Functional and Clinical Impact of Novel Tmprss6 Variants in Iron-Refractory Iron-Deficiency Anemia Patients and Genotype–Phenotype Studies
Contract grant sponsors: Fundación Ramon Areces (grant CIVP16A 1857 “Ayudas a proyectos de Investigación en Ciéncias de la Vida”); Ministry of Economy and Competitivity (MINECO; grant SAF2012-40106); Telethon Grant GGP12025 and Ricerca Finalizzata Ministero Sanità Rome RF-2010-2312048; Telethon Grant GGP09044 and PRIN-MIUR20128PNX83; European Rare Disease Project ERARE-115 HMA-IRON (2009); Spanish Ministry of Science and Innovation (RYC-2008-02352).
Communicated by David S. Rosenblatt
ABSTRACT
Iron-refractory iron-deficiency anemia (IRIDA) is a rare autosomal-recessive disorder characterized by hypochromic microcytic anemia, low transferrin saturation, and inappropriate high levels of the iron hormone hepcidin. The disease is caused by variants in the transmembrane protease serine 6 (TMPRSS6) gene that encodes the type II serine protease matriptase-2, a negative regulator of hepcidin transcription. Sequencing analysis of the TMPRSS6 gene in 21 new IRIDA patients from 16 families with different ethnic origin reveal 17 novel mutations, including the most frequent mutation in Southern Italy (p.W590R). Eight missense mutations were analyzed in vitro. All but the p.T287N variant impair matriptase-2 autoproteotylic activation, decrease the ability to cleave membrane HJV and inhibit the HJV-dependent hepcidin activation. Genotype–phenotype studies in IRIDA patients have been so far limited due to the relatively low number of described patients. Our genotype–phenotype correlation analysis demonstrates that patients carrying two nonsense mutations present a more severe anemia and microcytosis and higher hepcidin levels than the other patients. We confirm that TMPRSS6 mutations are spread along the gene and that mechanistically they fully or partially abrogate hepcidin inhibition. Genotyping IRIDA patients help in predicting IRIDA severity and may be useful for predicting response to iron treatment.
Introduction
Iron-refractory iron-deficiency anemia or IRIDA (MIM #206200; ORPHA209981) is an inherited-recessive anemia unresponsive to oral iron treatment but with a slow persistent response to intravenous iron injections [Finberg, 2009; De Falco et al., 2013]. Clinically, IRIDA is characterized by microcytic hypochromic anemia (low hemoglobin, low mean cell volume, and low mean corpuscular hemoglobin concentration) with low serum iron, low transferrin saturation, and normal/high ferritin levels. As an important hallmark, opposite to acquired anemias, serum hepcidin is inappropriately normal/high for the low iron status and accounts for the absent/delayed response to treatment [De Falco et al., 2013]. The degree of anemia is variable, although mostly mild, and more pronounced during childhood [De Falco et al., 2013].
IRIDA is caused by mutations in the liver-expressed gene TMPRSS6 (MIM #609862), which encodes a membrane-bound serine protease, matriptase-2 (MT-2) [Finberg et al., 2008; Folgueras et al., 2008; Du et al., 2008]. This serine protease plays an essential role in downregulating hepcidin, the key regulator of iron homeostasis [Muckenthaler, 2008; Silvestri et al., 2008]. IRIDA patients were reported in 2008 by Finberg and collaborators [Finberg et al., 2008]. Up to now, 35 IRIDA families with 53 patients of different ethnic origin have been reported representing a total of 41 different mutations in the TMPRSS6 gene [De Falco et al., 2013; Jaspers et al., 2013; Khuong-Quang et al., 2013].
Here, we describe 21 IRIDA patients belonging to 16 unrelated families, identify 17 novel TMPRSS6 mutations, and perform functional studies. Experimentally, we demonstrate that most of the missense MT-2 pathogenic variants are unable to autoactivate, unable to cleave membrane HJV, and partially unable to fully repress the HJV-dependent hepcidin activation.
Our results further add heterogeneity to the molecular genetics of IRIDA and extend previous findings. Moreover, and for the first time, we are able to perform a genotype–phenotype correlation analysis previously limited by the small number of cases reported in the literature.
Materials and Methods
Patients
Four laboratories offering diagnostic services for IRIDA analyzed the TMPRSS6 genomic sequence in 21 patients from 16 families. Five families were studied in Barcelona (IMPPC), seven in Naples (CEINGE, Biotecnologie Avanzate, s.c.a.r.l., Naples, Italy), and four in Paris (Genetic Department, Hospital Bichat, Paris, France). None of the patients included in this study have been previously reported.
The pedigrees of the families are detailed in Supp. Figure S1, their ethnic origin, clinical, genetic, and laboratory data are shown in Table 1.
| Pediatric Normal values normal ranges | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hgb (g/dl) | MCVb (fl) | Ferritinb (ng/ml) | Transf. sat.b (%) | Serum ironb (μg/dl) | Serum hepcidin (ng/ml) | ||||||
| Family, case number | TMPRSS6 variantsa | Sex | Origin | Age at diagnosis | (Age 1 m–12 y) 9.5–14.8c | (Age 1 m–8 y) 70–96c | (Age 0.5–15 y) 7–142d | (Age 0–11 y) 15%–39%e | (Age 6 weeks–14 y) 20–151f | (Age 5 m–17y) mean value 16.71 ± 14.74g | Treatment |
| A.II.1 | c.[860C>A];[860C>A] p.[T287N];[T287N] | M | Venezuela | 7y | 10.5 | 67.7 | 132 | 12 | 36 | 34.05 | Oral iron+ none |
| A.II.2 | c.[860C>A];[860C>A]p.[T287N];[T287N] | F | Venezuela | 1y | 10.3 | 61 | 367 | 6 | 26 | 43.05 | Oral iron |
| B.IV.1 | c.[2253_2254insT];[2253_2254insT]p.[(K752*)];[(K752*)] | M | Spain | 14m | 6.8 | 50 | 170 | 5 | 13 | 68.04 | Oral and IV iron |
| B.IV.2 | c.[2253_2254insT];[2253_2254insT]p.[(K752*)];[(K752*)] | M | Spain | 1y | 8.7 | 56.8 | 90 | 5 | 10 | 80.02 | Oral and IV iron |
| C | c.[860C>A];[860C>A]p.[T287N];[T287N] | M | Colombia | 4y | 9.7 | 69 | 30 | 6 | 15 | 40.76 | Oral and IV iron |
| D.II.1 | c.[76_80delGGTGA];[1817T>G]p.[(G26Wfs*14)];[L606R] | M | Spain | 5y | 9.7 | 61.7 | 40 | 4 | 13 | 85.76 | Oral and IV iron |
| E.II.1 | c.[1562A>G];[1562A>G]p.[(D521G)];[(D521G)] | F | Spain | 22m | 8.0 | 55.9 | 21 | 5 | 20 | 114.37 | Oral and IV iron |
| F. II.2 | c.[1213C>T];[1868G>C]p.[(Q405*)];[(S623T)] | M | Italy | 3y | 8.2 | 54 | 70 | 5 | 13 | 157.62 | Oral and IV iron |
| G.II.1 | c.[1714_1724del11];[1714_1724del11]p.[(G572Pfs*12)];[(G572Pfs*12)] | M | Turkey | 1y | 8.5 | 50.6 | 35 | 7 | 20 | 56.47 | Oral and IV iron |
| G.II.2 | c.[1714_1724del11];[1714_1724del11]p.[(G572Pfs*12)];[(G572Pfs*12)] | F | Turkey | 1y | 7.5 | 52.9 | 30 | 6 | 18 | 130.54 | Oral and IV iron |
| G.II.3 | c.[1714_1724del11];[1714_1724del11]p.[(G572Pfs*12)];[(G572Pfs*12)] | F | Turkey | 4m | 8.4 | 63.8 | 365 | 6.3 | 13 | 232.05 | Oral and IV iron |
| H.II.1 | c.[1223+1G>A];[1528T>C]Splicing defect p.[?];[C510R] | M | Turkey | 3.5y | 6.1 | 47.8 | 22.8 | 1 | 6 | 137.6 | Oral iron |
| I.I.1 | c.[1768 T>C];[1768 T>C] p.[W590R];[W590R] |
M | Italy | 42y | 10.2 | 66 | 55 | n.d. | n.d. | 120.25 | None |
| I.II.2 |
c.[1768 T>C];[1768 T>C] p.[W590R];[W590R] |
F | Italy | 3y | 6.1 | 55 | 23 | 2.3 | 7 | 80.25 | Oral and IV iron |
| J.II.1 |
c.[188delT];[1768T>C] p.[(L63Pfs*14)];[W590R] |
F | Italy | 2y | 8.5 | 57 | 76 | 3 | 13 | 29.82 | Oral and IV iron |
| K.II.1 |
c.[1004G>T];[1768T>C] p.[C335F];[W590R] |
F | Italy | 6m | 11.2 | 63.2 | 76 | 1 | 6 | 37.78 | Oral and IV iron |
| L.II.1 |
c.[1789C>T(;)1814C>G] p.[R597W(;)A605G] |
M | France | 3y | 8.2 | 58 | 68 | 4 | 8 | 122.2 | Oral and IV iron |
| M.II.1 |
c.[1468_1468+3dupGGTG(;)1813delG] Splicing defect p.[? (;)A605Pfs*8] |
M | Ireland | 1y | 8.4 | 57 | 98 | 4 | 11 | 132.8 | IV iron |
| N.II.1 |
c.[2101_2108dup];c.[2101_2108dup] p.[(W703Cfs*28)];[(W703Cfs*28)] |
F | Congo | 3m | 7.3 | 51 | 130 | 3 | 6 | 58.3 | IV iron |
| O.II.1 |
c.[741 G>T];[741 G>T] p.[W247C];[W247C] |
M | Belgium | 4.5y | 7.3 | 48.8 | 22 | 5 | 9 | 75.0 | IV iron |
| P.II.2 |
c.[1025C>T];[1768T>C] p.[(S304L)];[W590R] |
M | Italy | 1y | 11.2 | 63.2 | 76 | 2 | 6 | 164.2 | n.a. |
- Biochemical values are at diagnosis time. c., refers to the coding region nucleotide affected with A of the initiation ATG codon as 1; p., refers to the protein amino acid affected with the initiation methionine as 1.
- a Nucleotide numbering uses +1 as the A of the ATG translation initiation codon in the reference sequence (GenBank mRNA: NM_153609.2), with the initiation codon as codon 1 (www.hgvs.org/mutnomen). GenBank protein: NP_705837.2.
- b Reported Hg, MCV, serum ferritin, transferrin saturation, and serum iron values were done at diagnosis time and measured in nontreated patients.
- c Novak (1987).
- d Siimes, et al. (1974).
- e Andropoulos (2012).
- f Selected normal paediatric laboratory values from Pearson Education retrieved from http://wps.prenhall.com/wps/media/objects/354/362846/London%20App.%20B.pdf.
- g Mean and standard deviation for serum hepcidin in control children using the serum hepcidin-25 C-ELISA Kit from Bachem according to Choi et al. (2012).
- Hg, hemoglobin; MCV, mean corpuscular volume; transf. sat., transferrin saturation; y, years; m, months; HGVS, Human Genome Variation Society recommended mutation description; n.d., not determined; n.a., not available.
Written informed consent for genetic analyses was obtained from the probands and relatives of all families according to the guidelines of each institution and the study protocol conforms to the ethical guidelines of the 2002 Helsinki Declaration. The studies were in keeping within the regulations of the country in which the diagnosis was performed and approved by the corresponding clinical research ethics committee.
DNA Sequence Analysis
The conditions of DNA extraction, polymerase chain reaction, and sequence analysis used in the three laboratories were standard [Guillem et al., 2008; De Falco et al., 2010; Luscieti et al., 2013]. All exons, exon–intron boundaries and a varying amount of the 5′ and 3′ flanking sequence of the TMPRSS6 gene were examined using fluorescent chain-terminator cycle sequencing. Newly designed or previously reported primers were employed [Guillem et al., 2008; De Falco et al., 2010] (Supp. Table S1).
Plasmid Construction and Functional Assays
The pcDNA3.1-TMPRSS6-FLAG expressing vector was kindly provided by Prof. Carlos López-Otín (University of Oviedo, Spain). TMPRSS6, lacking the serine protease domain [Du et al., 2008], was described in Silvestri et al. (2008). To introduce pathogenetic variants, TMPRSS6 cDNA was mutagenized by using QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA), according to the manufacturer's protocol, or by standard protocol. Oligonucleotides used for site-directed mutagenesis are described in Supp. Table S1. Mutagenesis changes were verified by conventional Sanger sequencing.
Hepcidin Assay
Hepcidin concentrations in patient's plasma/serum samples were quantified by competition enzyme-linked immunoassay (C-ELISA) using the hepcidin-25 (human) enzyme immunoassay kit (Bachem, Torrance, CA)) or as previously described [Ganz et al., 2008] according to the manufacturer's protocol. Samples and standards were run in duplicate. Plasma samples were diluted one in 10 or one in 50 in supplied standard diluent (peptide-cleared human serum) and analyzed using a 10-point twofold serial dilution (maximum concentration, 25 ng/ml) standard curve. Hepcidin concentrations were interpolated from standard curves generated by a four-parameter logistic nonlinear regression model using Prism (Version 5.0d; GraphPad Software Inc., La Jolla, CA, USA). Appropriate dilutions were used to obtain readings inside the linear region of the curve.
Cell Culture and Reagents
Cell culture and reagents were from Invitrogen, Saint Aubin, France and Sigma–Aldrich, St. Louis, MO, USA. HeLa and Hep3B cells were cultured in DMEM and EMEM, respectively, supplemented with 2 mM l-glutamine, 200 U/ml penicillin, 200 mg/ml streptomycin, 1 mM sodium pyruvate, and 10% heat-inactivated fetal bovine serum at 37°C in 95% humidifier air and 5% CO2.
Luciferase Assay
Luciferase activity was analyzed as described previously [Pagani et al., 2008] with minor modification. Briefly, Hep3B cells, seeded at 70%/80% of confluency in a 48-multiwell plate, were transiently transfected with 250 ng of hepcidin promoter luciferase reporter construct in combination with 15 ng of pRL-TK Renilla luciferase vector (Promega, Madison, WI, USA) to normalize for transfection efficiency, with 50 ng of HJV-expressing vector and with 10 ng of wild-type or mutant MT-2. Eighteen hours after transfection, the medium was replaced with EMEM supplemented with 2% fetal bovine serum. Luciferase activity was determined 24 hr later, according to manufacturer's instructions (Dual Luciferase Reporter Assay; Promega). Relative activity was calculated as the ratio of firefly to renilla luciferase, expressed as a multiple of the activity of the reporter alone.
Western Blot Analysis
HeLa cells, seeded in 100-mm diameter dishes, were transiently transfected with 10 μg HJV and 3 μg of wild-type or mutant TMPRSS6-expressing vectors. When indicated, empty vector was used instead of TMPRSS6-expressing vectors. Eighteen hours after transfection, the medium was replaced with 4 ml of OptiMem and collected 24 hr later. Cells were lyzed in NET/Triton buffer (150 mM NaCl, 5 mM EDTA, and 10 mM Tris [pH 7.4] with 1% Triton X-100). Conditioned cell culture media were collected and concentrated using 3 kDa molecular weight (MW) cut-off ultrafiltration (Amicon Ultra; Millipore, Billerica, MA) columns. Proteins were quantified using the Bio-Rad Protein Assay (Bio-Rad, Bio-Rad Laboratories, Munich, Germany); 50 μg of protein extracts were subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). To analyze the TMPRSS6 serine protease domain, concentrated media were incubated overnight (o/n) at 4°C with 40 μl of anti-FLAG M2 affinity gel (Sigma–Aldrich), washed with NET buffer, resuspended in 25 μl of Laemmli sample buffer and boiled. β-mercaptoethanol (β-ME; 4%, v/v) was then added. Samples were loaded on 10% SDS-PAGE, transferred to Hybond C membrane (Amersham Biosciences, Piscataway, New Jersey, USA) and analyzed by standard Western blotting technique. Blots were blocked with 2% enhanced chemiluminescence Advance Blocking Agent (Amersham Biosciences) in TBS (0.5 M Tris–HGI pH 7.4 and 0.15 M NaCl) containing 0.1% Tween-20 (TBST) and incubated with anti-HJV (1:1,000; [Silvestri et al., 2008]) or anti-FLAG (1:1,000; Sigma–Aldrich).
Bioinformatic Prediction Methods
Prediction of possible impact of amino acid substitution on TMPRSS6 protein was done using the commonly used and previously published software SIFT version 4.0.3 (http://sift.jcvi.org) [Kumar et al., 2009] and PolyPhen-2 version 2.2.2 (http://genetics.bwh.harvard.edu/pph2/) [Adzhubei et al., 2010] using default parameters. Multiple sequence alignment of TMPRSS6 protein (MT-2) in several species was done using Clustal Omega software using default parameters (http://www.ebi.ac.uk/Tools/msa/clustalo/).
Statistics
We included in genotype–phenotype study 59 pediatric (from 1 to 18 years) patients (39 from the literature and 20 new recruited patients). To analyze the genotype–phenotype correlation, we divided the IRIDA patients into two groups: Group A (n = 34), patients with two missense mutations, and patients with one missense mutation in combination with another type of mutation (nonsense, frameshift, or splicing mutation); group B (n = 25), patients with two nonsense mutations, two frameshift mutations, two splicing mutations, one nonsense and one frameshift mutation, and one frameshift and one splicing mutation. Patients with only one reported mutation in the TMPRSS6 gene were excluded from the analysis. Obviously, the comparison was not possible in a particular parameter if the data were not available; therefore, the number of compared patients in each particular studied parameter could be lower than the total number of patients included in each group, the precise n value is shown in Table 2. A Student t-test and Mann–Whitney test were used to compare differences in quantitative variables between the two groups. Qualitative clinical data were compared using the chi-square test. Odds ratios and 95% confidence intervals were calculated to assess the relative risk of a more severe phenotype conferred by a specific genotype. A two-sided P value of less than 0.05 was considered statistically significant.
| Group A | Group B | P A versus B | |
|---|---|---|---|
| Male–female (33:26) | 20:14 | 13:12 | 0.60 |
| Age at diagnosis (years) | 4.20±3.85 | 3.20±4.16 | 0.34 |
| Hemoglobin (g/dl) | 8.68±1.34 | 7.85±1.20 | 0.02 |
| MCV (fl) | 59.46±6.17 | 54.52±5.30 | 0.0018 |
| Transferrin saturation (%) | 4.79±2.21 (30) | 4.34±2.00 | 0.44 |
| Serum iron (μg/dl) | 12.14±8.92 (20) | 12.64±4.70 (11) | 0.90 |
| Serum ferritin (μg/dl) | 96.48±109.47 (28) | 85.89±88.90 (18) | 0.72 |
- a Patients for whom the data were incomplete, were not included; data are reported as mean ± SD.
- Group A includes patients with two missense mutations and one missense plus other mutation. Group B includes patients with two null alleles (i.e., two nonsense mutations, two frameshift mutations, two splicing mutations, one nonsense and one frameshift mutation, and one frameshift and one splicing mutation) (also see Supp. Table S2).
- In case the number of patients compared for each parameter is lower than the total number in the group, the (n) value is indicated in brackets.
Results
We report 16 unrelated families of different ethnicities suffering from the autosomal-recessive disease IRIDA (see pedigrees in Supp. Fig. S1). Clinical and biochemical data of the 21 affected individuals are described in Table 1. All patients present with a severe/moderate microcytic hypochromic anemia (hemoglobin range: 6.1–11.2 g/dl; mean corpuscular volume (MCV) range: 47.8–69 fl) and were diagnosed mostly in infancy (in 20 out of 21 cases). Thalassemia and other hemoglobinopathies were excluded, as well as acquired malabsorption such as celiac disease. As known for IRIDA, patients serum iron and transferrin saturation are characteristically low, whereas ferritin is mostly low or normal (Table 1). Hepcidin levels in all tested patients are inappropriately normal or even high for the degree of anemia; this feature is a key hallmark for IRIDA diagnosis (Table 1). We observed that some patients fully responded to oral iron treatment with none or minimum requirements for intravenous iron therapy. Intravenous iron injections resulted in partial increase of hemoglobin in all treated patients.
Sequencing analysis of TMPRSS6 gene in 21 IRIDA patients from 16 unrelated families revealed 20 mutations, 17 were novel: nine missense (p.W247C, p.T287N, p.C335F, p.C510R, p.D521G, p.W590R, p.R597W, p.A605G, and p.L606R), two nonsense (p.Q405* and p.K752*), four frameshift (p.G26Wfs*14, p.L63Pfs*14, p.G572Pfs*12, and p.W703Cfs*28), and two splicing (c.[1224+1G>A] and c.[1468_1468+3dupGGTG]) (Table 1; Fig. 1). Three already reported mutations (p.A605Pfs*8, p.S623T, and p.S304L) [Finberg et al., 2008; Tchou et al., 2009] were found in compound heterozygous state with novel mutations (patients F.II.2, M.II.1, and P.II.2). The distribution of the mutations in the gene and in the different domains of the protein is shown in Figure 1. None of the novel variants has been previously reported in the examined databases (ENSEMBL: http://www.ensembl.org/, NCBI dbSNP: http://www.ncbi.nlm.nih.gov/SNP/, 1000 Genomes: http://browser.1000genomes.org/). The novel variants have been submitted to the LOVD public database: http://www.lovd.nl/TMPRSS6.
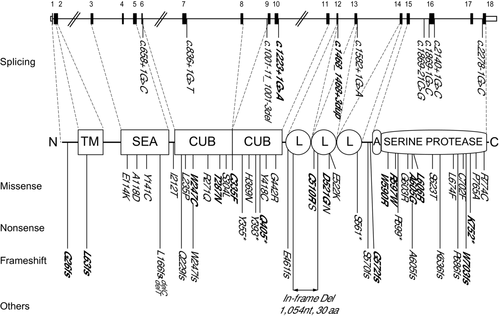
Only four nonsense mutations have been previously reported in TMPRSS6 gene (p.Y355*, p.Y393*, p.S561*, and p.R599* [Finberg et al., 2008; Guillem et al., 2008; De Falco et al., 2010]. Here, we report one patient (patient F.II.2; Table 1) bearing the nonsense mutation p.Q405* at the heterozygous state with a previously reported missense mutation S623T, [Tchou et al., 2009] and one family (Family B) with the nonsense mutation p.K752* at the homozygous state.
Mutations so far reported in the TMPRSS6 gene are all located in the extracellular domain of the protein. In this work, we report two frameshift mutations, one situated in the cytoplasmic domain and the other in transmembrane domain (p.G26Wfs*14 and p.L63Pfs*14, respectively). Therefore, mutations in the TMPRSS6 gene can affect also intracellular and transmembrane regions.
Bioinformatic Prediction
All the nine novel missense substitutions are bioinformatically predicted to be damaging or deleterious according to two commonly used programs (SIFT and PolyPhen-2) [Kumar et al., 2009; Adzhubei et al., 2010]. In addition, a multiple sequence alignment of MT-2 proteins among 24 species shows that all these amino acids are highly conserved through evolution (identical or equivalent amino acid in at least 92% of the sequences; Supp. Fig. S2).
Missense mutations at positions 510 (p.C510S) and 521 (p.D521N) were previously reported in IRIDA patients [Finberg et al., 2008; De Falco et al., 2010]; here, we report two patients (patient H.II.1 and E.II.1; Table 1) with different amino acid substitutions at the same positions (p.C510R and p.D521G). This fact suggests that cysteine 510 and aspartic acid 521 are essential residues that do not tolerate changes. Indeed, cysteines 510 and 335 are predicted to form a disulfide bond with cysteines 525 and 366, respectively (Uniprot Q8IU80). Therefore, mutations p.C510R or p.C510S and p.C335F may impair the structural folding of MT-2.
Frequency of TMPRSS6 Mutations
To date, 41 causative mutations of IRIDA have been described along the entire coding sequence of the TMPRSS6 gene (Fig. 1). The inclusion of the novel mutations reported in this work increases the number of reported causative mutations to 58 (41 previous described and 17 novel from this study). The majority of the IRIDA mutations (37.9%) are located in serine protease domain with nine mutations in exon 15 and seven mutations in exon 16 and surrounding exon–intron boundaries (Fig. 1). The most frequent mutation is the missense variant S304L, located in the first CUB domain, and described in seven patients from five different families.
We observed that W590R mutation is relatively frequent in patients of South Italy origin (present in five patients from four unrelated families, representing 8.6% of all reported IRIDA mutations). Further ongoing investigations will address whether the mutation W590R has a possible founder effect in South Italian population.
Genotype–Phenotype Correlations in IRIDA Patients
IRIDA patients have a variable spectrum of clinical severity with some patients presenting a relatively good partial response even to oral iron, whereas others are depending on intravenous iron infusions especially at young ages [De Falco et al., 2010; Khuong-Quang et al., 2013]. Therefore, we attempted to assess whether genotype–phenotype correlation studies may help the classification of IRIDA patients to predict their clinical severity and response to iron treatment. This assessment was made possible by the discovery of additional 17 new mutations. In this correlation study, we consider a total of 59 pediatric patients (from literature and newly reported patients from this study, see Supp. Table S2) and divided the patients in two groups (A and B) depending on their genetic defect (see Material and Methods for details and Supp. Table S2).
We first evaluated the age at diagnosis (age at first hematologic evaluation) and we found that it is higher in group A (4.20 ± 3.85) than in group B (3.20 ± 4.16); however, this difference is not statistically significant (P = 0.34) (Table 2). Among basal hematologic and iron parameters, hemoglobin levels are significantly lower in group B (n = 25; 7.85 ± 1.20 g/dl) than in group A (n = 34; 8.68 ± 1.34g/dl) (P = 0.02) (Table 2). In addition, MCV is also significantly lower in group B (n = 25; 54.52 ± 5.30 fl) than in group A (n = 34; 59.46 ± 6.17 fl) (P = 0.0018) (Table 2).
In both groups, transferrin saturation and serum iron levels are low, whereas serum ferritin values are mainly in the normal range; those are typical features of IRIDA patients. However, statistically significant differences were not found between group A and B when comparing transferrin saturation, serum ferritin, and serum iron levels (Table 2).
Finally, we also analyzed the hepcidin levels with regards to the genetic alterations. Hepcidin levels in all patients were tested with the same methodology (ELISA). As seen in Figure 2, patients bearing more severe mutations (group B) present statistically significant higher serum hepcidin levels (n = 12; 185.60 ± 131.97) than patients in group A (n = 18; 92.04 ± 51.11) (P = 0.05). These data are in agreement with our above observation regarding group B as the IRIDA patients with more severe anemia (lower hemoglobin and lower MCV).

Functional Characterization of Novel TMPRSS6 Missense Variants
We have experimentally assessed the functional consequences of eight out of nine new missense mutations reported in this work. Mutation D521G was not included because the functional effect of a reported mutation at the same residue, D521N, was previously described [Silvestri et al., 2009]. To characterize the missense mutations p.W247C, p.T287N, p.C335F, p.C510R, p.W590R, p.R597W, p.A605G, and p.L606R, we have studied their effect on the ability to cleave membrane HJV (m-HJV), to undergo autoactivation and to inhibit HJV-dependent hepcidin activation compared with wild-type MT-2 and the MASK variant, a truncated variant lacking the serine protease domain.
Wild-type MT-2 cleaves m-HJV causing the release of HJV fragments in the culture media [Silvestri et al., 2008]. These fragments are undetectable in the media of HeLa cells cotransfected with HJV and MT-2 missense variants under analysis, with the exception of the p.T287N mutant that behaves as the wild-type protein. Fragments are only barely visible in the presence of the p.R597W variant (Fig. 3A).
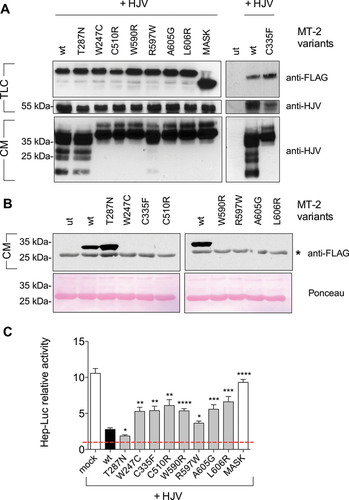
We also observed that MT-2 mutants, with the exception of the p.T287N variant, are unable to release the serine protease domain in the culture media (Fig. 3B), suggesting they are proteolytically inactive. This result demonstrates that the inability of these variants to cleave m-HJV is due to the lack of MT-2 autoactivation.
To study the effect of MT-2 mutants on hepcidin expression, Hep3B cells were transfected with the hepcidin promoter luciferase reporter construct (Hep-Luc). In the presence of HJV, hepcidin is strongly activated, whereas coexpression of HJV and wild-type MT-2 significantly decreases hepcidin [Silvestri et al., 2008] (Fig. 3C). The inactive variant MASK, which lacks the protease domain, fails to downregulate hepcidin in the presence of HJV. Coexpression of HJV and the MT-2 missense mutants under analysis led to a partial hepcidin inhibition (Fig. 3C). The proteolytic activity of MT-2 is partially conserved in the p.R597W mutant, which inhibits hepcidin more efficiently than the other pathogenic mutants, and fully conserved in p.T287N variant, that inhibits hepcidin even more efficiently than the wild-type protein.
Discussion
Up to date, 41 different TMPRSS6 variants have been identified in 51 patients from 33 unrelated families with IRIDA [De Falco, et al., 2013; Jaspers, et al., 2013] including missense, nonsense, frameshifts, and splicing defects. Here, we report 17 additional novel mutations (Fig. 1) and studied their functional consequences. In addition, we attempted a phenotype–genotype correlation in a subset of IRIDA patients.
Previous works showed that IRIDA-causative mutations are spread along all the functional domains of the large extracellular portion of the MT-2 protein [De Falco et al., 2013]. In fact, we here describe for the first time the only so far MT-2 mutations located in the cytoplasmic and transmembrane domain (frameshift mutations G26fs and L63fs; see Fig. 1). Therefore, any domain of MT-2 could be affected in IRIDA patients, which implies that the TMPRSS6 gene should be fully screened to detect IRIDA causal mutations. Interestingly, we describe here a new frequent mutation, p.W590R, present in 50% of the Italian families (four from literature and four from this work). All the patients with the p.W590R mutation originate from South Italy allowing us to hypothesize a founder effect.
In vitro, the activity of MT-2 can be monitored by using several functional assays: the release of HJV fragments in the culture media of HJV-MT2 cotransfected cells to investigate the ability of the protease to cleave its substrate, the release of the serine protease domain to investigate MT-2 autocatalytic cleavage, and the hepcidin promoter assay to analyze the ability of MT-2 to modulate hepcidin [Silvestri et al., 2008, 2009, 2009]. Seven out of the eight studied missense mutations (all but T287N) show impaired autoproteotylic activation, with a consequent decrease ability to cleave membrane HJV and to inhibit the HJV-dependent hepcidin activation. This is a common effect of MT2 pathogenic variants studied so far [Silvestri et al., 2008, 2009].
MT-2 variant appears to be a silent mutation in vitro, similar to the previously reported p.R271Q mutation [De Falco et al., 2010]. Indeed, it behaves as the wild-type protein maintaining autocatalytic proteolysis, cleavage of m-HJV and inhibition of hepcidin functions. It is possible that in vivo the T287N variant may be instable and that this effect is overruled in our in vitro overexpressing system. Interestingly, the three South American patients reported with the p.T287N mutations (patients A.II.1, A.II.2, and C.II.1) present mild anemia (Hb: 9.7–10.5 g/dl) and low values of serum hepcidin (34.1–40.8 ng/ml) in line with the in vitro data, suggesting a partially preserved MT-2 function. However, we cannot formally exclude that this variation represent an uncommon polymorphism in South American population in linkage disequilibrium with a pathogenic variant present in regions unscreened in this study (promoter or deep intronic variants).
For the first time, genotype–phenotype correlation analysis could be attempted in a consistent number of IRIDA patients to demonstrate that patients with severe mutations (group B) have statistically significant lower hemoglobin and MCV values and higher serum hepcidin levels than patients with two missense mutations or one missense mutation in combination with other type of mutations.
Few IRIDA cases have been reported to respond well to oral iron treatment [Khuong-Quang et al., 2013] and unpublished observations. We have attempted to correlate the different genotype of the two groups (A and B) in regards to the response to oral iron treatment. We consider a good response to oral iron an increase in hemoglobin value of at least 1 g/dl and we have analyzed a small subset of patients where data are available (n = 16, eight cases from the literature and eight cases from this work). We found that patients in group A present a better response to oral iron treatment than those in group B (32.3% vs. 20%); however, this difference is not statistically significant (P = 0.36) (ratio of good responders to oral iron therapy is 11 in group A vs. 5 in group B). These data may indicate a better oral iron response in patients bearing less deleterious mutations (i.e., two missense mutations or one missense plus another mutation type). Therefore, we hypothesized that the IRIDA genotype not only determine the severity of the anemia, including the hepcidin levels, but it may also help in predicting the responsiveness to oral iron treatment. This is in agreement with a recent retrospective study, where hepcidin levels were proven useful in identifying iron-deficiency anemic patients who did not respond to oral iron supplementation [Bregman et al., 2013].
Acknowledgments
The authors would like to thank all patients and their family members for their participation in the study. The authors thank Prof. G. Russo (U.O.C. Emato-OncologiaPediatrica, Catania, Italy) and Dr. N. Dodaro (U.O. di Pediatria, Cosenza, Italy) for referring families with W590R mutation. C.B., C.C., A.I., B.G., and M.S. conceived the study. M.S., L.D.F., L.S., C.C., and C.K. wrote the paper. Patient ascertainment and recruitment were carried out by B.A., I.Y., E.Y.-K., U.K., and M.F.-R. Sequencing and genotyping were carried out and interpreted by L.D.F., C.K., E.M., J.A., C.O., and M.S.. L.S. and M.R. performed functional and molecular studies. L.D.F. and M.B. performed genotype–phenotype analyses. All authors read, revised, and approved the final version of the manuscript.
Disclosure statement: No conflict of interest is declared by the authors.




