Impaired Development of Neural-Crest Cell-Derived Organs and Intellectual Disability Caused by MED13L Haploinsufficiency
Contract grant sponsors: Biomedical Medical Research Council (BMRC) of the Agency for Science, Technology and Research (A*STAR), Singapore; Singapore International Graduate Award (SINGA).
Communicated by Arnold Munnich
ABSTRACT
MED13L is a component subunit of the Mediator complex, an important regulator of transcription that is highly conserved across eukaryotes. Here, we report MED13L disruption in a translocation t(12;19) breakpoint of a patient with Pierre–Robin syndrome, moderate intellectual disability, craniofacial anomalies, and muscular defects. The phenotype is similar to previously described patients with MED13L haploinsufficiency. Knockdown of MED13L orthologue in zebrafish, med13b, showed early defective migration of cranial neural crest cells (NCCs) that contributed to cartilage structure deformities in the later stage, recapitulating craniofacial anomalies seen in human patients. Notably, we observed abnormal distribution of developing neurons in different brain regions of med13b morphant embryos, which could be rescued upon introduction of full-length human MED13L mRNA. To compare with mammalian system, we suppressed MED13L expression by short-hairpin RNA in ES-derived human neural progenitors, and differentiated them into neurons. Transcriptome analysis revealed differential expression of components of Wnt and FGF signaling pathways in MED13L-deficient neurons. Our finding provides a novel insight into the mechanism of overlapping phenotypic outcome targeting NCCs derivatives organs in patients with MED13L haploinsufficiency, and emphasizes a clinically recognizable syndromic phenotype in these patients.
Introduction
MED13L (MED13L; MIM #608771) is one of the subunit in the CDK8 module of Mediator Complex, a dissociable component of Mediator complex that has been described to have an activating or repressing functions in regulating transcriptions [Tsai et al., 2013]. Mediator complex regulates gene expression by physically linking transcription factors to RNA Polymerase II [Ansari and Morse, 2013; Davis et al., 2013; Nakamura et al., 2011; Tsai, et al., 2013]. Recent studies have shown that MED13L has a role in cancer pathways, specifically suppressing Retinoblastoma/E2F-induced growth [Angus and Nevins, 2012], and also targeted for degradation by FBW7 (F Box and WD repeat domain-containing 7) a tumor suppressor and a component of SCF (Skp-Cullin-F box) ubiquitine ligase [Welcker and Clurman, 2008] to disrupt CDK8-mediator association [Davis et al., 2013].
Several studies have identified structural variants (SVs) and mutations affecting MED13L in patients with heart defects, craniofacial anomalies, and intellectual disability (ID) [Muncke et al., 2003; Najmabadi et al., 2011; Asadollahi et al., 2013]. The first study reported a patient harboring a translocation-disrupting MED13L with a dextra-looped transposition of great arteries, 1 (dTGA1; MIM #608808) and ID, and three additional missense mutations in MED13L (p.Glu251Gly, p.Arg1872His, and p.Asp2023Gly) in mutation screening of dTGA patients’ cohort [Muncke et al., 2003]. Subsequently, copy-number variants encompassing MED13L were reported in three patients of which two were out-of-frame deletions, with conotruncal heart defect, moderate ID, hypotonia and facial anomalies, and one triplication involving a full-length MED13L, and a neighboring gene MAP1LC3B2 whom displayed a much milder phenotype with learning disability and no major facial dysmorphism [Asadollahi et al., 2013]. In addition, homozygous nonsynonymous variant p.Arg1416His was found in two siblings presenting isolated nonsyndromic ID without associated anomalies, suggesting that disruption of MED13L might underlie the overlapping phenotypes in these individuals.
Here, we report a new patient harboring a monoallelic translocation disrupting MED13L that displays remarkably similar phenotypes to previously reported patients harboring MED13L deletions, which strongly supports that haploinsufficiency of MED13L represents a clinically recognizable entity. Using zebrafish as a developmental model system, we demonstrated through generation and rescue of zebrafish phenotypes by morpholino (MO)-mediated knockdown of med13b, the zebrafish closest orthologue of MED13L, resulted in improper development of branchyal and pharyngeal arches due to defects in early migration of neural crest cells (NCCs), thereby recapitulating the craniofacial anomalies seen in human patients. Furthermore, we analyzed the gene expression changes upon MED13L knockdown in neurons derived from human embryonic stem cells (hESCs) and identified significant differences in expression of genes from Wnt and FGF signaling pathways.
Materials and Methods
Ethics Statement
Patient sample and information were collected after written informed consent from patient's parents was obtained and in accordance with local institutional review board approved protocols from National University of Singapore in Singapore, and CHRU de Montpellier, hospital Arnaud-de-Villeneuve, France.
Patient
The patient is the only child of healthy and unrelated parents. She was diagnosed with Pierre–Robin sequence at birth, characterized by cleft palate, glossoptosis, and retrognathia. She had multiple limb contractures and camptodactyly, including metatarsus adductus of the thumb, and bilateral equinovarus foot deformity. Her electroencephalogram showed epileptiform discharges with absence seizures. At 4 years old, she had moderate intellectual disability (IQ = 50) and language difficulties with the absence of speech. Other developmental milestones were significantly delayed and she required special education. Further speech development was delayed in isolated words, graphism, phonological programming, and global speech delay at the age of 14 years. MRI done at the age of 8 years revealed a ventricule enlargement in correlation with global atrophy. Physical examinations revealed dysmorphic features including flat occiput, hypertelorism, flat philtrum, bulbous nose, and broad nasal bridge. Strabismus and hirsutism were noted. She had scoliosis during puberty, about 14 years old. Chromosome analysis revealed a balanced translocation between chromosome 12 and 19 t(12;19) (q24;q12). Both parents have normal karyotypes and did not show any phenotypic abnormality. Array-CGH (Agilent 244K) performed on the patient's genomic DNA showed no additional chromosomal imbalances.
Genomic DNA
DNA sample was obtained from Epstein–Barr virus transformed lymphoblastoid cell lines from peripheral blood lymphocytes of the patient, and was extracted by QIAamp DNA Blood and Cell Culture DNA Kit (Qiagen, Valencia, CA) according to the manufacturer's instruction. Quality and quantity of the extracted DNA were measured using NanoDrop 1000 Spectrophotometer and agarose gel electrophoresis.
DNA–PET Sequencing
We constructed the DNA–PET library according to the method described in Hillmer et al. (2011). Genomic DNA (40 μg) was fragmented to 10 kb DNA fragments, ligated to long mate paired cap adaptors, and end repaired. After size selection, the library was amplified by PCR and subjected to high-throughput sequencing of 50 bp libraries using the SOLiD sequencers (v4) according to manufacturer's instructions (Life Technologies, Carlsbad, CA). Sequence tags were mapped to human reference sequence (NCBI Build 36) and paired using SOLiD system Analysis Pipeline Tool Bioscope, allowing up to 12 color code mismatches per 50 bp tag.
The majority of the PET sequences mapped accordingly to the reference genome NCBI Build 36 (concordant PETs or cPETs) with expected mapping orientation and expected mapping distances. The remaining portion of the PETs mapped discordantly to the reference genome (discordant PETs or dPETs), classified as those with incorrect paired-tag orientation and incorrect genomic distances. These abnormally oriented dPETs provided information for different types of structural variations as described in Hillmer et al. (2011) and Ng et al. (2012). Filtering of SVs were performed across 23 normal individuals (25 DNA-PET data sets), the pilot release set of 1000 Genome Project SV release set, previously published paired-end sequencing studies in 18 normal individuals, and Database of Genomic Variants (DGV). Sequences have been submitted to the Short Read Archive (http://trace.ncbi.nlm.nih.gov/Traces/sra) at the National Center for Biotechnology Information (NCBI) with the accession number SRP034864.
Sanger Sequencing Validation
Primers were designed by Primer3 program, and the amplicons spanned the breakpoints predicted by dPET clusters according to the human genome assembly NCBI Build 36. PCR was carried out with JumpStart REDAccuTaq LA polymerase (Sigma–Aldrich Inc., St. Louis, MO) in a 50-μl reaction volume and with 500 ng of genomic DNA template. The following program was used: (1) initial denaturation at 96°C for 30 sec; (2) 40 cycles of 15 sec at 94°, 30 sec at 58°C, 10 min at 68°; and (3) 68° for 10 min. PCR products showing single bands were purified by QiaQuick PCR Purification Kit (Qiagen) and used as templates for sequencing in both directions by Sanger sequencing. The sequencing of junction fragments was aligned to the human genome reference NCBI Build 36 sequence using Blat in the UCSC Genome Browser.
Quantitative RT-PCR
Total RNA was extracted using RNAeasy Mini kit (Qiagen). Reverse transcription of 2 μg RNA was performed in 20 μl of SuperScript III Reverse Transcriptase reaction buffer (Life Technologies) using random hexamer primers. qPCR reactions were performed in the Viia7™ Real Time PCR system (Life Technologies) with 100 ng of cDNA, 200 nM of each primer using the SybrGreen PCR Master Mix (Life Technologies). Measurements were performed in triplicates. Relative mRNA expression was obtained by using 2−∆∆Ct method and normalized against control sample with human ACTB. For zebrafish morphants, data were normalized against zebrafish 18s ribosomal RNA or actin.
Western Blot
Lymphoblastoid cells were lysed in RIPA buffer and protease inhibitor, and protein concentration was measured with a Bradford assay kit (Bio-Rad, Hercules, CA). Cell lysate (50 μg) was resolved on a 10% SDS-PAGE and transferred to a polyvinylidinefluoride membrane (Bio-Rad). After blocking in 5% milk for 1 hr, the appropriate primary antibodies were added: anti-MED13L (rabbit polyclonal antibody generated against the region of 550–600 amino acid, ab87831; Abcam, Cambridge, United Kingdom), anti-β-actin (sc-1616; Santa Cruz, Dallas, TX) for 1 hr. After washing with TBS/0.1% Tween-20 (TBS-T), horse-radish peroxidase-conjugated antirabbit or goat IgG secondary antibodies was added for 1 hr. After washing with TBS-T, signals were developed using Amersham ECL Select detection kit (GE Healthcare, Uppsala, Sweden). Western blot experiments were repeated twice.
MO Microinjection
Two antisense MO for zebrafish Mediator complex subunit 13-like, med13b (NM_001083838) sequences were designed by the manufacturer to target the start codon (med13b-1-Start: ATGGCAGAGCCCCTCGTTTGTTAGA) and the splice site between exon 14 and 15 (med13b-2-Splice: AGAACTCATCATCAAAGCGCAGTCC) (GeneTools, Philomath, OR). Subsequently, 4 ng of each MO was injected into the yolk of one to two cell stage embryos from wild-type AB zebrafish strains. Injected embryos were incubated at 28.5° until reaching the appropriate developmental stage. Consistent phenotype was observed in at least three independent experiments of around 90–150 embryos each. The embryos were then fixed in 4% paraformaldehyde in PBS (PFA/PBS) overnight in 4°C and stored in gradual increase of methanol hydration in 100% MeOH at −20°C overnight.
In Situ Hybridization
Whole mount in situ hybridizations were carried out as previously described by Thisse and Thisse (2008). Antisense DIG (Roche, BAsel, Switzerland) riboprobes were synthesized: med13b (cDNA clone ID: 7050861; GE Dharmacon, Lafayette, CO), sox10 (PCR-amplified with primers 5′-GGGATTCAGA GCGCGAGCGA-3′ and 5′-CGCGC ATTTAGGTGACACTATAGAAGTGACAGGTACTAGC ATC ATG TG-3′), twist1b (PCR amplified with primers 5′-CCCTCCGTGA CGCAGGAGGA-3′ and 5′-CGCGC ATTTAGGTGACACTATAGAAGTG TTCGTTGAGTGTGTGTGTTT-3′), and islet1 (PCR amplified with primer 5′-TCCAGGCTCAAACTCCAC-3′ and 5′-GGATCCATTAACCCTCACTAAAGGGAATGTCCGACCGTTTACTTACAG-3′).
MED13L mRNA Synthesis
Full-length MED13L cDNA (NM_015335.4) was amplified by using primers hMED13L-F 5′-AGGATCATGACTGCGGCAGC-3′ and hMED13L-R 5′-TCGCGGAGGATCATGACT-3′ from human cell line GM12878 cDNA. Full-length MED13L cDNA was subcloned into TOPOII Dual Promoter vector by using Topo TA Cloning Kit (Life Technologies). The validity and orientation of clones were confirmed by PCR and sequencing. Capped mRNA was generated by using SP6 mMESSAGE Machine T3 Kit (Life Technologies) according to manufacturer's instruction and purified by using RNAeasy mini kit (Qiagen). mRNA rescue experiments were performed by coinjection of 4 ng med13b-MO and 150 pg of MED13L mRNA.
Alcian Blue Staining
Embryos were fixed at 5 dpf with 4% PFA for 2 hr at room temperature. After fixation, embryos were dehydrated to ethanol (50%) for 10 min at room temperature. Embryos were then transferred to Alcian Blue staining solution (0.4% Alcian Blue [w/v] in 70% ethanol) to rock at room temperature overnight. To remove the pigmentation in older embryos, embryos were bleached the next day in 3% H2O2 and 2% KOH for 20 min. To clear the tissue, embryos were soaked in 20% glycerol/0.25% KOH for 30 min, and prepared for imaging in the same solution.
Neural Progenitor Cells Maintenance
NPC differentiation of ESCs was done based on established protocols [Li et al., 2011]. Cells were first plated in clumps on matrigel-coated 6 well-plates using mTESR1 media (StemCell Technologies, Vancouver, British Columbia, Canada). The following day, the media was changed with NPC media composed of DMEM-F12/neurobasal media (1:1) supplemented with N2 and B27 without vitamin A supplements (Gibco, Life Technologies) and CHIR99021 (4 μM) (StemCell Technologies), SB431542 (3 μM) (Millipore, Billerica, MA), Compound E (0.1 μM) (Millipore), and human LIF (10 ng/ml) (Millipore). After 7 days, cells were passaged using accutase and grown in the same basal media with CHIR99021 (3 μM), SB431245 (2 μM), and additional growth factors human LIF (10 ng/ml), EGF (20 ng/ml) (Life Technologies), and bFGF (20 ng/ml) (Life Technologies).
shRNA Transfection
Two sequences targeting MED13L sequences were designed and cloned into pSUPER neo vectors (Oligoengine, Seattle, WA). Two shRNAs were transfected into H1-NPCs by using Fugene 6 transfection reagent (Promega, Madison, WI). After 48 hr, G418 (Gibco, Life Technologies) was added into the culture for selection of stably transfected cells. The surviving cells were expanded for NPCs characterization and neuronal differentiation.
Neuronal Differentiation
Neuronal differentiation was performed according to the protocol described by Brennand et al. (2011). NPCs were plated at a density of 200,000 cells per 6 well on a Poly-L-Ornithine/Laminin-coated coverslips, and grown in neuronal differentiation media (DMEM-F12/neurobasal media [1:1] supplemented with N2 and B27 supplements [Gibco, Life Technologies], GDNF [20 ng/ml] [R&D Systems, Minneapolis, MN], BDNF [20 ng/ml] [R&D Systems], dibutyryl-cyclic AMP [1 mM] [Sigma–Aldrich Inc.], and ascorbic acid [200 nM] [Sigma–Aldrich Inc.]). H1-NPCs-derived neurons were differentiated for 4 weeks to achieve TUJ1 and MAP2-positive neuronal cells. For characterization, neurons were passaged using accutase and replated at a very low density (5,000 cells per 24 well) on coverslips.
Immunocytochemistry
Cells were plated on ethanol-treated coverslips and fixed with 4% paraformaldehyde in PBS at 4°C and incubated with TBS containing 5% of fetal bovine serum, 1% BSA (Sigma–Aldrich Inc.), and 0.1% of Triton X-100 (Sigma–Aldrich Inc.) for 45 min at room temperature. Following are the primary antibodies used: Nestin (ab22030, 1:500), Sox2 (sc17320, 1:200), Vimentin (sc7557, 1:100), Map2 (A2320, 1:1000), and Tuj1 (PRB-435P, 1:2000). Each primary antibody was incubated with fixed cells overnight at 4°C, and cells were subsequently stained with secondary antibody conjugated to Alexa Fluor 594 or 488 (Molecular Probes, Life Technologies, Carlsbad, CA) (4 μg/ml) for 1 hr at room temperature in the dark. Images were captured using a ZEISS AxioObservor DI inverted fluorescence microscope (Carl Zeiss, Oberkochen, Baden-Wurttemberg, Germany).
Microarray
Human Ref-12 Expression BeadChip microarrays (Illumina, San Diego, CA) were used for genome-wide expression analysis. For hybridization, cRNAs from duplicate or triplicate samples were synthesized and labeled using TotalPrep RNA Amplification Kit (Ambion, Austin, TX), following the manufacturer's instructions. Scanned data from the BeadChip raw files for all samples were retrieved and background corrected using BeadStudio, and subsequent analyses were completed in GeneSpring GX. Data were normalized both within and between arrays, and corrected for multiple testing according to Benjamini–Hochberg. We defined genes as significantly differentially expressed when FDR was <0.05.
Data Analysis
Gene ontology and pathway enrichment analysis were performed by using DAVID Functional Annotation Bioinformatics Microarray Analysis and PantherDB Classification system. Differentially expressed gene lists were exported through these systems and top GO category were considered for P < 0.05 and after multiple testing correction with Benjamini–Hochberg.
Results
Identification of MED13L Disruption in a Patient with Mild ID and Dysmorphism
We analyzed a patient with Pierre–Robin sequence at birth, moderate ID, limb defects, and dysmorphic features. Prior cytogenetic analysis revealed a de novo balanced translocation between chromosome 12 and 19 t(12;19)(q24;q12). We performed a large-insert genome paired-end tag (DNA–PET) sequencing approach as described previously [Hillmer et al., 2011; Yao et al., 2012; Utami et al., 2014] to fine map the translocation breakpoint, which generated approximately 66 million sequence reads and achieved 150× physical coverage. Two abnormally oriented paired clusters corresponding to the t(12;19) translocation were identified. The breakpoint on chromosome 12 (chr12:114,971,734–114,971,744, hg18) disrupted MED13L and no coding genes were found in the chromosome 19 breakpoint (chr19:35,769,937-35,769,968, hg18). Sanger sequencing validation confirmed the breakpoint junction at intron 4 of MED13L with a 1-bp microhomology in derivative chromosome 12 at position, likely resulting in a truncated MED13L protein at amino acid position Ser160 (Fig. 1A). The heterozygous disruption was confirmed by capillary sequencing on coding exons of MED13L that did not reveal any additional mutation in the flanking exons (Supp. Fig. S1). Both mRNA and protein expression of MED13L were reduced in the patient's lymphoblastoid cell lines compared with two healthy controls, as shown by qPCR and Western blot analysis (Supp. Fig. S2). MED13L mRNA is ubiquitously expressed in human tissues with the highest levels in cerebellum, fetal brain, and skeletal muscle (Supp. Fig. S3). We compared the affected regions of MED13L mutations in previously published subjects [Muncke et al., 2003; Najmabadi et al., 2011; Asadollahi et al., 2013] (Fig. 1B) and their clinical findings are summarized in Table 1. It was previously suggested that variants within N- or C-MED13 domains were likely to cause cardiac phenotypes [Asadollahi et al., 2013]. However, the translocation breakpoint in our patient is within N-terminal MED13 domain, and cardiac defect was not present at birth and early adulthood. Interestingly, during the review of this manuscript, two additional patients with deletions within the N-MED13 domain were reported, and no cardiac anomalies were noted in both patients [van Haelst et al., 2014], suggesting a reduced penetrance for congenital heart defect in patients with MED13L haploinsufficiency.
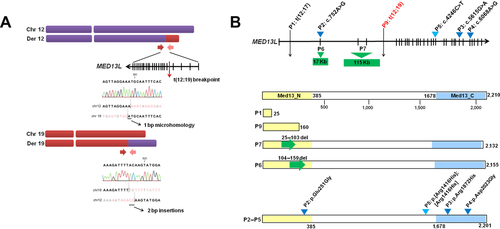
| Patient 1 [Muncke et al., 2003] | Patient 2 [Asadollahi et al., 2013] | Patient 3 [Asadollahi et al., 2013] | Patient 4 [Asadollahi et al., 2013] | Patient 5 (this study) | |
|---|---|---|---|---|---|
| MED13L SVs | t(12;17)(q24.1;q21) (intron 1) | Deletion in exon 2(c.71-?_310+?del) | Deletion in exon 3–4(c.311-?_479+?del) | Triplication of the whole gene (c.1?_6574+?) (Flanagan et al., 1991) | t(12;19)(q24;q21) (intron 4) |
| Inheritance | De novo | De novo | De novo | De novo | De novo |
| Neurological features | |||||
| Intellectual disability | + | + | + | (+) | + |
| Language delay | NR | + | + | NR | + |
| Speech | Nearly absent | Delayed | Absent | NR | Absent |
| Ataxia | + | NR | NR | NR | NR |
| Motor skills | + | + | + | NR | + |
| Hypotonia | - | + | + | + | NR |
| Dysmorphic features | |||||
| Facial/skull prominence | Microcephaly | Broad forehead with nevus simplex | Broad forehead, prominent occipital protuberance | NR | Plagiocephaly |
| Cranial | NR | Macroglossia, micrognathia | Micrognathia | NR | Pierre–Robin sequence: cleft palate, glossoptosis, microretrognathia |
| Ears | NR | Large, low-set ears | Large, low-set ears | NR | NR |
| Eyes | NR | Upslanting palpebral fissures | Upslanting palpebral fissures | NR | Hypertelorism, strabismus |
| Nose | NR | Flat nasal root, bulbous nose | Flat nasal root, bulbous nasal tip | Broad nasal bridge | Broad nasal bridge |
| Mouth | NR | Deep philtrum, facial hypotonia with open mouth appearance | Deep and short philtrum, hypotonic with open mouth appearance | NR | Flat philtrum |
| Digital | NR | NR | Bowed legs, overlapping fifth toe | NR | Metatarsus adductus, camptodactyly, bilateral equino-varus deformity |
| Cardiac manifestation | |||||
| Septal defect | pVSD, open foramen ovale, coA | Pulmonary atresia, supracardial TAPVC, VSD, ToF | ToF | pVSD | NR |
| Transposition of arteries | dTGA | - | - | - | - |
- CoA, coarctation of the aorta; dTGA, dextro-looped transposition of great arteries; pVSD, perimembranous ventricular septal defect; TAPVC, total anomalous pulmonary venous connection; ToF, tetralogy of fallot; ID, intellectual disability; DD, developmental delay; SD, speech delay; LD, language delay; NR, not reported; (+), learning disability.
Knockdown of med13b Impairs Cranial NCCs Migration
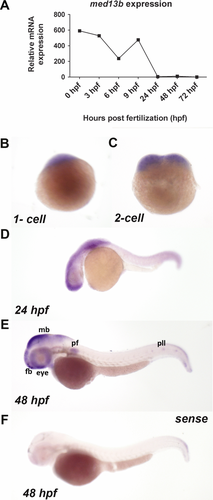
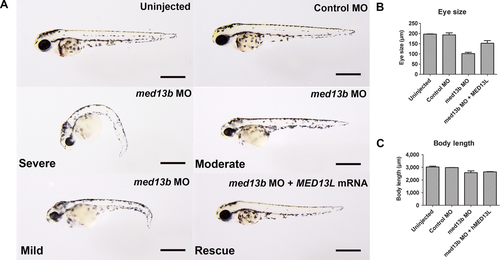
To investigate the role of MED13L underlying the broad phenotypic spectrum observed in these patients, we examined whether a knockdown of med13b, with 65% protein sequence similarity to human protein (Supp. Fig. S4), could model the observed clinical features seen in patients. First, we checked the mRNA expression of med13b across developmental stages between 1 and 72 hr postfertilization (hpf) by qPCR, and observed maternal and zygotic transcripts during embryonic development (Fig. 2A). To determine spatiotemporal expression of med13b, we performed in situ hybridization of med13b and confirmed maternal expression at one to two cell stage in the first blastomeres, supporting a role during early embryogenesis (Fig. 2B and C). At 24 hpf and 48 hpf, zygotic med13b expression became restricted to the forebrain, midbrain-hindbrain boundary, eye, pectoral fin, and the lateral line (Fig. 2D and E), whereas sense probe showed no specific hybridization signal (Fig. 2F). Some of these tissues require derivatives of neural crest lineage to develop: pectoral fin arises from trunk neural crest, lateral line arises from cranial placodal ectoderm, and eye development involves migration of NCCs to the periocular mesenchyme [Langenberg et al., 2008]. med13b suppression by splice-site antisense MO oligonucleotides resulted in developmental defects affecting eyes, head, and antero-posterior axis seen at 2 days postfertilization (dpf) (Fig. 3A). More than 95% of the embryos were affected with a curved body axis, underdeveloped head, pericardial edema, and microphtalmia, which were categorized as “severe” for 40%, and as “mild-to-moderate” for 55% of the analyzed embryos. Coinjection of approximately 150 pg full-length human MED13L mRNA with med13b MO was sufficient to rescue the eye size of the morphants, and fully restore head development, whereas body length was partially rescued (Fig. 3B and C).
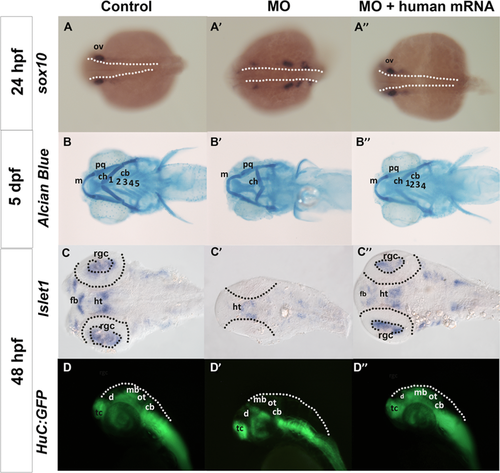
One of the striking observations among patients with MED13L haploinsufficiency is the presence of craniofacial dysmorphisms (including upslanting palpebral fissures, flat nasal root, bulbous nose, broad forehead, deep philtrum, and palatal problems) (See Table 1). To study the implication of med13b loss of function in craniofacial and cartilage structure development, we checked the migration of cranial NCCs in the morphant embryos by using sox10 that is expressed in the early migrating cranial and trunk NCCs. At 24 hpf, the expression of sox10 was strongly present in the cranial ganglia and otic vesicle in control embryo, whereas the NCCs migration was delayed in the morphants, shown by clustered expression of sox10-positive cells along the migratory cranial ganglia at a more posterior location (Fig. 4A and A′). Upon coinjection with human MED13L mRNA, the location of sox10 positive cells were comparable to that of control embryos (Fig. 4A), suggesting that med13b is required for proper NCCs migration. Further examination of cartilage development on 5-day-old morphant embryos stained by Alcian Blue, a time point when all craniofacial cartilage had been fully formed, resulted in a severely distorted ceratohyal cartilage (Fig. 4B). This phenotype was fully restored upon human MED13L mRNA introduction (Fig. 4B). Similar pharyngeal skeletal defects have been observed in the zebrafish bielak mutants 5 dpf larvae, with ceratohyal pointing caudally, suggesting a role in the specification of craniofacial skeleton [Neuhauss et al., 1996]. These data clearly support that defective cranial NCC migration contributes to craniofacial cartilage defects upon disruption of med13b in these embryos.
Loss of med13b Affects Neuronal Distribution in Zebrafish Brain
Another shared clinical feature among patients with MED13L disruptions is mild to moderate intellectual disability. To explore the impact of med13b knockdown in neuronal development, we used islet1 as a marker of differentiating motor neurons, which is expressed in the retinal ganglion cells (RGCs), hypothalamus, and ventral telencephalon. Med13b morphants showed a lack of islet1 expression in RGCs and forebrain, and reduced expression in the hypothalamus and telencephalon, which was restored upon human MED13L mRNA coinjection, suggesting impaired neuronal development in early brain patterning (Fig. 4C and C”). To better examine events of neural differentiation, we further analyzed distribution of neuroblasts in the developing zebrafish brain using HuC:GFP (green fluorescent protein) transgenic line [Park et al., 2000], where cells that start to develop as neurons initiate expression of GFP under the regulation of HuC promoter. At 48 hpf, we observed a decrease in the GFP expression in the optic tectum of the midbrain in the morphant embryos (Fig. 4D’), whereas rescued embryos showed expression relatively similar to that in control embryos (Fig. 4D”). These results suggest that med13b may be involved in early events of neural differentiation in the zebrafish embryo.
Knockdown of MED13L in hESC-Derived Neural Progenitors Did Not Affect Neuronal Differentiation
We further attempted to validate defects observed in zebrafish morphants in human cells. To this end, we used H1-hESCs-derived neural progenitor cells (H1-NPCs), which were differentiated into NPCs using an established protocol. Knockdown of MED13L in these cells by using two short hairpins RNA targeting MED13L resulted in 50%–60% suppression of MED13L mRNA after 2 days of treatment with G418 antibiotic selection, relative to the shRNA targeting scrambled sequence as a control (Supp. Fig. S5). The protein expression of nestin, SOX2, and vimentin, markers of NPCs, were not remarkably different between shScrambled and shMED13L1/2 cells shown by immunocytochemistry, indicating that MED13L knockdown did not alter the cell fate, as they still retain NPC characteristics (Supp. Fig. S6). NPCs were able to self-renew and proliferate until a limited number of times before they became postmitotic. The proliferative capacity of shMED13L1/2 NPCs was slightly increased by 5%–8% compared with shScrambled NPCs based on quantification of 5-ethynyl-2′-deoxyuridine (EdU)-incorporated cells and Ki67-positive cells (Supp. Fig. S7). The NPCs were maintained at low passage and differentiated into a mixed neuronal population, including glutamatergic and GABAergic neurons according to the established protocol [Brennand et al., 2011]. At 4 weeks, shMED13L neurons showed comparably similar expression of early differentiating neuron marker, TUJ1, and mature neuron marker, MAP2, with shScrambled cells, suggesting no significant differences of differentiated neurons in cultures upon MED13L knockdown in contrast to findings in zebrafish morphant (Supp. Fig. S8). This might be explained by a lack of information to determine specific brain regions in the in vitro model.
Transcriptome Profile of MED13L-Deficient Neurons Revealed Dynamic Expression Changes of Cranial NCCs Genes
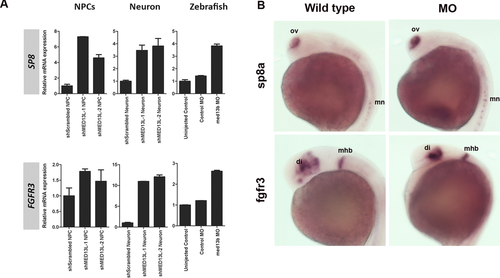
To explore the gene expression changes upon MED13L knockdown, we conducted exploratory microarray experiments comparing shMED13L1/2 to shScrambled neurons at week 4, as this time point is a critical transition period between neural progenitors to become postmitotic neurons. We identified 1,117 genes showing significant expression changes in shMED13L neurons (Supp. Fig. S9). These deregulated gene list was submitted to DAVID annotation, and significant (P < 0.05) gene ontology terms were enriched in the developmental processes, which corroborated the functional role of MED13L in regulating other genes during development (Supp. Fig. S9). The top two genes being upregulated were: SP8, a zinc finger transcription factor gene known to be crucially involved in craniofacial development [Kasberg et al., 2013]; and FGFR3, fibroblast growth factor (FGF) receptor 3 gene, a repressor of bone growth that were found nearly 10-fold upregulated in shMED13L neurons. Both were confirmed to be upregulated in shMED13L NPCs and med13b morphant embryos by qRT-PCR (Fig. 5A). Furthermore, higher expressions of sp8a at olfactory vesicle and motor neurons, and fgfr3 at diencephalon and midbrain-hindbrain boundary were demonstrated in med13b MO embryos compared with wild type by in situ hybridization (Fig. 5B). Four other genes, which zebrafish orthologues are known to be expressed in NCCs development according to ZFIN database, such as pharyngeal arches (Calcr), lateral line (Rspo3), and pectoral fin (Hoxa5 and Atp1a2), were significantly downregulated (Log2 FC = −3.83 to −2.39, P < 0.05), suggesting that MED13L suppression also affects the regulation of genes that are important for the development of NCCs derivatives (Supp. Fig. S10). We next performed pathway-enrichment analysis using PANTHER Web tools in the 1,117 genes and revealed 16 components within canonical Wnt pathway being significantly differentially expressed in shMED13L1/2 neurons (Supp. Fig. S10). We validated eight of the genes that were selected based on their known counterpart as the main component (ligands, antagonist, receptors, and downstream target gene) of canonical Wnt pathway, by qRT-PCR and confirmed microarray-predicted changes in the genes tested (Supp. Table S1), for neurons and NPCs, as well as med13b morphant embryos, supporting the notion that Wnt signaling is deregulated in cells lacking MED13L.
Discussion
Our work provides a mechanistic insight into the functional consequences of MED13L disruption in human patients. Here, we functionally demonstrated that depletion of its orthologue in zebrafish model could partly phenocopy the craniofacial abnormalities in human and the observed phenotype can be reversed by introducing intact human mRNA, suggesting that gene dosage is required for normal gene function. Previous reported cases with shorter length or presumably truncated MED13L protein displayed more severe phenotypes [Muncke et al., 2003; Asadollahi et al., 2013; van Haelst et al., 2014], compared with patients carrying single amino acid substitutions associated with either isolated dTGA or ID, indicating a distinct molecular mechanism between single-point mutations and haploinsufficiency of MED13L [Muncke et al., 2003; Najmabadi et al., 2011]. Patients with missense mutations have either isolated heart defects or isolated ID, whereas patients with MED13L haploinsufficiency displayed moderate ID and craniofacial anomalies, with a reduced penetrance of cardiac defects. Our in vivo model demonstrated that early migration defects of cranial NCCs toward the branchyal arches contributed to the craniofacial defect seen in 5 dpf morphants, suggesting that med13b is required for early craniofacial development. This could partly be explained by the enrichment of cranial NCCs genes including components of Wnt and FGFs signaling pathways in the transcriptome profile of MED13L-deficient neuronal cells. FGF signaling is known to play a role in promoting the fate of NCCs, and required for migration and patterning of the cranial NCCs into the pharyngeal arches. Fgf8 is secreted by the anterior neural ridge (ANR), and is required for cranial NCCs proliferation, as well as the development of forebrain and midbrain structures. Loss of FGF signaling leads to a failure of pharyngeal arch cartilage development [Sarkar et al., 2001; David et al., 2002]. A Recent study has shown that SP8, one of the top upregulated genes in our transcriptome data, promotes Fgf8 expression in the ANR and olfactory pit signaling centers that regulate survival, proliferation, and differentiation of the NCCs to form facial skeleton and the anterior brain [Kasberg et al., 2013]. Studies in frogs and mice have shown that the induction of NCCs is dependent on the activation of Fgf and Wnt pathways in the paraxial mesoderm [Kengaku and Okamoto, 1993; Mayor et al., 1995; Hong et al., 2008]. Wnt signaling induces the protrusion of the frontonasal and maxillary prominences, which eventually form the facial skeleton [Helms et al., 2005; Tapadia et al., 2005; Brugmann et al., 2006]. Conditional knockout mice of β-catenin, the key nuclear effector in the canonical Wnt signaling, resulted in brain malformation and failure of craniofacial development [Brault et al., 2001]. Interestingly, other subunit in the CDK8 module of Mediator complex, MED12, has been shown to interact physically with β-catenin [Kim et al., 2006] and recent genetic studies in Drosophila and C. elegans have revealed that mutations in other Mediator subunits MED1, MED6, MED12, MED13, and MED23 variously affect developmental processes regulated by Wnt signaling [Zhang and Emmons, 2000; Treisman, 2001; Zhang and Emmons, 2001; Janody et al., 2003; Yoda et al., 2005]. Our work further extends the involvement of MED13L as part of Mediator complex, showing that upon knockdown significantly deregulate genes within Wnt signaling pathway.
There are seven additional patients with ID and congenital anomalies documented by DECIPHER [Firth et al., 2009], consisted of five deletions and two duplications encompassing MED13L. Complete description of additional patients with MED13L disruption becomes clinically relevant to extend the definition of MED13L haploinsufficiency syndrome, as initially postulated by Asadollahi et al. (2013). This could be explored further by performing additional mutation screening within MED13L for patients that were tested negative for DiGeorge syndrome, or other neurocristopathies. Considering the coexistence of ID in patients with MED13L mutations and our functional validation showing abnormal regulation of neurogenesis in the developing brain of med13b-depleted zebrafish embryo, we further recommend adding MED13L to the ID-linked screening panel for clinical genetic testing of ID patients.
In summary, we have presented a mechanistic correlation in patients sharing a single gene disruption and provided an in vivo model of MED13L loss-of-function that strongly suggests an implication of defective NCCs migration during craniofacial development mimicking the human patients. Taken together, our study effectively demonstrates that haploinsufficiency of MED13L is attributable to the ID and craniofacial phenotype in our patient.
Acknowledgments
We thank the patient and family members for their participation. This study makes use of data generated by the DECIPHER Consortium. A full list of centers who contributed to the generation of the data is available at http://decipher.sanger.ac.uk and via email from [email protected]. We thank Teddy Young and David Greenald for providing reagents, and Cathleen Teh, Hongyuan Shen, William Goh for the helpful advice in zebrafish transgenic lines and protocols.




