Ferroportin Diseases: Functional Studies, a Link Between Genetic and Clinical Phenotype
Contract grant sponsors: DHOS (general french healthcare management organization); INSERM; French Reference Centre for Rare Iron Overload Diseases of Genetic Origin.
Communicated by Michel Goossens
ABSTRACT
Ferroportin (FPN) mediates iron export from cells and this function is modulated by serum hepcidin. Mutations in the FPN gene (SLC40A1) lead to autosomal dominant iron overload diseases related either to loss or to gain of function, and usually characterized by normal or low transferrin saturation versus elevated transferrin saturation, respectively. However, for the same mutation, the phenotypic expression may vary from one patient to another. Using in vitro overexpression of wild-type or mutant FPN proteins, we characterized the functional impact of five recently identified FPN gene mutations regarding FPN localization, cell iron status, and hepcidin sensitivity. Our aim was to integrate functional results and biological findings in probands and relatives. We show that while the p.Arg371Gln (R371Q) mutation had no impact on studied parameters, the p.Trp158Leu (W158L), p.Arg88Gly (R88G), and p.Asn185Asp (N185D) mutations caused an iron export defect and were classified as loss-of-function mutations. The p.Gly204Ser (G204S) mutation induced a gain of FPN function. Functional studies are useful to determine whether or not a FPN gene mutation found in an iron overloaded patient is deleterious and to characterize its biological impact, especially when family studies are not fully informative and/or additional confounding factors may affect bio-clinical expression.
Introduction
Iron is essential for major cellular processes, including erythroid function, but it has deleterious effects on health when present in excess. Iron homeostasis must, therefore, be tightly regulated. In addition to the HFE protein, additional proteins involved in iron homeostasis have been identified, including ferroportin (FPN; MIM #604353), hepcidin (MIM #606464), hemojuvelin (MIM #608374), transferrin receptor 2 (MIM #604720), and DMT1 (MIM #600523) [Reviewed in Evstatiev and Gasche, 2012]. Mutations in the genes encoding these proteins or their regulators cause iron metabolism disorders [Brissot et al., 2011; Pietrangelo et al., 2011].
Hepcidin, a protein produced and secreted by hepatocytes, has been described as a modulator of iron metabolism [Krause et al., 2000; Nicolas et al., 2001; Park et al., 2001; Pigeon et al., 2001]. The identified target of hepcidin is FPN [Nemeth et al., 2004], a transmembrane protein responsible for iron export from cells to plasma, present mostly in macrophages and at the basolateral surface of enterocytes [Abboud and Haile, 2000; Donovan et al, 2000; McKie et al., 2000]. Excess iron induces an increase in hepcidin concentration, resulting in the internalization and degradation of FPN protein [De Domenico et al., 2007; Nemeth et al., 2004]. Intracellular iron is then sequestered by binding to ferritin within cells, limiting the increase in plasma iron concentration and transferrin saturation.
Mutations of the FPN gene (SLC40A1) (ref: NG_009027.1; NM_014585.5) may lead to iron overload, also known as hemochromatosis type 4 or FPN disease [Brissot et al., 2011; Pietrangelo et al., 2011]. Unlike other genetic iron overload diseases, FPN disease displays autosomal-dominant inheritance [Montosi et al., 2001] with incomplete penetrance [Le Lan et al., 2011; Pelucchi et al., 2008]. Phenotypes are usually classified into two groups: “loss of function” or “gain of function.” In the loss-of-function phenotype (also called type A FPN disease), mutations affect the localization of FPN to the cell membrane and/or iron export function [Schimanski et al., 2005], leading to iron sequestration within the cell. Patients display hyperferritinemia, normal or low transferrin saturation, and iron accumulation, principally within the macrophages [Montosi et al., 2001]. The gain-of-function phenotype is similar to that of the classical HFE form of hemochromatosis and is referred as type B FPN disease. In this case, mutations affect the interaction between hepcidin and FPN, at the binding or signaling step, which normally leads to internalization and degradation of the FPN protein. Iron is thus continually exported from the cells to the plasma, due to increased expression of FPN protein on cell membrane. Patients with type B FPN disease have high levels of transferrin saturation, hyperferritinemia [Njajou et al., 2001], and iron overload, mostly affecting hepatocytes [Drakesmith et al., 2005]. Multiple mutations in FPN gene have been described so far [Mayr et al., 2010; Rice et al., 2009; Wallace et al., 2010]. However, when faced with a patient displaying both iron overload and a nucleotide change in the sequence of the FPN gene, it can be difficult to demonstrate that the mutation is deleterious and is involved in the development of the observed iron overload, particularly if family study is not possible or is uninformative. In addition, it may be difficult to classify the disease as being due to a gain or loss of FPN protein function, particularly in the presence of confounding factors that may modify the phenotype, such as metabolic syndrome, excessive alcohol intake, and digenism [Le Lan et al., 2011]. Such factors may lead to the coexistence of patients with clinical phenotype evoking either gain or loss of function for the same mutation, even within the same family. Therefore, new approaches must be engaged to permit characterization of the impact of FPN sequence alterations in such cases.
We investigated the cellular effects of mutations identified in bio-clinically characterized patients having iron overload disease with FPN gene mutations [Le Lan et al., 2011], by performing functional studies for the newly discovered mutations, NM-014585.5: c.473G>T p.Trp158Leu (W158L), c.610G>A p.Gly204Ser (G204S), and c.1112G>A p.Arg371Gln (R371Q), and for two mutations previously reported in patients but not previously characterized in vitro, c.262A>G p.Arg88Gly (R88G) [Cunat et al., 2007] and c.553A>G p.Asn185Asp (N185D) [Morris et al., 2005]. These studies were performed to have an understanding of the impact of the mutations presenting, from in silico analysis, a potential deleterious impact. We compared our observations with the in vitro phenotypes of the c.230C>A p.Ala77Asp (A77D) and c.190T>A p.Tyr64Asn (Y64N) variants, corresponding to the type A and type B phenotypes, respectively [Drakesmith et al., 2005; Schimanski et al., 2005]. For this purpose, we carried out transient transfection experiments with expression vectors encoding mutated or wild-type FPN (FPN-wt) in the embryonic kidney cell line HEK 293T, which are classically used in FPN studies. The biological impact of mutations was evaluated on the cellular distribution of the FPN protein, the cellular iron storage, and export and the effects of hepcidin on FPN expression. Our results reinforce the usefulness of functional studies, particularly in front of new FPN mutations detected for patients presenting established iron overload with confounding factors for iron metabolism alteration or family discrepancies in the disease expression.
Materials and Methods
Genetic Study
The SLC40A1 (NM_014585.5) gene mutations, used in these functional studies, were c.262A>G p.Arg88Gly (R88G) (rs387907374), c.473G>T p.Trp158Leu (W158L) (rs387907375), c.553A>G p.Asn185Asp (N185D) (rs387907376), c.610G>A p.Gly204Ser (G204S) (rs387907377), and c.1112G>A p.Arg371Gln (R371Q) (rs387907378); nucleotide numbering reflects cDNA numbering with +1 corresponding to the A of the ATG (translation initiation codon) in the reference sequence. Our variants have been submitted to NCBI dbSNP (http://www.ncbi.nlm.nih.gov/sites/varvu?gene=SLC40A1). They were previously reported in patients by Le Lan et al. (2011), who investigated clinical, biochemical, imaging, histological, and genetic data for 70 affected subjects from 33 families with 19 mutations. Informed consent for molecular studies was obtained from all participants, in accordance with the Helsinki Declaration. The CCPPRB (Comité Consultatif pour la Protection des Personnes se prêtant à des Recherches Biomédicales) of Rennes University Hospital approved the study on May 11, 2005 (reference number 04/42-523).
FPN Expression Vectors and Mutagenesis
The full-length wild-type human FPN (NM_014585.5) cDNA clone MGC:26152 IMAGE:4823308) was obtained from Open Biosystems and inserted into the pEGFP-N3 expression vector, between the KpnI and XmaI sites, to generate a C-terminal GFP (Green Fluorescent Protein)-tagged protein (FPN–GFP). The FPN mutations identified in patients were reproduced with the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA). Specific primers were used to generate all the constructs used from the wild-type FPN–GFP (Supp. Table S1). Construct integrity was checked by DNA sequencing with the BigDye Terminator v3.1 Kit and an AbiPrism 3130xl DNA sequencer (Applied Biosystems, Carlsbad, CA).
Cell culture and Transfection
HEK-293T human epithelial kidney cells were maintained in DMEM (Dulbecco's modified Eagle medium) supplemented with 10% fetal bovine serum (Invitrogen, Camarillo, CA). They were transiently transfected with FPN constructs at 50% confluence, after cell seeding, with jetPEI DNA transfection reagents, according to the manufacturer's protocol (Polyplus Transfection SA, Illkirch, France).
Confocal Immunofluorescence Studies
HEK293T cells were grown in Ibitreat eight-well microslides (Ibidi, Martinsried, Germany) and transfected with wild-type or mutated FPN–GFP constructs. Twenty hours after transfection, cells were either left untreated or were treated with 1 μM hepcidin for 3 hr. Before imaging, we replaced the culture medium with DMEM medium without red phenol supplemented with HEPES (20 mM). CellMask deep red plasma membrane stain (Invitrogen, Life Technologies) was used to label cell membranes just before imaging. We used a CSU-X1 spinning-disk confocal microscope (Nikon TI-E) with a 60× oil immersion objective (NA 1,4). Images were acquired with Metamorph® Software (Molecular Devices, Sunnyvale, CA, USA).
Data Analysis
For quantification of the FPN–GFP membrane signal, we used the Open Source image processing package Fiji, which is based on ImageJ software which is a public domain, Java-based image processing program developed at the National Institutes of Health, NIH. (Schneider et al., 2012). The CellMask deep red plasma membrane signal was used to define the surface of the cell and of the cell membrane. The FPN membrane signal is presented in arbitrary units, corresponding to the ratio of membrane FPN–GFP intensity to total FPN–GFP intensity (for the normalization of quantification).
Intracellular Ferritin Measurements
HEK293T cells were transfected with FPN constructs. Forty-eight hours later, cells were harvested and sonicated in 100 mM HEPES, 150 mM NaCl buffer (pH 7.5). After centrifugation, the supernatant was assayed for ferritin and total proteins, by immunoturbidimetric and colorimetric methods, respectively, on a multiparametric AU2700 Olympus automated analyzer (Olympus corporation, Tokyo, Japan). Results are expressed as a ratio of ferritin (μg)/total protein (g). Experiments were performed in triplicate, two to three times. When hepcidin treatment (1 μM) was realized, cells were exposed or not to hepcidin 24 hr after transfection and harvested 24 hr later (48 hr after transfection).
Statistical Analysis
Statistical analyses were performed with the nonparametric Mann–Whitney U test; P < 0.05 was considered significant.
Results
Localization of FPN Mutations in the Protein
From the adapted FPN model of Liu et al. (2005), we show the distribution of previously described mutations, including N185D and R88G, and of the newly identified mutations W158L, G204S, and R371Q (Fig. 1).

The G204S and R371Q mutations affect intermembrane segments, an extracellular domain between transmembrane domains 5 and 6 for G204S and a cytosolic loop between transmembrane domains 8 and 9 for R371Q. To our knowledge, no mutation has previously been studied at either of these sites.
In silico analysis of the impact of mutations was performed using Alamut V2.3, which permits multiple in silico mutations analysis using public softwares (SIFT; Align GVGD, Polyphen 2, Mutation T@ster). All the mutations studied were predicted in silico as possible or probably deleterious at least by one analyzer software.
FPN Expression in HEK Cells
Wild-type or mutant FPN–GFP fusion proteins were produced in HEK 293T cells. We analyzed the effect of the mutations on the intracellular distribution of the protein (Fig. 2).
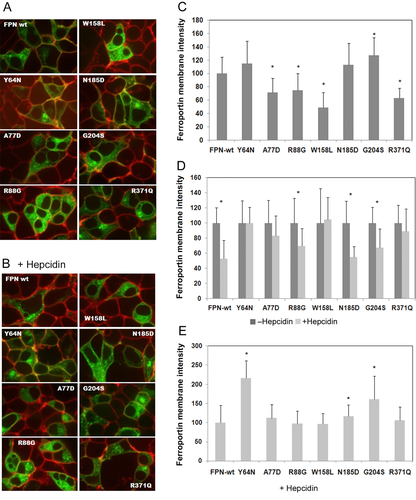
FPN-wt was found mostly at the cell surface and in the cytoplasm (Fig. 2A). Cells transfected with the mutant Y64N, N185D, or G204S FPN constructs displayed identical localization patterns. By contrast, the A77D, R88G, W158L, and R371Q mutant FPN proteins were mostly present in the cytoplasm, with only weak membrane staining, particularly in cells transfected with the W158L mutant construct. The membrane signal was quantified (Fig. 2C) and was significantly weaker than the wild-type signal for the A77D, R88G, W158L, and R371Q mutations, and significantly stronger for the G204S mutation.
Following treatment of the cells with hepcidin (Fig. 2B), the membrane signal disappeared and cytoplasmic fluorescence decreased with the FPN-wt construct. The membrane signal decreased but did not disappear, and the cytoplasm signal decreased very slightly in cells transfected with the N185D and G204S mutant constructs. Hepcidin affected FPN staining only slightly, either at the membrane or in the cytoplasm, in cells producing the R88G and R371Q mutant proteins. We observed no effect of hepcidin on FPN localization in cells producing the Y64N, A77D, and W158L mutant proteins. The quantification of the membrane signal after hepcidin treatment is presented in Figure 2 D and E. Decrease of the membrane signal, resulting in hepcidin effect compared with untreated cells, is presented in Figure 2D. In hepcidin-treated cells, the FPN signal at the membrane was significantly weaker than in the corresponding untreated controls, for FPN-wt and for the R88G, N185D, and G204S mutant proteins. We normalized the FPN membrane signal in treated cells producing mutated proteins with respect to treated cells producing FPN-wt (Fig. 2E). The values obtained reflect the real mutation impact on hepcidin sensitivity of mutated FPN. They were found to be significantly higher for the Y64N, N185D, and G204S mutant proteins, but not for the other mutant proteins (Fig. 2E).
Impact of Mutations on Intracellular Ferritin Levels
We investigated the effect of FPN mutations on cellular iron storage and export, by determining intracellular ferritin concentration. Following the export of intracellular iron by FPN, intracellular iron-depleted ferritin is ubiquitinated and degraded [De Domenico et al., 2006]. In addition, the IRE/IRP system limits the ferritin mRNA translation when cells are iron deprived [Pantopoulos, 2004]. Both phenomena lead to a decrease in intracellular ferritin concentration that reports a functional FPN protein for the present experiment.
We checked the effect of the various FPN mutant proteins, by comparison with FPN-wt (Fig. 3). Cells transfected with the A77D mutant construct and used as a control for the loss-of-function phenotype had significantly higher ferritin concentrations than cells transfected with FPN-wt. Similarly, cells producing the R88G, W158L, and N185D FPN mutant proteins had higher cellular ferritin concentrations than cells expressing the FPN-wt construct, accounting for a loss of function of the mutated proteins. As expected, the Y64N mutant construct, which was used as a control for the gain-of-function phenotype, resulted in lower ferritin concentrations than those obtained with FPN-wt. Cells transfected with the G204S and R371Q mutant constructs also had significantly lower ferritin concentrations than cells transfected with the wild-type construct accounting for iron export functional proteins associated with increase iron export raising the question of hepcidin resistance for both mutants.
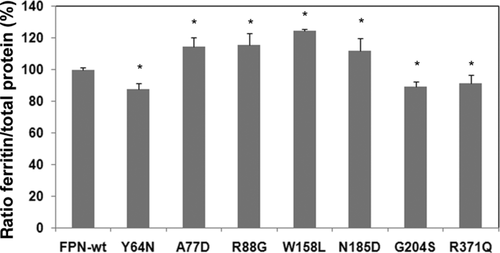
We investigated whether hepcidin resistance was conferred by G204S and R371Q mutant proteins, which are associated, respectively, with normal or partial FPN membrane localization and with decrease in intracellular ferritin concentration, by determining intracellular ferritin concentrations in hepcidin-treated and hepcidin-untreated HEK293T cells (Fig. 4). We used the Y64N mutant construct as a positive control for the FPN form of hepcidin resistance [Drakesmith et al., 2005]. Ferritin levels were not significantly higher in cells transfected with the Y64N or G204S construct, suggesting that the G204S FPN mutant protein is resistant to hepcidin. In cells transfected with the R371Q construct, ferritin levels increased in the presence of hepcidin, suggesting that this mutant protein remained sensitive to hepcidin.

Integration of Functional and Bioclinical Data
Together, the functional data show that: (1) R88G, W158L, and N185D provoke a loss of function of FPN, related either to abnormal localization or deficiency in iron export; (2) G204S-mutated FPN induces a gain of function related to hepcidin resistance; and (3) R371Q-mutated protein did not provoke export iron defect, nor hepcidin resistance, despite a lower expression of the protein on cell membrane. The summary of the different in vitro tests is shown in Table 1.
| Celllular localization | FPN membrane | Ferritin assay | ||||||
|---|---|---|---|---|---|---|---|---|
| Mutation | in basal condition | localization under | Ferritin level | Hepcidin | Mutation | |||
| FPN protein | Nucleotide | Amino acid | Membrane | Cytosol | hepcidin exposure | decrease | effect | impact |
| Wild type | +++ | + | − | + | ||||
| Y64N | c.190T>A | p.Tyr64Asn | +++ | + | + | − | − | Gain |
| A77D | c.230C>A | p.Ala77Asp | +/− | +++ | − | + | ND | Loss |
| R88G | c.262A>G | p.Arg88Gly | +/− | +++ | − | + | ND | Loss |
| W158L | c.423G>T | p.Trp158Leu | − | +++ | − | + | ND | Loss |
| N185D | c.553A>G | p.Asn185Asp | +++ | ++ | +/− | + | ND | Loss |
| G204S | c.610G>A | p.Gly204Ser | +++ | + | + | − | − | Gain |
| R371Q | c.1112G>A | p.Arg371Gln | +/− | ++ | − | − | + | None |
- Nucleotide numbering reflects cDNA numbering with +1 corresponding to the A of the ATG translation initiation codon in the reference sequence SLC40A1 (NM_014585.5); the protein change is associated according to the reference NP_055400.1 mutation number in this case corresponding to the position of the mutation on the reference protein sequence.
- ND, not determined.
According to iron parameters, including transferrin saturation levels usually used for FPN diseases type A or B classification, we determined the phenotypic expression of the disease for each patient and compared the phenotype with the functional impact of the considered mutation (Table 2). For detailed and complete clinical findings for this cohort, see Le Lan et al. (2011). Probands of the present study exhibiting other mutations in genes involved in iron metabolism were excluded, except those carrying heterozygote HFE p.Cys282Tyr mutation or p.His63Asp mutation (Table 2). Some discrepancies appeared in probands between clinical phenotype and functional data concerning: two patients of three carrying the mutation R88G, the patient carrying the mutation N185D, and one of the five patients carrying the G204S mutation.
| Patient | Mutation | Mutation associated | Serum iron (μmol/L) | Transferrin saturation (%) | Serum ferritin (μg/L) | LIC MRI (mg/g dw) | Location of iron deposits | Clinical phenotype | Functional results |
|---|---|---|---|---|---|---|---|---|---|
| 1 | R88G | HFE p.His63Asp+/− | 30.8 | 74 | 5765 | Mixed, mainly hepatocytes | Gain | Loss | |
| 2 | R88G | 8.2 | 11 | 2131 | 310 | ND | Loss | Loss | |
| 3 | R88G | 44.5 | 79 | 21665 | Mixed, mainly macrophages | Gain/Loss | Loss | ||
| 4 | W158L | HFE p.His63Asp+/− | 22.5 | 35.5 | 14829 | Macrophage, spleen | Loss | Loss | |
| 5 | W158L | 13.5 | 29 | 1405 | 150 | ND | Loss | Loss | |
| 6 | N185D | HFE p.Cys282Tyr+/− | 40.8 | 83 | 6970 | Mixed, mainly hepatocytes | Gain | Loss | |
| 7 | G204S | HFE p.His63Asp+/+ | 32 | 91.4 | 3755 | Mixed, mainly hepatocytes | Gain | Gain | |
| 8 | G204S | HFE p.His63Asp+/− | 43 | 88 | 1380 | Mixed, mainly hepatocytes | Gain | Gain | |
| 9 | G204S | HFE p.His63Asp+/− | 39.6 | 79 | 855 | 170 | ND | Gain | Gain |
| 10 | G204S | HFE p.His63Asp+/− | 23.2 | 44 | 744 | 170 | ND | Loss | Gain |
| 11 | G204S | HFE p.His63Asp+/− | 37 | 79 | 5418 | 320 | Mixed, mainly hepatocytes | Gain | Gain |
| 12 | G204S | 24.9 | 47.4 | 761 | 210 | ND | Gain | Gain | |
| 13 | R371Q | HFE p.Cys282Tyr+/− | 23.7 | 40 | 777 | 140 | ND | Loss | N |
- LIC: Liver Iron Concentration; MRI: Magnetic Resonance Imaging; dw: dry weight; ND: not determined; N: normal.
Discussion
Mutations within the FPN gene induce the development of iron overload with usually two phenotypic presentations: either low to normal transferrin saturation (FPN disease related to a loss of function) or high transferrin saturation (FPN disease associated with a gain of function). However, recent studies [Pelucchi et al., 2008], including one from our group on a large cohort of patients [Le Lan et al., 2011], have suggested that the phenotype associated with a given mutation may vary between patients. Furthermore, the penetrance of the disease is incomplete [Le Lan et al., 2011; Pelucchi et al., 2008]. Thus, we investigated the relationship between mutations and phenotypes, by exploring the effect of FPN mutations on the cellular distribution of the FPN protein, on cellular iron stores and on FPN sensitivity to hepcidin, which regulates its cell membrane expression.
The G204S FPN mutation was showed by functional studies to induce a gain of function type disease. However, patients carrying the G204S mutation had heterogeneous phenotypes [Le Lan et al., 2011; Santos et al., 2011]; one of the five probands (patient 10) showed a loss-of-function phenotype. Such phenotypic variability was also found within families, suggesting that environmental or genetic cofactors may affect disease expression [Le Lan et al., 2011]. The hypothesis that unrecognized factor(s) could mask iron metabolism disturbance has also to be considered.
On the basis of functional data, we assigned the W158L, N185D, and R88G mutations to the loss-of-function disease group. For the W158L mutation, the results of functional studies were in agreement with the bioclinical findings. These results are consistent with those recently reported for another substitution (W158C) of the same amino acid [Mayr et al., 2011]. Considering the N185D FPN mutation, the clinical phenotype of the proband (patient 6) was surprisingly suggestive of a gain of function. However, acquired cofactors may be responsible for the apparent gain-of-function phenotype, because the proband and his brother (who shared the same phenotypic iron profile) had excessive alcohol consumption, a factor previously reported decreasing hepcidin expression [Ohtake et al., 2007] and therefore susceptible to increase transferrin saturation. Furthermore, the proband had cirrhosis, which may also decrease hepcidin production [Détivaud et al., 2005]. Nevertheless, their mother, who also carried the mutation and was not alcoholic, presented normal transferrin saturation [Le Lan et al., 2011], consistent with loss-of-function disease and with our functional study data. For the R88G-mutated FPN, clinical phenotype differed between the three probands. Patients 1 and 3, who had high levels of transferrin saturation, presented overweight related to metabolic syndrome reported to slightly increase hepcidin [Barisani et al. 2008, Ruivard et al. 2009], which may modify the clinical presentation of the disease. It is noteworthy that similar clinical observations have been reported by others for the FPN mutation R88T [Bach et al., 2006]. In addition, patient 1 exhibited a non insulin-dependent diabetes and was heterozygous p.His63Asp in HFE gene. Furthermore, from available bioclinical file, we cannot definitively exclude excessive alcohol intake for patient 3, as well as other unidentified genetic elements. All these factors are difficult to integrate and could interfere to modify the phenotype, supporting the interest of functional studies to assess the deleterious character of the mutation.
Considering R371Q FPN mutant, our in vitro functional results suggested that the R371Q mutation is not deleterious. This conclusion is supported by the absence of iron overload in relatives carrying the mutation, as assessed by determination of ferritin concentrations (data not shown). However, no obvious confounding factor was found that could participate to the iron overload observed in the proband, but the existence of other genetic factor(s) cannot be excluded.
Taken together, our data suggest that in front of an iron-overloaded patient, evaluated through MRI (Magnetic Resonance Imaging) or liver biopsy, and exhibiting a not previously characterized mutation in the FPN gene, functional studies are helpful, especially in the following cases: when there is no available informative family study and/or when there are discrepancies in the mutation expression between patients and/or when there are discrepancies between several in silico predictions.
Discrepancies between bioclinical and functional data are likely related to multiple environmental and genetic factors. For the later point, it is noteworthy that a majority of probands (9/13) were presented with nucleotide change in HFE, either p.His63Asp at heterozygous (n = 6) or homozygous (n = 1) state or p.Cys282Tyr at heterozygous state (n = 2). Nevertheless, these HFE genotypes are not reported to induce iron overload.
Our functional data also support the idea that transferrin saturation is not the better parameter to classify FPN diseases.
Conclusion
Functional studies are, after careful bioclinical evaluation of the patients, useful to decipher the impact of FPN gene mutations. The approach used in our study made it possible to classify the mutations found in patients as causing a gain or a loss of function or having no likely impact. Likely in the future, new functional tests will be developed to improve the understanding of the mutation impact on the FPN protein function, expression, or regulation.
Acknowledgments
The authors would like to thank Stéphanie Dutertre and Marine Lambert for assistance at the Microscopy Rennes Imaging Center (MRic) facilities of Federative Institute 140, and the physicians who referred patients to the French Reference Center for Rare Iron Overload Diseases of Genetic Origin: Dr. Bettan L, Villeneuve St George; Dr. Corront B, Annecy; Prof. Couzigou P, Bordeaux; Dr. Dudicourt JC, Cesson Sevigné; Dr. Laroussi N, Grenoble; Dr. Roget L, Grenoble.
Disclosure statement: Lénaïck Détivaud performed the experimental work, designed research of functional studies, analyzed the data, and wrote the manuscript; Marie-Laure Island contributed to cloning and to design research of functional studies; Anne-Marie Jouanolle, performed genetic studies contributed to design research and to write the paper; Martine Ropert performed the hepcidin assay, biochemical test in functional studies and analyzed the data; Edouard Bardou-Jacquet contributed to functional studies and patients characterization; Caroline Le Lan performed patients characterization and analyzed bioclinical data; Annick Mosser contributed to Genetic studies; Patricia Leroyer contributed to functionnal studies; Yves Deugnier contributed to patients characterization; Véronique David contributed to genetic studies, to design research and to write the paper; Pierre Brissot contributed to patients characterization, and to design research, analyzed the data, and wrote the paper; Olivier Loréal designed research, analyzed the data, and wrote the paper.
The authors declare no conflict of interest.




