A Novel SHOC2 Variant in Rasopathy
Contract grants sponsors: National Cancer Institute (R00CA126161); National Institute of General Medical Sciences (P20GM103486); NIH Common Fund, through the Office of Strategic Coordination/Office of the NIH Director (1U01HG007674).
Communicated by Reed Pyeritz
ABSTRACT
Rasopathies are a group of genetic disorders caused by germline mutations in multiple genes of the Extracellular signal-Regulated Kinases 1 and 2 (ERK1/2) pathway. The only previously identified missense mutation in SHOC2, a scaffold protein of the ERK1/2 pathway, led to Noonan-like syndrome with loose anagen hair. Here, we report a novel mutation in SHOC2(c.519G>A; p.M173I) that leads to a Rasopathy with clinical features partially overlapping those occurring in Noonan and cardiofaciocutaneous syndromes. Studies to clarify the significance of this SHOC2 variant revealed that the mutant protein has impaired capacity to interact with protein phosphatase 1c (PP1c), leading to insufficient activation of RAF-1 kinase. This SHOC2 variant thus is unable to fully rescue ERK1/2 activity in cells depleted of endogenous SHOC2. We conclude that SHOC2 mutations can cause a spectrum of Rasopathy phenotypes in heterozygous individuals. Importantly, our work suggests that individuals with mild Rasopathy symptoms may be underdiagnosed.
Rasopathies are phenotypically similar syndromes with overlapping features. These autosomal dominant disorders typically present with distinctive craniofacial features, a wide spectrum of congenital heart defects, short stature, and variable neurocognitive impairments [Rauen, 2013]. Rasopathies are caused by mutations that deregulate the RAS-mediated Extracellular signal-Regulated Kinases 1 and 2 (ERK1/2) signal transduction pathway [Allanson and Roberts, 2011; Tartaglia et al., 2011].
Noonan syndrome (NS; MIM #163950) is the most common Rasopathy, affecting between one in 1,000 and one in 2,500 children worldwide. Individuals with NS typically have distinct craniofacial features that may include wide-spaced, and down-slanting palpebral fissures with ptosis and epicanthal folds, a short nose with a depressed nasal bridge and anteverted nares, low-set ears with prominent helices, which may be posteriorly rotated, and a high arched palate. Cardiac defects occur in 50%–80% of individuals with pulmonic stenosis and hypertrophic cardiomyopathy (HCM) being the most common. Other findings include short stature, intellectual disability in up to 30% of patients, ophthalmologic, renal and musculoskeletal defects, lymphatic dysfunction, bleeding diathesis, cryptorchidism, and predisposition to leukemia [Allanson et al., 2010]. Mutations associated with NS are found in nonreceptor protein tyrosine phosphatase PTPN11 (50%); the Ras guanine nucleotide exchange factor SOS1 (13%); small GTPases KRAS, NRAS, and RIT1; and the serine/threonine kinase RAF1 (3%–17%). Additionally, the molecular cause for the disorder is not known for ∼20% of patients with NS [Allanson and Roberts, 2011].
Cardiofaciocutaneous syndrome (CFC; MIM #115150) is a rare Rasopathy with craniofacial features that are similar to but often coarser than those in NS [Rauen, 2012]. Cardiac findings are similar to those in NS and include cardiac arrhythmias, heart defects, and HCM in frequency similar to those of NS and Costello syndrome [Rauen, 2006; Yoon et al., 2007; Siegel et al., 2011]. Most individuals have short stature, a broad forehead, macrocephaly, bitemporal narrowing, shallow orbital ridges, cutaneous abnormalities (such as sparse hair with abnormal texture, dystrophic nails, ichthyosis, hyperpigmentation, and hyperkeratosis), and cognitive delay [Yoon et al., 2007; Allanson et al., 2011]. Brain anomalies, such as crowding of the posterior fossa or Chiari I malformations, are not uncommon. At least four genes are associated with CFC: BRAF (∼75%–80%), MAP2K1 and MAP2K2 (∼10%–15%), and KRAS (<5%) [Allanson et al., 2011; Rauen, 2012; Rauen, 2013].
SHOC2 (MIM #602775) is a scaffold protein in the ERK1/2 pathway, which tethers RAS, RAF-1, and the catalytic subunit of protein phosphatase 1c (PP1c). Recruitment of PP1c enables dephosphorylation of the S259 residue of RAF-1 resulting in the stimulation of RAF-1 kinase activity by phosphorylation of RAF-1 at Ser338 [Rodriguez-Viciana et al., 2006b]. The only previously reported pathogenic SHOC2 variant (c.4A>G; p.S2G) is associated with Noonan-like syndrome with loose anagen hair (NS/LAH). Most reported individuals with NS/LAH have a prominent forehead, macrocephaly, growth hormone deficiency, sparse, loose and slow-growing anagen hair, hyperpigmented skin with eczema or ichthyosis, mild psychomotor delays, hypernasal voices, and attention deficit hyperactivity disorder that improves with age [Mazzanti et al., 2003; Cordeddu et al., 2009]. Cardiac defects appear to include an over-representation of mitral valve dysplasia and septal defects in comparison with classic NS. Other reports have expanded the clinical phenotype to include structural brain anomalies and myelofibrosis [Gripp et al., 2013]. The p.Ser2Gly mutation creates a new recognition site for N-terminal myristoylation, causing aberrant targeting of SHOC2 protein to the plasma membrane and impaired translocation to the nucleus upon stimulation with growth factor.
We report here the identification of a novel SHOC2 functional variant (c.519G>A; p.M173I) in two individuals who do not have classic symptoms of NS, CFC or NS/LAH, but display features overlapping all three conditions. In this study, we have examined the EGF signal transduced through the SHOC2 p.M173I variant in order to demonstrate that it alters the ability of SHOC2 to accelerate ERK1/2 phosphorylation. We provide evidence that the M173I mutation leads to changes in the assembly of the SHOC2–RAS–RAF-1–PP1c complex.
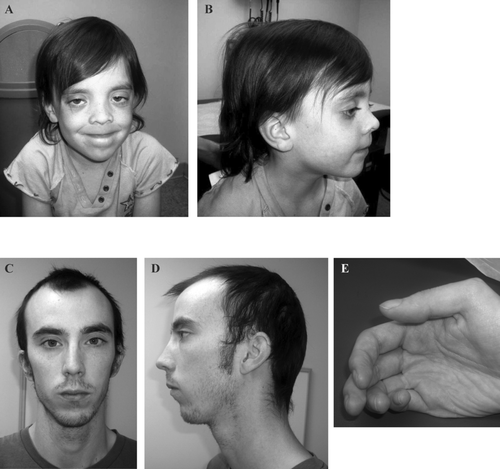
The first patient is a 5-year-old female referred for evaluation of right optic nerve hypoplasia, bilateral nystagmus, speech delay, poor hair growth, and hyperactive behavior. In addition, magnetic resonance imaging showed multiple irregularities of unknown significance in the midbrain, pons, and medulla. She is the oldest child of nonconsanguineous parents of European ancestry. She is the 6 pound 9 ounce product of a 34 weeks gestation, who was delivered by emergency C-section due to cephalopelvic disproportion. Milestones, including walking at 11–12 months of age and speech onset, were normal. The family history was significant, as both parents required special education classes in high school. She had average stature (47th percentile), macrocephaly (98th percentile), sparse and slow-growing hair, which was not loose, normal skin pigmentation, bilateral ptosis and nystagmus, normal chest with wide-spaced nipples, and clubbed nails (Fig. 1A and B). A Simulconsult database query (http://www.simulconsult.com/run/index.html), using the keywords ptosis, macrocephaly, optic nerve hypoplasia, and poor hair growth, suggested CFC as a possible diagnosis. A chromosome microarray was negative for copy number variants. DNA sequencing of a Rasopathy gene panel including BRAF, CBL, HRAS, KRAS, MAP2K1, MAP2K2, NRAS, PTPN11, RAF1 SHOC2, and SOS1 identified a novel heterozygous SHOC2 variant c.519G>A; p.M173I. Nucleotide numbering reflects cDNA numbering with +1 corresponding to the A of ATG translation initiation codon in the reference sequence for human SHOC2 (GenBank: NM_007373.3), according to journal guidelines (http://www.hgvs.org/mutnomen/). The initiation codon is codon 1. This variant was not found in 6,500 asymptomatic individuals. Both parents were tested, and our proband's father had a heterozygous copy of the p.M173I variant. He was 6’3” tall, had intellectual disability, a high-arched palate, sparse hair, and clubbed nails (Fig. 1C–E). Echocardiograms and renal ultrasounds on both patients were negative (Supp. Table S1 provides a comparison of our patients with classic findings in NS, CFC, and NS/LAH). This novel c.519G>A variant has been submitted to the NCBI ClinVar database (http://www.ncbi.nlm.nih.gov/clinvar/) (SCV000147871.1).
To evaluate the functional consequences of the SHOC2 p.M173I variant, we performed a series of experiments in cells lacking endogenous SHOC2 protein (see Supp. Materials and Methods). Cos1 cells with constitutive knock-down of SHOC2 (Cos1-LV1) described in our earlier studies were utilized [Galperin et al., 2012]. In these cells, phosphorylation of MEK1/2 and ERK1/2 is severely impaired upon stimulation with physiological amounts of EGF. The single-base substitution (c.519G>A) identified in both patients was introduced into SHOC2 in the tagRFP-N1 vector. In addition to p.M173I, six “silent” variants were also introduced in the SHOC2 cDNA to render it resistant to shRNA without changing its amino acid sequence [Galperin et al., 2012]. Cells were transfected with wild-type (WT) SHOC2-tRFP and the SHOC2 (M173I)-tRFP, and then stimulated with a low (physiological) concentration of EGF (0.2 ng/ml). We observed that while the constitutive depletion of SHOC2 led to a dramatic decrease in the extent of phosphorylation of MEK1/2 and ERK1/2 upon EGFR activation (Fig. 2A, lanes 4–6), transient expression of the WT SHOC2-tRFP rescued EGF-induced ERK1/2 phosphorylation to the extent of the endogenous SHOC2 (Fig. 2A, lanes 10–12). As expected, the maximum increase in ERK1/2 phosphorylation was observed 7 min after stimulation of cells with EGF. Conversely, the SHOC2 (M173I)-tRFP mutant expressed to a similar level as WT SHOC2-tRFP was only able to restore ∼10% above the basal level of EGF-induced ERK1/2 activity (Fig. 2A, lanes 7–9 and Fig. 2B). Overexpression of the SHOC2 (M173I)-tRFP in parental Cos1 cells did not lead to changes in ERK1/2 activity (Supp. Fig. S1A and B). We also did not observed changes in AKT phosphorylation. These findings indicated that the M173I mutation altered the function of the SHOC2 protein.
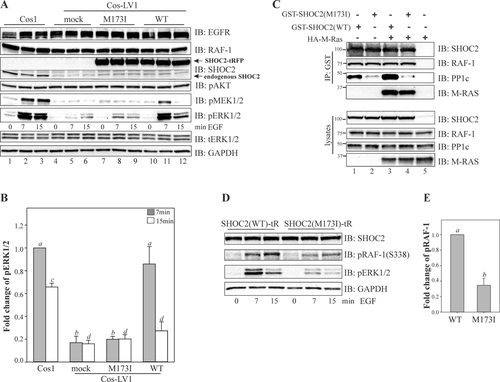
The Met173 amino acid residue of SHOC2 is located in the fourth leucine-rich repeat (LRR4) of the SHOC2 LRR domain and is conserved among all SHOC2 vertebrate orthologues (Supp. Fig. S2A). We speculated that the disparity in the capacity of the SHOC2-M173I mutant to enhance ERK1/2 signaling was due to alterations in protein stability. To assess the physical effect of p.M173I, we analyzed a molecular model of the full LRR domain of SHOC2 [Jeoung et al., 2013]. From both the model and the alternating hydrophilic/hydrophobic pattern found in a beta-sheet, Met173 is predicted to be a solvent-facing residue located on the concave surface of the SHOC2 LRR domain. The M173I mutation can be readily accommodated without any perturbation to surrounding residues suggesting that the point mutation does not destabilize the fold of SHOC2 (Supp. Fig. S2B). To experimentally test the stability of the SHOC2 mutant in comparison with that of WT SHOC2, we measured the half-life of WT and the SHOC2 (p.M173I) mutant in Cos-LV1 cells. To accomplish this, Cos-LV1 cells were transiently transfected with WT SHOC2-tRFP or the SHOC2 (M173I)-tRFP mutant. We found that the M173I substitution in SHOC2 did not affect the half-life of this long-lived protein (∼12 h) (Supp. Fig. S2C), suggesting that protein stability was not affected by the M173I variant.
To examine whether the reduced ability of SHOC2-M173I to enhance activity of the ERK1/2 pathway is due to altered subcellular localization of SHOC2, we investigated the subcellular localization of SHOC2-M173I. Fluorescence microscopy analysis showed that SHOC2-tRFP and SHOC2 (M173I)-tRFP were distributed in both the cytosol and nucleus (Supp. Fig. S2D). We next compared the cytosolic/nuclear distribution of the SHOC2-M173I mutant to WT SHOC2 by subcellular fractionation of Cos-LV1 cells transiently expressing either WT SHOC2-tRFP or SHOC2 (M173I)-tRFP proteins (Supp. Fig. S2E). Our results suggest that the subcellular distribution of SHOC2 (M173I) variant was unaltered.
Next, we investigated whether the M173I mutation altered the capacity of SHOC2 to tether RAS and RAF-1 proteins to the scaffold complex. We expressed WT and the SHOC2 (p.M173I) variant in 293FT cells and tested their ability to interact with HA-M-RAS and RAF-1. The capacity of SHOC2-M173I to form a complex with M-RAS and RAF-1 was comparable to that of WT SHOC2 (Fig. 2C), showing that the p.M173I substitution does not affect SHOC2–RAS–RAF-1 complex assembly. These observations were not entirely surprising as we have previously determined that an N-terminal domain of SHOC2 mediates binding of RAS [Jeoung et al., 2013].
Since SHOC2 has been shown to function as a regulatory subunit of PP1c resulting in activation of RAF-1 kinase [Rodriguez-Viciana et al., 2006b], we hypothesized that the M173I mutation might affect the SHOC2 interaction with PP1c. Although SHOC2 does not contain a canonical PP1c binding motif [R/K]-x(0,1)-[V/I]-x-F, the sequences in LRR4 of SHOC2 contained a hydrophilic motif 169KKLR172 that is found in several PP1c-binding proteins [Bennett et al., 2006]. To determine whether p.M173I is part of a PP1c-binding motif and whether mutation affects SHOC2-PP1c binding affinity, we transfected 293FT cells with the WT or the M173I mutant of GST-SHOC2 and tested for their interaction with PP1c using immunoprecipitation. Results in Figure 2C (lanes 2 and 4) show that the GST-tagged M173I mutant of SHOC2 failed to precipitate endogenous PP1c effectively, suggesting that the M173I substitution alters the interaction of SHOC2 and PP1c. The function of PP1c in the SHOC2–RAS–RAF-1 complex is associated with activation of RAF1 kinase activity [Rodriguez-Viciana et al., 2006b]. Thus, we assessed whether phosphorylation of RAF-1 at Ser 338, a step that is required for efficient RAF-1 activation, and levels of phospho-ERK were altered concomitantly. In cells expressing WT SHOC2-tRFP, stimulation with EGF led to a dramatic increase in RAF-1-(S338) phosphorylation (Fig. 2D and E). Conversely, in cells expressing SHOC2 (M173I)-tRFP, only a mild increase in RAF-1-(S338) phosphorylation was observed, supporting the hypothesis that the M173I substitution in SHOC2 altered the interaction of SHOC2 with PP1c thus contributing to decreased RAF-1/ERK1/2 activity.
The c.519G>A; p.M173I substitution reported here is only the second SHOC2 mutation reported in patients with Rasopathy symptoms. Phenotypic features of patients with the M173I mutation are significantly different from those reported for patients with the c.4A>G; p.S2G SHOC2 mutation. While NS/LAH patients usually present with significant short stature, intellectual disability, cardiac anomalies, and multiple cutaneous findings [Cordeddu et al., 2009; Gripp et al., 2013], our proband has craniofacial features suggestive of NS and sparse slow-growing hair that is more characteristic of CFC. She does not have short stature, a cardiac anomaly, hyperpigmentation, ichthyosis, or loose anagen hair. Her developmental delays are relatively mild compared with many patients with CFC or NS/LAH, especially considering that both of her parents have mild intellectual disability. Her father has tall stature and very subtle clinical features that do not clearly denote a Rasopathy, other than his sparse hair and clubbed nails. We thus conclude that SHOC2 mutations can cause a spectrum of Rasopathy phenotypes of varying severity. This is not surprising, given that variable phenotypes have been reported for most other Rasopathy-associated genes. A recent study by Justino et al. (2014), re-emphasized the clinical heterogeneity of all neuro-CFC syndromes, the absence of consensus on distinct diagnostic criteria and the necessity of multigene panels for molecular diagnosis. Clinical heterogeneity associated with the SHOC2 c.4A>G; p.S2G variant has also been reported [Cordeddu et al., 2009; Hoban et al., 2012; Justino et al., 2014].
The p.M173I SHOC2 substitution causes loss of function in the ERK1/2 pathway. While the majority of mutations associated with Rasopathies are gain-of-function mutations, loss-of-function mutations in PTPN11 have been reported to cause Leopard syndrome, a Rasopathy with the added feature of lentigenes [Kontaridis et al., 2006]. CFC can be caused by both loss- and gain-of-function mutations in B-RAF. [Rodriguez-Viciana et al., 2006a]. This implies that the deregulation of the ERK1/2 pathway rather than upregulation is the critical factor in the causation of Rasopathies.
In summary, our results agree with a substantial set of data suggesting that deregulation of the ERK1/2 pathway has a significant effect on development. Our report of patients with mild Rasopathy symptoms caused by a novel SHOC2 mutation should improve the diagnostic accuracy of patients with findings in the Rasopathy spectrum and provide new evidence for understanding the pathogenesis of these disorders. Our data show that p.M173I is a functional variant by demonstrating that it causes an alteration in ERK1/2 activity and that reduced binding of SHOC2 to the catalytic subunit of PP1c is the cause of the ERK1/2 pathway dysregulation. A recent study by Young et al. (2013) proposed two PP1c binding motifs that are closely localized on the exposed convex side of SHOC2, SLVK, and KIPF [Young et al., 2013]. An attractive hypothesis for our observations is that these motifs are alternatively utilized for efficient PP1c recruitment to the SHOC2 scaffolding complex. Future studies will be needed to confirm this conclusion, to decipher the physiological role of the p.M173I SHOC2 variant in development, and to generate a comprehensive model of SHOC2 function.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Drs. Matthew S. Gentry, Rebecca Dutch, Charles Waechter, Louis Hersh, and Stacy Smith for critical reading of the manuscript, Dr. Craig Vander Kooi for help with SHOC2 modeling, the Viral Production Core at the Department of Molecular and Cellular Biochemistry (University of Kentucky) for assistance with production of lentiviruses.
Disclosure statement: The authors declare no conflict of interest.




