plasmids with NotI, followed by mRNA synthesis using the mMESSAGE mMACHINE kit (Ambion, Thermo Fisher Scientific, Waltham, MA, USA).
2.4.3 Zebrafish maintenance and injection
Adult zebrafish were maintained on a 14 h day/10 h night cycle at Karolinska Institutet zebrafish core facility. Embryos were produced by mass spawning, injected at 1-cell stage, and maintained at 25°C until used.
2.4.4 Zebrafish complementation studies
Rescue experiments were performed by coinjecting in vitro-synthesized wt- or truncIFT81 mRNA with a translation-blocking morpholino (MO) (Kallakuri et al., 2015), used to knockdown endogenous ift81 expression.
Presence of wt- and truncIFT81 mRNA after injection was confirmed by RT-PCR of RNA samples from injected embryos one day post injection. Primers used were: F 5′ CAGGATGAAACTGTGGCTGACAC 3′ and R 5′ GCTTTGCATCTGCTGCAGCTTGG 3′.
For rescue experiments, the ift81-MO was injected at a subeffective concentration, 0.2 mM in the injection mix, resulting in mild phenotypes in the majority of the embryos when injected alone. Complementation studies were performed by coinjecting the MO with wtIFT81, truncIFT81, or with a combination of both IFT81 mRNAs (at 100 ng/μL in injection mix). All injections were performed in triplicate.
Embryos were assessed for early developmental defects at the 8–10 somite stage, that included body axis length and notochord and somite shape. Phenotypes were classified into class I for embryos moderately affected, class II when severely affected, and class III when gastrulation or organogenesis was severely disrupted (Leitch et al., 2008; Lindstrand et al., 2016). Statistical analysis was performed with GraphPad Prism 7, using ANOVA and t-tests for comparisons. Images were acquired using an Olympus IX73 wide field fluorescence microscope.
2.4.5 Western blot
Cultured fibroblasts from P1 and a sex-matched control were lysed with 1X RIPA Buffer (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 1 mM PMSF (Sigma-Aldrich, St. Louis, MO, USA) and 1X EDTA-free protease inhibitor cocktail (Hoffman-La Roche Ltd, Basel, Switzerland). Total cellular protein was recovered in the supernatant after centrifugation and concentration was measured using a BCA assay (Thermo Fisher Scientific, Waltham, MA, USA). Ten micrograms of protein was denatured in Laemmlli buffer (BioRad) and separated using a 4–20% mini-Protean TGX SDS-PAGE (BioRad, Hercules, CA, USA) along with a prestained protein standard (Thermo Fisher Scientific, Waltham, MA, USA) to determine molecular weights. Proteins were transferred onto a PVDF membrane and subsequent immunoblotting was performed using standard protocols as described previously (Hofmeister et al., 2018). Antibodies used were polyclonal anti-IFT81 against amino acids (aa) 131–431 (Catalog number 10604-2-AP; 1:600, Proteintech, Chicago, IL, USA) and secondary HRP anti-rabbit (1:20,000). Anti-acetylated tubulin (Catalog number T6793; 1:1000, Sigma-Aldrich, St. Louis, MO, USA) and secondary HRP anti-mouse (1:20,000) were used for loading controls.
2.5 Ethics
The study was approved by the ethics committee of Karolinska Institutet (ethics permit number 2014/983-31/1) and written informed consent was obtained from the parents of both patients. Wild-type zebrafish were maintained according to standard procedures and with permission from the Stockholm Ethical Board for Animal Experiments (ethics permit number 13063-2017).
3 RESULTS
3.1 Patient 1: IFT81 intragenic homozygous duplication
3.1.1 Array-CGH and WGS
In P1, a homozygous intragenic duplication was identified by array-CGH in IFT81 (NM_014055.3), affecting exons 9 and 10 (Figure 2A and B). The terminal abnormal probes were located at chr12:110576651-110576711 and chr12:110588973-110589026, defining the minimal duplication size to 12.4 kb (chr12:110576651-110589026). The first flanking normal probes were located at chr12:110576015-110576075 and chr12:110593055-110593115, giving a maximum duplication size of 17.1 kb (chr12:110576015-110593115). The duplication was confirmed and shown to be in tandem orientation using WGS and further confirmed by breakpoint PCR and Sanger sequencing of the breakpoint junction (Figure 2C–E). After breakpoint sequencing, the coordinates were (chr12:g.110593351–110576466) (Hg19) and the exact size of the duplication could be determined to be 16.9 kb. The wild-type protein sequence of IFT81 contains 676 aa and the tandem duplication found in IFT81 was predicted to disrupt the ORF and cause a truncation of the peptide sequence to 348 aa (Figure 2F).
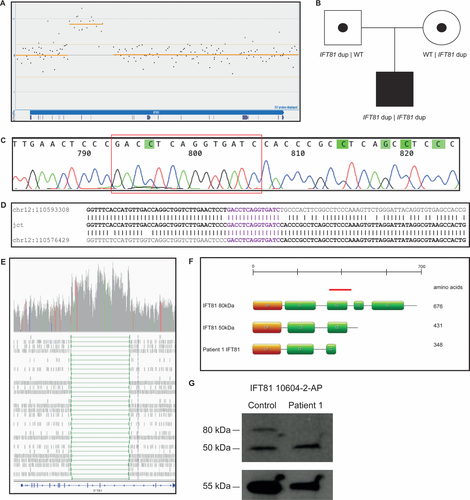
Breakpoint junction analysis revealed both breakpoints to be located in Alu elements from the same subfamily (AluSp) that share 85% of nucleotide similarity in the breakpoint areas. Breakpoint junction was mapped within a 14 bp overlap, suggestive of an Alu-Alu mediated formation of the duplication and the creation of a fusion Alu element (Figure 2D, Supporting Information Figure S1).
The intragenic duplication involving two exons of IFT81 was found through segregation studies to be on both alleles, hence in four copies, and inherited from heterozygous parents (Figure 2B). We then performed a loss of heterozygosity analysis using WGS data from 13 unrelated individuals, including nine samples with known nonconsanguinity, four known consanguineous samples, and P1 from the present study. The result showed that P1 is not consanguineous since his data cluster with those from nonconsanguineous samples (Figure 3). Notable, it was found that a 4.8 Mb region on chromosome 12 (q23.3–q24.12), including IFT81, a 1.6 Mb region on chromosome 20 (q13.12) and a 2.2 Mb region on chromosome 17 (17q24.1–q24.2) had significantly higher amounts of homozygous variants than expected (P = 0.01, Mann–Whitney–Wilcoxon test). Both parents originate from the small Swedish island of Gotland, and are therefore likely to have a common ancestor. No other rare homozygous SNVs or CNVs were identified within the ROHs.

3.1.2 Western blot revealed loss of full-length 80 kDa IFT81 isoform
IFT81 undergoes alternative splicing resulting in at least two protein isoforms (Ensembl, ENSG00000122970). Western blot for IFT81 using total protein from control fibroblasts shows two bands of approximately 80 kDa and 50 kDa, predicted to correspond to those two IFT81 isoforms (676 aa, NM_014055.3, and 431 aa, NM_031473.3) (Figure 2G). In contrast, the band corresponding to the 80 kDa isoform is absent in fibroblasts from P1 (Figure 2G). The 50 kDa band corresponding to the 431 aa isoform was still observed, suggesting that this isoform is not affected or that the 50 kDa band represents a nonspecific product. No band corresponding to the truncated protein (348 aa, 40 kDa) was present, suggesting that disruption of the ORF results in nonsense-mediated decay (NMD) of the truncated NM_014055.3 transcript.
3.1.3 Zebrafish studies
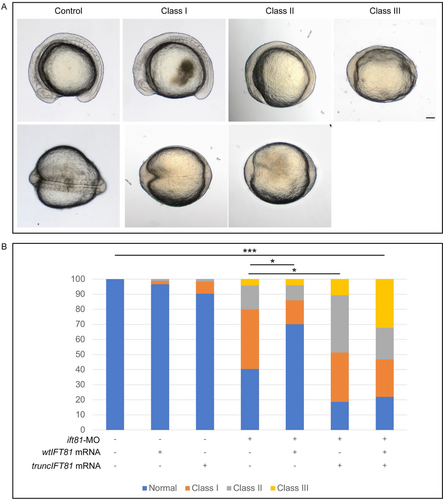
In zebrafish there is no clear indication for the existence of an alternative splice form and we therefore wanted to assess the impact of expressing truncIFT81 in this animal model. Comparison of human and zebrafish protein sequences revealed that they share 70% identity and 84% similarity. Knockdown of endogenous ift81 using a translation blocking MO and mutating endogenous ift81 in zebrafish results in a curved body, vascular defects and kidney cysts, common features of ciliopathies (Dharmat et al., 2017; Kallakuri et al., 2015; Sun et al., 2004). For the complementation experiments, we injected the translation blocking ift81-MO at a subeffective concentration, 0.2 mM in injection mix, resulting in mostly mildly affected body axis, notochord, and somites (classified as Class I, Figure 4A), and only a smaller percentage of affected embryos showing severe body axis shortening, kinked notochord and thinner and broader somites (Class II, Figure 4A), or severe organogenesis or gastrulation defects (Class III, Figure 4A) (adapted from Leitch et al., 2008). Coinjection of ift81-MO with wtIFT81 (0.2 mM and 100 ng/μL in injection mix, respectively) was able to rescue the phenotype, with the majority of the injected embryos showing a normal phenotype (P-value Normal vs. Normal, 0.003; P-value Class I vs. Class I, 0.018; Class II and III, nonsignificant; Figure 4B). Coinjection of ift81-MO with truncIFT81 was, however, unable to rescue the phenotype. In fact, expression of truncIFT81 in combination with the ift81-MO worsens the phenotype with a statistically significant decrease in Normal (P = 0.03) and increase in Class II (P = 0.026) phenotypic embryos (Class I and Class III nonsignificant). Furthermore, coinjection of ift81-MO with wt- and truncIFT81 was also unable to rescue the loss of Ift81 phenotype (Figure 4B), suggesting that in zebrafish truncIFT81 has a dominant negative effect.
3.2 Patient 2: MATN3 intragenic duplication
3.2.1 Array-CGH and WGS
In P2, a heterozygous intragenic duplication was identified by custom array-CGH within MATN3, affecting exons 2–5 (NM_002381.4) (Figure 5A). The terminal abnormal probes were located at chr2:20199299–20199356 and chr2:20208164–20208224, defining the minimal duplication size to 8.9 kb (chr12: 20199299–20208224). The first flanking normal probes were located at chr2:20197797–20197857 and chr2:20210032–20210092, giving a maximum duplication size of 12.2 kb (chr12: 20197857–20210032). Segregation analysis revealed that the duplication was de novo (Figure 5B). WGS and breakpoint junction PCR and Sanger sequencing indicated a tandem orientation of the duplicated segment (Figure 5C–E). After breakpoint sequencing, the genomic coordinates were chr2:20198536–20208996 (Hg19) and the exact size of the duplication was 10.4 kb. Breakpoint junction sequence analysis showed that both breakpoints were located within Alu elements from different subfamilies (AluJo and AluJb) that share 59% sequence similarity in the breakpoint areas (Figure 5D). The distal breakpoint was located in the very end of the AluJo element, immediately followed by an AT-rich simple repeat (Figure 5C and D) while keeping the general Alu structure, which suggest formation of a hybrid Alu element in this junction, similar to the duplication in P1 (Supporting information Figure S1).
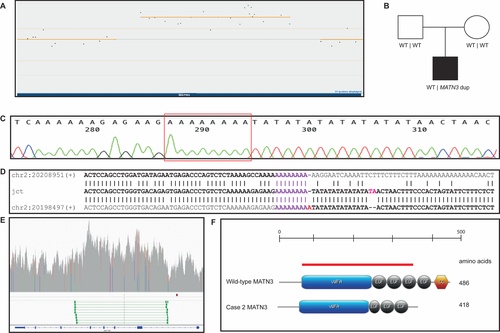
The wild-type protein sequence of MATN3 contains 486 aa and the tandem duplication found in MATN3 was predicted to disrupt the ORF, truncating it to a peptide of 418 aa, resulting in a complete loss of the last two domains of the MATN3 protein (Figure 5F). Unfortunately, no fibroblasts for protein studies were available for this patient.
4 DISCUSSION
In summary, we have identified a homozygous pathogenic intragenic duplication in IFT81, causing a milder form of Jeune syndrome and a heterozygous putative pathogenic intragenic duplication in MATN3, likely causing a mild MED phenotype.
IFT81 (Intraflagellar Transport 81) is a protein-coding gene consisting of 19 exons that encodes a core component of the intraflagellar transport B-complex (IFT-B). It is involved in anterograde transport from the base to the tip of cilia (Perrault et al., 2015). All six components of the IFT-A complex have been associated with skeletal ciliopathies (Huber & Cormier-Daire, 2012) and so far 4 out of 16 members of the IFT-B complex have been associated with skeletal ciliopathies (IFT52, IFT80, IFT172, IFT81) (Beales et al., 2007; Duran et al., 2016; Halbritter et al., 2013; Zhang et al., 2016). Compound heterozygous mutation in IFT81 (p.Leu29Phe;p.Arg512* and p.Leu262*;c.1303_1305delCTT, respectively) have been shown to cause phenotypes within ATD and short-rib polydactyly syndrome type II (Duran et al., 2016). An ift81 zebrafish mutant showed somite stage enrichment of gene expression in organs typically associated with ciliopathies: notochord, otic vesicle, pronephric duct, and around the cerebral ventricles, indicating an essential role of ift81 in embryonic development of kidneys and brain (Sun et al., 2004). Here, we show that expression of the truncated IFT81 transcript is not able to rescue the loss of endogenous ift81 and may have a negative role in embryogenesis in zebrafish. The dominant negative effect observed in the zebrafish studies is, however, not observed in the heterozygous carrier parents, both clinically unaffected. This result in addition to the lack of protein expression in P1 suggests that the truncated transcript is degraded by NMD. Interestingly, Western blot studies with protein extracted from P1 fibroblasts showed that the predicted truncated IFT81 was not translated in that particular cell type, whereas a shorter IFT81 isoform seems to still be present. However, to our knowledge, the antibody used for Western blot in the present study has not been applied in other studies, hence we cannot exclude that the second band is due to unspecific binding unrelated to IFT81.
Short-rib thoracic dysplasia caused by null mutations in their corresponding genes is often not compatible with life (Huber & Cormier-Daire, 2012). Protein studies of P1 in the present study showed a complete lack of the canonical IFT81 isoform, but suggest expression of a shorter IFT81 isoform. Only two other cases with IFT81 mutations and short-rib thoracic dysplasia/ATD phenotypes have been reported previously; Duran et al. reported two patients with compound heterozygous mutations who died at 19 months of age (ATD) and a few minutes after birth (short-rib thoracic dysplasia type II), respectively (Duran et al., 2016). P1 reported here is now 8 years old and developing well, although reporting frequent upper respiratory tract infections. He also presents some learning difficulties in regular elementary school, suggesting that the phenotype variability in IFT81-related Jeune syndrome is possibly related to the mutation type.
Heterozygous mutations in MATN3 are known to cause autosomal dominant MED type 5 (MED5). MATN3 (Matrillin-3) is member of the von Willebrand factor A domain containing protein family, expressed in the cartilage during development, then at low levels in adult articular cartilage, although its expression is induced during osteoarthritis (Deak, Wagener, Kiss, & Paulsson, 1999; Pullig, Weseloh, Klatt, Wagener, & Swoboda, 2002; Wagener, Kobbe, & Paulsson, 1997). A study by the European Skeletal Dysplasia Network (ESDN) with a cohort of 56 patients with MED found that in 24%, the MED was caused by heterozygous mutations in MATN3, while a Korean study of 55 individuals with MED detected heterozygous mutations in MATN3 in 55% of the studied individuals (Jackson et al., 2012; Kim et al., 2011). The results from the Korean study are in close agreement with a Japanese study describing pathogenic variants in MATN3 in 9 out of 19 affected individuals (47%) (Mabuchi et al., 2004). Most of the previously reported disease-causing variants are missense variants located in the vWF domain of MATN3 that appear to cause a loss-of-function due to a delay of the folding of the A-domain, resulting in retention of mutant matrillin-3 in the rER both in vitro (Cotterill et al., 2005; Otten et al., 2005) and in vivo (Leighton et al., 2007; Nundlall et al., 2010). Only one mutation located outside the vWF domain has been described previously in a 32 year old patient with MED (Maeda, Nakashima, Horikoshi, Mabuchi, & Ikegawa, 2005). In P2, the identified intragenic MATN3 duplication is predicted to cause a premature stop codon. The truncated product is either degraded by NMD or a shorter protein is produced and if the truncated protein is translated, the vWF domain would still be intact.
Overall, there is a known broad phenotypic variability in MATN3-related MED (Jackson et al., 2012; Kim et al., 2011) and most patients have severe involvement of both hips and knees. The phenotype in P2 is on the milder end of the MED5 spectrum with currently no hip involvement and mild radiographic changes in the epiphyses.
The breakpoint junctions of both IFT81 and MATN3 intragenic duplications map within Alu elements. We suggest that those CNVs are mediated by Alu sequences through nonallelic homologous recombination (NAHR) (Shaw & Lupski, 2005) or fork-stalling template switching (FoSTeS)/microhomology-mediated break-induced replication (MMBIR) (Boone et al., 2014; Mayle et al., 2015). Both duplications are also likely to have created Alu-Alu fusion elements (AluSp-AluSp and AluJo-AluJb, respectively) (Gu et al., 2015). Alu elements are suggested to be prone to nonallelic recombination or to facilitate template switching during repair by replication-based mechanisms due to their high nucleotide similarity as well as ubiquitous presence in the human genome. In fact, Alu-Alu mediated CNVs are proposed to account for 0.3% of human genetic diseases, but this number is likely to be underestimated (Deininger & Batzer, 1999), particularly within genes on which they are predicted to play an important role generating exonic CNVs (Song et al., 2018).
Intragenic exonic deletions and duplications still remain a technical challenge for molecular diagnosis since they frequently evade detection. Intragenic CNVs are often too large for prompt detection using single-nucleotide variant and indel tools whereas too small for most of the CNV detection tools used regularly in diagnostic laboratories, such as WES and low-resolution clinical arrays. The examples provided in this present study support the hypothesis that Alu elements have a large impact in creation of CNVs, and that intragenic CNVs are an important, yet still overlooked cause of disease in humans.
To our knowledge, only point mutations have been described previously as pathogenic variants in MATN3 and IFT81. We show that customized array-CGH targeting specific genes or WGS are powerful tools when WES results are negative. In addition, we suggest that a shorter IFT81 isoform is able to partially compensate the function of the full-length isoform, resulting in a milder form of Jeune syndrome.
ACKNOWLEDGMENTS
We thank both patients and their families for participating in this study. We also thank the staff at Karolinska core fish facility for excellent fish maintenance and UPPMAX for the use of computer infrastructure resources and the support from the National Genomics Infrastructure (NGI) Stockholm at Science for Life Laboratory in providing assistance in massive parallel sequencing. G.G. was supported by grants from Stiftelsen Samariten, Promobilia, Stockholm County Council (ALF project 20150143), Sällskapet Barnavård and Stiftelsen Frimurare. A.L. was supported by the SciLifeLab national sequencing projects grant, the Swedish Research Council [2013-2603, 2017-02936], the Swedish Society for Medical Research big grant, the Marianne and Marcus Wallenberg foundation [2014.0084], the Stockholm City Council, the Ulf Lundahl memory fund through the Swedish Brain Foundation and the Erik Rönnberg Foundation. M.P. and A.H. were supported by grants from Karolinska Institutet funding for doctoral education (KID).
CONFLICT OF INTEREST
The authors declare no conflict of interest.