Deletions in the 3′ Part of the NFIX Gene Including a Recurrent Alu-Mediated Deletion of Exon 6 and 7 Account for Previously Unexplained Cases of Marshall–Smith Syndrome
Communicated by Ravi Savarirayan
ABSTRACT
Marshall–Smith syndrome (MSS) is a very rare malformation syndrome characterized by typical craniofacial anomalies, abnormal osseous maturation, developmental delay, failure to thrive, and respiratory difficulties. Mutations in the nuclear factor 1/X gene (NFIX) were recently identified as the cause of MSS. In our study cohort of 17 patients with a clinical diagnosis of MSS, conventional sequencing of NFIX revealed frameshift and splice-site mutations in 10 individuals. Using multiplex ligation-dependent probe amplification analysis, we identified a recurrent deletion of NFIX exon 6 and 7 in five individuals. We demonstrate this recurrent deletion is the product of a recombination between AluY elements located in intron 5 and 7. Two other patients had smaller deletions affecting exon 6. These findings show that MSS is a genetically homogeneous Mendelian disorder. RT-PCR experiments with newly identified NFIX mutations including the recurrent exon 6 and 7 deletion confirmed previous findings indicating that MSS-associated mutant mRNAs are not cleared by nonsense-mediated mRNA decay. Predicted MSS-associated mutant NFIX proteins consistently have a preserved DNA binding and dimerization domain, whereas they grossly vary in their C-terminal portion. This is in line with the hypothesis that MSS-associated mutations encode dysfunctional proteins that act in a dominant negative manner.
Introduction
Marshall–Smith syndrome (MSS; MIM #602535) is a very rare congenital multisystem disorder that was first described as a distinct syndrome in 1971 [Marshall et al., 1971]. Approximately 50 patients have been published in the literature so far. The major clinical symptoms observed in most patients with MSS include developmental delay, respiratory difficulties, failure to thrive, and skeletal abnormalities [Shaw et al., 2010]. Delay in motor and cognitive development is usually moderate to severe and associated with truncal hypotonia, peripheral hypertonia, and limited or absent speech abilities. A variety of nonspecific brain abnormalities have been described. Upper airway obstruction and aspiration pneumonia represent the predominant respiratory complications in MSS. They mainly account for a previously reported high mortality of the disease, but airway support and other supportive treatment increasingly allows survival into adulthood. A distinct skeletal phenotype is part of the syndrome. It is characterized by strikingly advanced maturation not only of carpal ossification but also of epiphyseal ossification of short and long tubular bones in infancy and childhood. In addition, hand radiographs typically show wide, bullet-shaped middle phalanges. Cranial dysplasia as well as the frequent development of kyphoscoliosis and short stature underscores the generalized nature of skeletal involvement. The radiographic changes and distinctive facial appearance are diagnostic. The latter includes a high forehead, underdeveloped midface with proptosis and anteverted nares, and retrognathia. Full lips and gingival hypertrophy are other frequent findings.
In 2010, Malan et al. reported that distinct heterozygous mutations in NFIX (MIM #164005) are responsible for either MSS or a Sotos-like syndrome (MIM #614753), depending specifically on their impact on nonsense-mediated mRNA decay (NMD) [Malan et al., 2010]. Deletions of the entire gene and a nonsense mutations resulting in NMD lead to NFIX haploinsufficiency, which was found to be associated with a Sotos-like overgrowth syndrome and mild intellectual deficits. In contrast, frameshift and splice-site mutations identified in patients with MSS were shown to escape NMD, suggesting that the more severe phenotype of MSS is due to a dominant-negative effect of mutant NFIX proteins. Disease-causing NFIX mutations represented de novo events, in line with the known sporadic occurrence of MSS.
The Nuclear Factor I (NFI) family comprises four members in vertebrates, NFIA, NFIB, NFIC, and NFIX, which have distinct but overlapping expression patterns. NFI proteins share a conserved N-terminal DNA-binding and dimerization domain and a C-terminal transcriptional activation and/or repression domain (CTF/NFI domain). They bind as either homo- or heterodimers to specific DNA motifs and play important roles in the regulation of gene expression in many tissues [Gronostajski et al., 1985; Gounari et al., 1990; Gronostajski, 2000].
Nine MSS patients carrying NFIX mutations were initially reported [Malan et al., 2010]. Here, we report the results of genetic investigations in a larger cohort of 17 MSS patients and for the first time demonstrate that deletions in the 3′ part of the NFIX gene including a recurrent Alu-mediated deletion of exon 6 and 7 account for about one quarter of all MSS cases.
Materials and Methods
Study Cohort
The study cohort included 17 unrelated individuals affected by MSS. Four patients originally belonged to a cohort previously examined for NFIX point mutations, from which nine other patients with a proven mutation were reported by Malan et al. (2010). These four individuals had tested negative by NFIX sequencing and were included in this study for further genetic analysis. The remaining 13 cases were recruited independently. The clinical data of all affected individuals was reviewed by experienced clinical dysmorphologists (R.H., M.Z.), and the clinical diagnosis was confirmed on the basis of the typical craniofacial appearance, characteristic radiographic features and moderate to severe developmental delay as minimal criteria. All patients had further abnormalities supporting the diagnosis. Patient age ranged from 0.6 to 39 (median 7.5 years). All of them were sporadic cases. There was no instance of parental consanguinity. A summary of clinical symptoms is given in Table 1. Facial features of eight individuals participating in this study are shown in Figure 1, documenting the typical phenotype. All had a remarkably similar facial phenotype, which gradually changes over time, except for patient P2 in whom facial and skeletal signs were less marked. However, he was clinically still diagnosed as having MSS and did meet the inclusion criteria for this study. Five of the patients in our study cohort had been included in a previous clinical review article [Shaw et al., 2010], whereas the remainder represent unpublished cases. DNA from leukocytes was available from all patients. Lymphoblastoid cell lines (LCLs) and fibroblasts were available from four and one affected individual, respectively. In most cases, DNA samples from both parents were available for study. Informed consent was given by the parents or a legal guardian of each patient participating in this study. The study was approved by the local Ethics Boards.
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | P16 | P17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient # Shaw et al. (2010) | − | − | − | 6 | − | − | − | − | − | − | − | − | 15 | 14 | − | 3 | 9 |
| Gender | F | M | M | F | M | M | M | M | M | F | M | M | M | F | F | F | F |
| Age at the last exam (yr) | 6 | 24 | 6 | 7.5 | 39 | 15 | 14 | 7 | 3 | 16 | 1 | 2.8 | 13 | 14 | 3 | 0.5 | 10 |
| Age mother/father (yr) | 34/45 | 22/23 | 34/38 | 24/32 | 26/29 | 28/29 | 35/32 | 19/20 | 30/30 | 24/NA | 25/28 | 29/33 | 35/33 | 33/40 | 24/30 | 18/23 | 32/51 |
| Short stature | + | + | + | + | + | + | + | + | − | + | − | − | + | + | − | − | + |
| Intellectual disability | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | NA | + |
| Brain MRI/CT | BA | N | BA, CCH | CCH | N | N | NA | NA | NA | NA | NA | NA | NA | N | N | CH | N |
| Airway obstruction | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + |
| Abnormal bone maturation | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Scoliosis/kyphosis | + | + | + | + | + | + | + | − | − | + | − | + | + | + | + | − | + |
| Cardiac anomalies | ASD | VES | PDA | − | − | − | − | − | − | − | − | + | − | − | − | − | − |
| Umbilical hernia | − | + | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − |
- M, male; F, female; N, normal; BA, brain atrophy; CCH, corpus callosum hypoplasia; CH, cerebellar hypoplasia; ASD, atrial septal defect; VES, ventricular extrasystolia; PDA, persistent ductus arteriosus; NA, not available/applicable.
Mutation Screening of NFIX
Genomic DNA was isolated from peripheral blood leukocytes according to standard protocols. Specific oligonucleotide primers (Supp. Table S1) were designed with the help of Primer3 software (http://frodo.wi.mit.edu/) for amplification of the 11 coding exons and the flanking intronic regions of the NFIX gene. PCR conditions are available on request. Mutational analyses were performed using BigDye Terminator v1.1 chemistry on an ABI 3500xl DNA analyzer (Applied Biosystems, Foster City, CA). All obtained sequences were compared with the NFIX reference sequence deposited in the public databases (ENST00000592199). Designation of mutations follows the guidelines of the human genome variation society (http://www.hgvs.org/mutnomen/). All given genomic positions refer to GRCh37/hg19.
NFIX MLPA Analysis
We developed a MLPA (multiplex ligation-dependent probe amplification) assay to identify deletions and duplications of one or more NFIX exons. Probes for each exon of the gene were designed according to the recommendations for probe design by MRC Holland (http://www.mrc-holland.com; pdf file protocol synthetic probe design—updated Jan 2012). Probes for exon 2 through 11 are located within the respective exon, and another probe is located ∼660 bp upstream to exon 1 because the high GC content of exon 1 did not allow the design of a robust MLPA probe within this exon. Sequences of MLPA probes are available in Supp. Table S2). For further deletion refinement, we designed additional intronic probes for intron 6 and intron 7 (Supp. Table S2). MLPA was performed using the P300 reference kit (MRC Holland, Amsterdam, The Netherlands) according to the manufacturer's instructions. DNA samples from multiple normal controls (minimum three) were processed for comparison in each experiment. An automated capillary sequencer (ABI 3500xl; Applied Biosystems, Foster City, CA) was used for detection, and data analysis was performed with the Sequence Pilot software (JSI Medical Systems, Kippenheim, Germany).
Breakpoint Analysis of NFIX Deletions
In order to identify deletion breakpoints, deletion-spanning genomic PCR products were generated using appropriate oligonucleotide primers (Supp. Table S3). Selective amplification of the deletion fragment was achieved by adapting the elongation time used in the PCR protocol in order to suppress amplification of the larger wild-type allele. PCR products were partially sequenced in serial fragments until the junction site was eventually identified.
NFIX Expression Analysis by RT-PCR
LCLs or fibroblast cultures were established from five patients and divided into two subcultures, which were grown with and without puromycin, an inhibitor of NMD. Puromycin was added to the medium at a concentration of 100 μg/ml (for fibroblast cell cultures) or 200 μg/ml (lymphoblastoid cell cultures). After 12 h of incubation, the cells from each subculture were harvested. RNA was extracted from lymphoblastoid or fibroblast cell cultures using the RNeasy MinElute Cleanup Kit (QIAGEN, Hilden, Germany). PCR amplification and sequencing of a cDNA fragment containing the respective mutations was performed after reverse transcription of mRNA with the first-strand synthesis system (Invitrogen, Darmstadt, Germany). Oligonucleotide primer sequences are given in Supp. Table S4. For larger deletions, the existence of wild-type and deleted cDNA fragments was first demonstrated by agarose gel electrophoresis. cDNA fragments were cut and extracted from agarose gels using the MinElute Gel Extraction Kit (QIAGEN) and subsequently sequenced in order to verify their sequence.
Parental Origin of the Recurrent NFIX Exon 6 and 7 Deletion
To determine on which parental allele the deletion arose, we genotyped trios (patient and both parents) for two common single nucleotide polymorphisms located within the deleted region rs66502314 in intron 6 and rs8111617 in intron 7 (dbSNP). Sequences of oligonucleotide primers used for amplification and sequencing of the genomic fragment containing these SNPs are given in Supp. Table S5.
Results
NFIX Sequencing
Direct bidirectional sequencing of all coding exons of NFIX including the exon-flanking intronic regions revealed eight different heterozygous mutations in 10 patients of this study cohort Table 2. Only one of these mutations, a transition at the donor-splice site of exon 6 (c.955+1G>A), has previously been reported [Malan et al., 2010], whereas the remainder represent novel mutations. They included an insertion of 4 bp (c.1048_1049insAGCG) in exon 7, two duplications of 1 bp (c.1090dup and c.1147dup), a 14 bp deletion (c.1146_1159del) an insertion-deletion (c.1242_1246delinsT) in exon 8, a 43 bp duplication (c.1392_1402+32dup) at the exon 9/intron 9 boundary consisting of the last 11 exonic and the adjacent 32 intronic base pairs, and a 1 bp deletion (c.1456del) in exon 10. For all patients from which parental DNA samples were available (eight out of 10) the de novo origin of the respective mutation was confirmed (Supp. Fig. S1). In seven individuals affected by MSS including the four cases originally belonging to the previously published cohort [Malan et al., 2010], no NFIX mutation was identified by conventional sequencing.
| Patient | Location | cDNA level | Protein level | Status |
|---|---|---|---|---|
| P1 | Exon 8 | c.1146_1159del | p.Y383Dfs*35 | n.a. |
| P2 | Exon 9–intron 9 | c.1392_1402+32dup | p.S468Ffs*9 | De novo |
| P3 | Exon 8 | c.1090dupG | p.A364Gfs*59 | De novo |
| P4 | Intron 5–intron 7 | c.819-592_1079-808del | p.S273Rfs*63 | De novo |
| P5 | Intron 5–intron 7 | c.819-700_1079-915del | p.S273Rfs*63 | De novo |
| P6 | Exon 10 | c.1456delC | p.(R486Gfs*6) | De novo |
| P7 | Exon 8 | c.1090dupG | p.(A364Gfs*59) | De novo |
| P8 | Exon 7 | c.1048_1049insAGCG | p.(P350Qfs*74) | De novo |
| P9 | Intron 6 | c.955+1G>A | p.(P320Sfs*143) | De novo |
| P10 | Exon 8 | c.1147dupT | p.(Y383Lfs*40) | n.a. |
| P11 | Exon 6–intron 6 | c.863_955+1320del | p.(A290Vfs*183) | De novo |
| P12 | Exon 10 | c.1456delC | p.(R486Gfs*6) | De novo |
| P13 | Intron 5–intron 6 | c.818+561_956-804del | p.(S273Rfs*104) | n.a. |
| P14 | Intron 5–intron 7 | c.819-484_1079-700del | p.(S273Rfs*63) | De novo |
| P15 | Intron 5–intron 7 | c.819-471_1079-687del | p.(S273Rfs*63) | De novo |
| P16 | Intron 5–intron 7 | c.819-732_1079-948del | p.(S273Rfs*63) | n.a. |
| P17 | Exon 8 | c.1242_1246delinsT | p.(A414Hfs*48) | De novo |
- n.a., parental samples not available; NFIX reference sequence ENST00000592199; all variants have been submitted to: http://databases.lovd.nl/shared/genes/NFIX.
NFIX MLPA Analysis
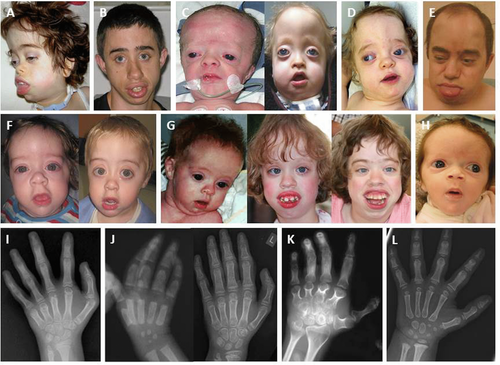
The seven patients without a NFIX mutation detectable by conventional sequencing were further analyzed for exonic deletions or duplications using a self-designed MLPA assay including specific probes upstream to exon 1 and for each of the exons 2–11 of the gene. In five patients (P4, P5, P14, P15, and P16), this analysis indicated a reduced copy number of exons 6 and 7 suggesting a heterozygous deletion. To verify this result and study the dimensions of the deletions in more detail, two additional intronic MLPA-probes in intron 6 and intron 7 of NFIX were analyzed. The five DNA samples consistently showed also reduced signals for these intronic probes. Therefore, the deletion breakpoints could be narrowed down to between intron 5 and a position distal to the NFIX intron 7 MLPA probe (Fig. 2A). In two additional patients (P11 and P13), MLPA analysis indicated a heterozygous deletion for the exon 6 probe only, suggesting a maximum deletion size between the position of the exon 5 and the intron 6 MLPA probes (Fig. 2A). Where parental DNAs were available (five of the seven deletion cases), we could uniformly demonstrate a normal signal distribution with MLPA analysis in both parents, thus confirming the de novo origin of the NFIX deletion (Supp. Fig. S2).
Breakpoint Sequencing of NFIX Deletions
In the genomic DNA samples from each of the five patients with the exon 6 and 7 deletion identified by MLPA, a deletion-spanning genomic PCR showed a smaller fragment of similar size, shortened by approximately 6 kb (Fig. 2B). Partial sequencing of this fragment disclosed the distal and proximal breakpoints to be located within two AluY repeats in intron 5 and intron 7, respectively, of the NFIX gene. These elements have 92% sequence identity and are oriented in parallel (Fig. 2A). The deletion size in the five patients was almost identical: 5.930 bp in patients P4, P14, and P15; 5,931 bp in patients P5 and P16 (Table 3). Breakpoint junction sites were flanked by 5 to 32 bp of perfect microhomology in all five cases (Supp. Fig. S3). The breakpoints of the exon 6 deletions in patient P11 and P13 were analyzed in a similar manner and were found to be unrelated to the AluY elements in intron 5 and intron 7. In fact, intron 5 was completely conserved in patient P11, whereas the deletion of 1,413 bp had its breakpoints within exon 6 (g.13,186,393; hg19), right at the exon 6 MLPA probe binding site, and in intron 6 (g.13,187,805). The deletion identified in patient P13 was found to span 3,223 bp comprising the entire exon 6 with breakpoints in intron 5 (g.13,185,401) and in intron 6 (g.13,188,623) (Fig. 2C and D).
| 5′-Breakpoint | 3′-Breakpoint | ||||
|---|---|---|---|---|---|
| Patient | Localization | Start | Localization | End | Deletion size (bp) |
| P16 | AluY – intron 5 | 13,185,616 | AluY – intron 7 | 13,191,546 | 5,931 |
| P5 | AluY – intron 5 | 13,185,649 | AluY – intron 7 | 13,191,579 | 5,931 |
| P4 | AluY – intron 5 | 13,185,757 | AluY – intron 7 | 13,191,686 | 5,930 |
| P14 | AluY – intron 5 | 13,185,865 | AluY – intron 7 | 13,191,794 | 5,930 |
| P15 | AluY – intron 5 | 13,185,878 | AluY – intron 7 | 13,191,807 | 5,930 |
| P13 | Intron 5 | 13,185,401 | Intron 6 | 13,188,623 | 3,223 |
| P11 | Exon 6 | 13,186,393 | Intron 6 | 13,187,805 | 1,413 |
Expression of Mutant NFIX mRNA
To study the consequences of MSS-associated mutations identified in this cohort, we analyzed RNA extracted from LCLs (P1–P4) or fibroblasts (P5) of five patients. In all of them, the wild-type as well as the mutant alleles could be amplified by RT-PCR. No substantial differences in the expression of the mutant allele were observed between subcultures with and without puromycin treatment, thus indicating that the mutant mRNA is not cleared by NMD (Fig. 3A–C and Supp. Fig. S4C). Furthermore, we could verify the consequences of these five mutations on the mRNA sequence (Supp. Fig. S4). In patients P1 and P3, cDNA sequencing confirmed that the deletion of 14 bp (Fig. 3A) and the duplication of one nucleotide, respectively, in exon 8 were similarly present in the mRNA, both leading to a shift of the reading frame (Supp. Fig. S4A and C). For the 43 bp duplication of 11 exonic and 32 intronic base pairs at the exon9/intron9 boundary identified in patient P2, we could demonstrate that an aberrant mRNA is produced through splicing at the duplicated splice site. This again results in a shift of the reading frame (Fig. 3B and Supp. Fig. S4 B).
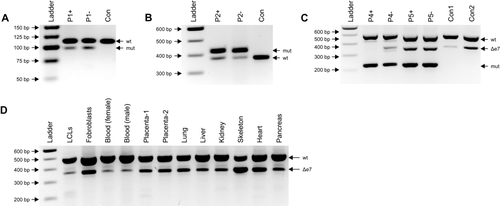
In cDNA of patients P4 and P5 with the Alu-mediated deletion of exon 6 and 7, three fragments of different size were identified through denaturating agarose gel electrophoresis of a RT-PCR product which was generated by the use of deletion-spanning oligonucleotide primers located in exons 4 and 8 (Supp. Table S4 and Fig. 3C). Sequencing confirmed these fragments to represent the wild-type, an endogenous known isoform lacking exon 7 (Δe7), and a mutated cDNA fragment lacking exon 6 and 7 (Supp. Fig. S4 D). This indicates that the exon 6 and 7 deletion, too, results in a stable mRNA that is translated into a mutant NFIX protein. The Δe7-isoform, which can only originate from the wild-type allele, could also be demonstrated in control cDNA from various tissues and is less abundantly expressed in leukocytes and lymphoblastoid cells compared to other tissues (Fig. 3D).
Parental Origin of NFIX Exon 6 and 7 Deletions
In five patients with proven NFIX deletions and DNA samples available from both parents, we examined the parental origin of the deletion. Genotyping of two common SNPs in intron 6 (rs66502314) and intron 7 (rs8111617) showed an informative constellation in three cases with the recurrent NFIX exon 6 and 7 deletion (Supp. Table S6). In all of them, the SNP genotypes indicated that the deletion had occurred on the paternally inherited allele. No informative SNP marker could be identified within the deleted NFIX regions in the patients P11 (partial exon 6 deletion) and P15 (exon 6 deletion).
Discussion
In this study, we identified NFIX mutations in all individuals of a cohort of 17 patients with MSS. Four patients originally belonged to the same cohort where NFIX mutations were first identified and reported in nine individuals with a positive test result [Malan et al., 2010]. Thus, the overall number of patients recruited by the members of the collaborative group is 26 with a NFIX mutation detection rate of 100%. Our findings provide no evidence of genetic heterogeneity of this clinically distinct syndrome.
We demonstrate for the first time that deletions of either exon 6 or exons 6 and 7 are responsible for the MSS phenotype and account for a previously unexplained, significant proportion of cases with MSS. Given a total patient population of 26 individuals as the basis of this study, we conclude that these deletions account for approximately one quarter of cases with MSS. Taken together, in the joint cohort comprising the previously published nine patients and the 17 participants of this study, the spectrum of mutations includes 16 small insertions/deletions (61%), three splice site mutations (12%), and seven larger exonic deletions (27%). MSS-associated mutations significantly cluster within the genomic region between exons 6 and 10. The common consequence of all of them is the production of transcripts with variable alterations of the reading frame at their 3′ ends (Fig. 4). As far as mutations could be studied on the mRNA level, it was confirmed that MSS-associated mutations uniformly escape NMD [(Malan et al., 2010] and data presented here]. We could show that this is also true for the recurrent NFIX exon 6 and 7 deletion. These findings thus corroborate the hypothesis that MSS-associated mutations are not simple inactivating (causing haploinsufficiency) but rather confer dominant negative effects.

MSS-associated mutations are predicted to lead to mutant NFIX proteins that have in common the preservation of the N-terminal part of the protein. Notably, none of the mutations is simply truncating a C-terminal part of the protein. Instead, the C-terminus of the predicted mutant proteins varies grossly in amino acid sequence and size (Fig. 4). DNA-binding and dimerization capacities of NFIs are known to be mediated by the N-terminal ∼200 amino acids (aa) of the proteins. This region contains a MH1 (MAD homolog1-dwarfin-type) domain that was shown to be sufficient for NFI dimerization and low-affinity site-specific DNA binding [Dekker et al., 1996]. The N-terminal third of the ∼200 aa region forms a stable alpha-helical subdomain that can bind DNA non-specifically, and, when fused to the MH1 domain, increases NFI DNA binding affinity ∼100-fold [Dekker et al., 1996]. The C-terminal portion of the protein represents a domain that is specifically conserved among the NFI family (CTF/NFI domain) and mediates transcriptional activation or repression particularly through a proline-rich region of ∼100 aa at its C-terminus. When linked to heterologous DNA-binding domains this 100 aa proline-rich domain can stimulate transcription five- to ten-fold in mammalian and drosophila cells [Mermod et al., 1989; Martinez et al., 1991; Seipel et al., 1992]. Generally, NFI proteins may either activate or repress transcription in a cell-type- and promoter-specific manner [Gronostajski, 2000]. These functions may be mediated by interaction with basal transcription factors or histones as well as through competition with other transactivators [Gronostajski, 2000, for reviews see: Hanna-Rose and Hansen, 1996; Manley et al., 1996; Pazin and Kadonaga, 1997]. Taking this into account, the predicted nature of MSS-associated mutant proteins suggests that they have preserved DNA binding and dimerization capacities, whereas the abnormal C-termini of mutant NFIX proteins are likely to disrupt the ability of DNA-bound NFI dimers to appropriately interact with binding partners in a nonspecific manner. A dominant negative effect may be explained by the mutant NFIX proteins’ competition with wild-type NFIs at the DNA binding sites and/or by their adverse effect on the wild-type component within a mutant-wild-type heterodimer. Further functional studies are required to elucidate the precise effects of MSS-associated NFIX mutant proteins.
Notably, despite the diversity of MSS-associated mutant NFIX proteins (Fig. 4), the patients’ phenotype is quite consistent with no obvious correlation between phenotype and specific alterations of the C-terminal portion of these proteins. Only a single patient, patient P2, displayed a somewhat milder MSS phenotype: his NFIX mutation introduces an ectopic exon 9 splice donor site without abrogating the authentic splice site. Production of a normally spliced mRNA from the mutant allele might ameliorate the phenotype by changing the stoichiometry between mutant and wild-type gene product in favor of the latter. This suggests that specific NFIX mutations may exist that will eventually fill the gap within the phenotypic spectrum between classical MSS and the Sotos-like phenotype caused by NFIX haploinsufficiency. In contrast to MSS, Sotos-like syndrome has been proposed to result from haploinsufficiency for NFIX, based on patients with two partial NFIX gene deletions and one nonsense mutation in the third exon of the gene [Malan et al., 2010]. More recently, two missense mutations (p.Leu60Pro, p.Arg121Pro) and one small in frame deletion (p.E53_E59del) were identified in patients with a Sotos-like phenotype [Priolo et al., 2012; Yoneda et al., 2012]. The common functional consequence of these mutations affecting the DNA-binding/dimerization domain is supposed to be the loss of the mutants’ ability to bind to DNA, thus probably representing loss-of-function mutations in contrast to the proposed dominant-negative effects of MSS-associated mutants.
There are different NFIX isoforms known whose differential roles and functions are so far barely understood. As an incidental finding of our experiments we could confirm the presence of an isoform lacking exon 7 (Δe7) in lymphoblastoid cells and other tissues (Fig. 3C and D). Notably, several mutations of exon 7 have been found in typical MSS patients. Such mutations are predicted to allow the production of a normal Δe7-isoform, the presence of which, however, is obviously not able to rescue or ameliorate the phenotype. This observation may be considered as a hint that the physiological role of the Δe7-isoform is only a minor one with regard to its impact on those developmental processes that are perturbed in MSS. Further research is required to clarify the differential expression and functions of NFIX isoforms.
Most of the mutations identified so far are private mutations observed only in one or two cases. In contrast, the exon 6 and 7 deletion described here is the only one that has been found as a recurrent mutation. We postulate that the predisposition for this deletion to occur de novo is explained by the local genomic architecture, namely the mediation by AluY repeats located within introns 5 and 7 of NFIX. Alu elements occur with a copy number of over one million in the human genome. Through their ability to act as retrotransposons and hotspots for intrachromosomal and interchromosomal recombination they are believed to contribute significantly to the dynamic nature and diversity of the human genome [Batzer and Deininger, 2002; Zhang et al., 2009]. The high GC content of their sequence (∼62.7%), and the remarkable sequence similarity (70–100%) among Alu subfamilies predispose Alu elements to successful recombination. Besides their role in genomic diversity and dynamics, Alu-mediated deletions have been identified as disease-causing mutations in numerous monogenic disorders, for example, Lynch syndrome [Li et al., 2006], hereditary breast and ovarian cancer [Mazoyer, 2005], von Hippel-Lindau disease [Franke et al., 2009], hereditary spastic paraplegia [Boone et al., 2011], and many others. It has been estimated that Alu/Alu-mediated genomic rearrangements are involved in 0.3% of human genetic diseases regardless of type, recurrent or non-recurrent rearrangements [Deininger and Batzer, 1999]. Different models have been developed to explain the occurrence of copy number changes in relation to the local genomic architecture [reviewed in [Cooper et al., 2011]]. The main mechanisms include non-allelic homologous recombination (NAHR), which occurs predominantly at low-copy repeats but has also been discussed to be involved in Alu-mediated deletions, and microhomology-mediated events such as fork stalling and template switching (FoSTeS) and/or microhomology-mediated break-induced replication (MMBIR). There is increasing evidence that the mechanisms of microhomology-mediated copy number change underlie most events of copy-number change, particularly those mediated by Alu elements [Hastings et al., 2009]. Accordingly, Vissers et al. (2009) demonstrated that Alu-related microhomology-mediated repair mechanisms prevail in rare pathogenic copy number changes. The finding of micohomology stretches of 5–32 bp at the junction sites of the recurrent NFIX exon 6 and 7 deletion supports such a microhomology-mediated mechanism. In contrast to NAHR, FoSTeS/MMBIR are replication-based mechanisms occurring during mitosis. In this context it is noteworthy that the Alu-mediated deletion described here was found to be located on the paternally inherited allele in the three informative cases. This is in line with previous studies demonstrating an excess of paternal origin of unbalanced de novo structural chromosome abnormalities, particularly of interstitial deletions [Thomas et al., 2006; Sibbons et al., 2012]. It was postulated that these errors occur in premeiotic divisions of germ cells through other mechanisms than NAHR, relating the excess of paternal origin to the higher amount of mitotic divisions in the male than in female germ cell line [Thomas et al., 2006]. No association with increased paternal age was found.
In summary, we demonstrate that MSS is genetically homogeneous in our cohort of patients, the spectrum of MSS-associated NFIX mutations is broad and includes also larger deletions in the 3′ part of the gene. Dominant negative effects of mutant NFIX proteins that retain the capacity of DNA binding and dimerization but lead to perturbed transactivation appear to be specifically related to this distinct phenotype.
Acknowledgments
The authors wish to express their gratitude to the patients and their families for participation in this study. We thank Susan Engelberg, Nicole Epperlein and Ilka Kramer for excellent technical support.
Disclosure statement: The authors declare no conflict of interest.




