The Natural History of NAFLD, a Community-Based Study at a Large Health Care Delivery System in the United States
Abstract
Nonalcoholic fatty liver disease (NAFLD) is a global public health problem. However, the natural history of NAFLD is incomplete. This is a retrospective cohort study of patients identified with NAFLD by diagnosis codes in a large, community-based health care delivery system. The objectives were (1) to follow patients from initial NAFLD presentation through progression to cirrhosis and/or decompensated cirrhosis to liver cancer, liver transplant, and death for up to 10 years; and (2) to conduct disease progression analysis restricted to patients with NAFLD identified as having diabetes at baseline. A total of 98,164 patients with full NAFLD and 26,488 with diabetes were divided into three baseline prevalent states: (1) no cirrhosis, (2) compensated cirrhosis, and (3) decompensated cirrhosis. In baseline patients without cirrhosis, annual rates of compensated cirrhosis, decompensated cirrhosis, and death were 0.28%, 0.31%, and 0.63% per year, respectively. With baseline compensated cirrhosis, the annual rates of decompensation and death were 2.4% and 6.7% per year. Finally, in those with decompensated cirrhosis at baseline, the death rate was 8.0% per year. In those without cirrhosis and with cirrhosis at baseline, the rates of liver cancer and death were increased approximately 2-fold in the diabetic subpopulation compared with the full NAFLD cohort. Age and comorbidities increased with increasing disease severity. Cox proportional hazards regression analysis showed that cirrhosis was strongly associated with death and liver cancer, and that diabetes was associated with a significant increase in the hazard of both liver cancer and death (2.56 [2.04-3.20] and 1.43 [1.35-1.52]), respectively. Conclusion: The findings of this community-based study further our understanding of the natural history of NAFLD and demonstrate that diabetes is a major factor in the progression of this disease.
Abbreviations
-
- BMI
-
- body mass index
-
- CHF
-
- congestive heart failure
-
- CI
-
- confidence interval
-
- CKD
-
- chronic kidney disease
-
- HR
-
- hazard ratio
-
- ICD-9
-
- International Classification of Diseases, 9th Revision
-
- ICD-10
-
- International Classification of Diseases, 10th Revision
-
- KP
-
- Kaiser Permanente
-
- KPSC
-
- Kaiser Permanente Southern California
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NASH
-
- nonalcoholic steatohepatitis
Nonalcoholic fatty liver disease (NAFLD) is a global public health problem estimated to affect 24% of people worldwide.(1) NAFLD is diagnosed in the absence of secondary causes of liver disease and consists of a spectrum ranging from nonalcoholic fatty liver to nonalcoholic steatohepatitis (NASH) to cirrhosis.(2) Previous studies suggest that nonalcoholic fatty liver (simple steatosis) can be slowly progressive or nonprogressive, whereas NASH often demonstrates histopathological progression to advanced fibrosis and cirrhosis.(3) It is also recognized that those with advanced fibrosis are less likely to improve and that they are at risk of liver-related mortality, including hepatic decompensation, liver cancer, liver transplantation, and death.(4-7)
Additional studies suggest that patients with diabetes may progress more rapidly to advanced histological disease and consequent adverse clinical outcomes.(8, 9) Furthermore, NAFLD has been shown to be independently associated with an increased incidence of cardiovascular events in patients with diabetes.(10-12)
Reports on the natural history of NAFLD in the United States include population-based studies, modeling studies, analyses of clinical trial data, and meta-analyses. The population-based studies include analyses of data from the National Health and Nutrition Examination Survey,(13, 14) reports of specific populations such as residents of Olmsted County, Minnesota, a majority White population (402 of 435 [92%] White),(15) and studies in American military veterans with over 90% males.(16, 17) Registry studies include analyses of data from sources such as the Surveillance, Epidemiology and End Results registry(18) and the Organ Procurement and Transplantation Network.(19)
Studies of populations outside the United States include an analysis of four European Union primary care databases (United Kingdom, Netherlands, Italy, and Spain)(20) and investigations of Swedish population–based cohorts.(21-23) These studies and other published analyses contribute to an increasing understanding of the natural history of NAFLD. However, there is a dearth of studies that describe the progression of NAFLD in community-based, multispecialty practice settings. To address this gap in the literature, the aims of this study are:
- To determine the rate of progression from NAFLD to cirrhosis or complications of cirrhosis;
- To determine risk factors associated with the progression from NAFLD to cirrhosis or complications of cirrhosis; and
- To determine the rate of progression from baseline status to the final outcomes of liver cancer, liver transplant, and all-cause death.
Methods
Design
This was a retrospective cohort study conducted in patients identified as having a diagnosis of NAFLD/NASH and assessing their disease progression and liver-related outcomes.
Setting
Kaiser Permanente Southern California (KPSC) is a nonprofit, integrated health services delivery system with over 4.0 million health plan members. The KPSC membership is socio-economically diverse and broadly representative of the racial and ethnic groups living in Southern California.(24) Each Kaiser Permanente (KP) member has a unique medical record number that can be used to link various clinical and administrative databases. All aspects of care and interactions with the health care delivery system are captured in a continuously updated, comprehensive electronic medical record that is available for research purposes. KP patients are seen exclusively in the KP system except for selected care offered at contracted facilities in certain impacted specialties and emergency services outside of the KP system. All external billing codes and clinical codes for care delivered in non-KP settings are captured by a detailed, continuously updated external claims system and entered in the research database.
Patients
To be eligible for inclusion in the study cohort, patients needed to have a qualifying NASH or NAFLD diagnosis from January 1, 2008, to December 31, 2017. Supporting Table S1 lists the qualifying diagnosis codes. It should be noted that before October 2015 there were no NASH diagnosis codes included in the International Classification of Diseases (ICD), 9th revision (ICD-9); NASH codes were included in the updated ICD-10 listing.
A total of 245 charts were reviewed to evaluate nonspecific liver disease ICD-9 and ICD-10 codes. Of the 245 cases reviewed, only four were considered NAFLD or NASH for a positive predictive value of 2%. Thus, these codes were excluded from our case finding methodology (see Supporting Table S2).
In addition to ICD-9/10 codes, the electronic algorithm for the decompensation events incorporated procedure codes for paracentesis and upper endoscopy (EGD). Patients with a paracentesis code were also required to have a diagnosis code for either ascites or spontaneous bacterial peritonitis and have a current or subsequent diagnosis of cirrhosis.
Charts flagged with a procedure code for EGD were considered decompensated only if they also had a code for upper gastrointestinal (GI) bleeding. A procedure code for transjugular intrahepatic portosystemic shunt any time during the study period qualified as a decompensating event.
In addition to diagnosis codes, hepatic encephalopathy was identified by pharmacy dispenses and by laboratory data. A lactulose or rifaximin prescription was required to also have an associated diagnosis of hepatic encephalopathy and no diagnosis of constipation or >3 dispenses and at least one of the following: (1) platelets <100,000; (2) total bilirubin >2.5; (3) propranolol or nadolol dispense; (4) ammonia test result; or (4) prescribed by a GI specialist (see Supporting Tables S3-S5 for details on the electronic algorithm for compensated and decompensated cirrhosis). Due to the observational design of the study, most diagnoses were assigned by the patients’ primary care physicians, and these diagnoses were made based primarily on clinical information and not on liver histopathology. In practice, the clinical manifestations of NAFLD can sometimes occur before a NAFLD diagnosis is made. For this study, if a complication of NAFLD is documented in the patient’s medical record with a subsequent NAFLD diagnosis, it was assumed that NAFLD was present at the time of the complication and contributed to the complication. Therefore, the index date is the date of a NAFLD diagnosis or the date of a NAFLD complication followed by a qualifying diagnosis, whichever comes first. Complications of NAFLD include compensated and decompensated cirrhosis. At baseline, patients were categorized into three mutually exclusive groups: no cirrhosis, compensated cirrhosis, and decompensated cirrhosis.
Additional inclusion criteria included age of 18 years or older on the index date and 12 months of continuous membership in the KPSC health plan, allowing for a less than 2 consecutive month gap in membership during the pre-index date baseline period. Patients were excluded if they had evidence of alcohol-induced liver damage, alcohol abuse and dependence, other liver diseases (primary biliary cirrhosis, Wilson Disease, hemochromatosis, autoimmune hepatitis, primary sclerosing cholangitis, Gaucher’s disease, or toxic liver disease), chronic hepatitis (hepatitis B or hepatitis C), and human immunodeficiency disease (see Supporting Table S6 for a listing of exclusion codes). Liver cancer and liver transplants were identified using diagnosis codes and procedure codes, respectively. Mortality data were obtained from the California Department of Public Health Vital Statistics report.
To validate the electronic algorithm, we performed a chart review on 330 patients. One hundred and nine randomly selected charts identified with NAFLD by the electronic algorithm were reviewed, and the following was recorded: body mass index (BMI) >30 (yes/no), elevated liver tests (yes/no), prediabetes or diabetes (yes/no), and imaging consistent with fatty liver and/or cirrhosis (yes/no). Elevated liver tests were defined as two or greater values of ALT >40 U/L for men and >30 U/L for women more than 6 months apart, total bilirubin >1.3 mg/dL, or alkaline phosphatase >113 U/L. This chart review revealed that 103 of 109 (95%) had consistent imaging, and 91 of 109 (83%) had elevated liver tests. The 6 patients without consistent imaging all had prediabetes/diabetes and BMI >30.
To validate the algorithm for cirrhosis, 111 randomly selected charts identified by the electronic algorithm with compensated cirrhosis were reviewed. Additionally, 110 charts identified by the algorithm with decompensated cirrhosis were reviewed. This review showed that 98 of 111 (sensitivity = 88%) had true cirrhosis, as evidenced by imaging (liver nodularity with or without evidence of portal hypertension), and/or had a diagnosis of cirrhosis made by a specialist in gastroenterology/hepatology. Most of these patients had other abnormalities including elevated liver tests and transient elastography values consistent with cirrhosis. Ninety-one of 110 (sensitivity = 83%) had true decompensation by imaging (ascites: 76% of chart-review population) and/or other clinical criteria, including endoscopy with variceal bleeding and/or hepatic encephalopathy diagnosed and treated by a gastroenterologist/hepatologist. Chart review was also performed on 60 randomly selected cases to validate the electronic algorithm for comorbidities. We found >93% concordance for all of the comorbidities analyzed.
Outcomes
The primary objective of the study was to follow patients from their initial NAFLD disease presentation through its natural history progression of cirrhosis and/or decompensated cirrhosis to the clinical outcomes of liver cancer, liver transplant, and death. The secondary objective was to conduct a natural history disease progression analysis restricted to patients with NAFLD identified as having diabetes at baseline. Patients were followed from cohort entry until they disenrolled from the health plan, death, or the end of study follow-up on December 31, 2018. Patients identified with cirrhosis and decompensated cirrhosis at baseline were defined as having prevalent disease.
Statistical Analysis
The patients were categorized into three groups at baseline: no cirrhosis, compensated cirrhosis, and decompensated cirrhosis. We compared the demographics and comorbidities of patients at baseline among these three groups. Continuous variables were presented as mean (SD) and analyzed by the Kruskal-Wallis test. Categorical variables were presented as n (%) and analyzed by chi-square test. Cox proportional hazards regression model was applied to investigate the effects of initial diseases on liver cancer, liver transplant, and death outcomes. The Cox proportional hazards model was adjusted for these potential confounders: age, gender, race/ethnicity, congestive heart failure, chronic kidney disease, hypertension, diabetes, and obesity.
In addition, we established the disease-progression patterns of patients from initial disease stage to the latest disease stage diagnosed by the end of follow-up for each group. Both mean years and conditional probability of progressing from one disease stage to the next were calculated and presented for each disease progression pattern.
The statistical analyses were performed using the SAS EG ver. 7.1 software (SAS, Cary, NC), and P values <0.05 were considered significant.
Results
The source population for the study included 3,534,604 patients (Fig. 1). After applying inclusion and exclusion criteria, 98,312 remained. The mean (SD) and median (interquartile range) follow-up time (in years) for the entire study was 4.83 (2.97) and 4.13 (2.22, 7.21), respectively. Those who had undergone liver transplant or who were diagnosed with liver cancer before cohort entry were excluded, reaching a total sample size of 98,164. Patients were divided into groups identified in the following prevalent states at baseline: no cirrhosis (n = 90,071, 91.8%), compensated cirrhosis (n = 4,814, 4.9%), and decompensated cirrhosis (n = 3,279, 3.3%).
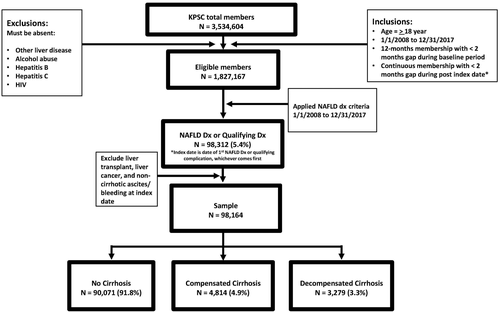
Table 1 describes the demographics and comorbidities of the general non-NAFLD KPSC population after applying the inclusion and exclusion criteria (n = 1,827,167) compared with the prevalent state at baseline: no cirrhosis, compensated cirrhosis, and decompensated cirrhosis. Females are predominant, age increases with disease severity, and race/ethnicity reflect that of the KPSC population. Mean BMI is over 30 in all groups with NAFLD, and comorbidities generally increase with increasing disease severity. Notably, diabetes is 2-4-fold higher in the patients with NAFLD compared to the general population. Congestive heart failure (CHF), chronic kidney disease (CKD), and hypertension also show a marked increase with progressive hepatic fibrosis.
| KPSC Population* (n = 1,827,167) | No Cirrhosis (n = 90,071) | Compensated Cirrhosis (n = 4,814) | Decompensated Cirrhosis (n = 3,279) | |
|---|---|---|---|---|
| Age, mean (SD) | 48.5 (17.47) | 50.4 (13.90) | 61.8 (14.77) | 61.3 (15.21) |
| Male sex, n (%) | 831,130 (45.5%) | 40,836 (45.3%) | 2,284 (47.4%) | 1,452 (44.3%) |
| Race/ethnicity, n (%) | ||||
| White | 757,669 (41.5%) | 30,563 (33.9%) | 2,371 (49.3%) | 1,547 (47.2%) |
| African American | 184,342 (10.1%) | 4,748 (5.3%) | 312 (6.5%) | 359 (10.9%) |
| Hispanic | 502,179 (27.5%) | 38,863 (43.1%) | 1,577 (32.8%) | 958 (29.2%) |
| Asian/Pacific Islander | 190,306 (10.4%) | 9,375 (10.4%) | 347 (7.2%) | 279 (8.5%) |
| Native American | 19,288 (1.1%) | 1,051 (1.2%) | 48 (1%) | 54 (1.6%) |
| Other | 173,383 (9.5%) | 5,471 (6.1%) | 159 (3.3%) | 82 (2.5%) |
| BMI, kg/m2, mean (SD) | 28.6 (6.30) | 33.1 (6.72) | 31.9 (7.51) | 31.5 (7.44) |
| CHF, n (%) | 37,891 (2.1%) | 1,856 (2.1%) | 637 (13.2%) | 639 (19.5%) |
| CKD, n (%) | 156,923 (8.6%) | 8,441 (9.4%) | 1,370 (28.5%) | 1,146 (34.9%) |
| Diabetes, n (%) yes | 210,010 (11.5%) | 22,959 (25.5%) | 2,115 (43.9%) | 1,414 (43.1%) |
| Hypertension, n (%) yes | 551,437 (30.2%) | 40,498 (45%) | 3,309 (68.7%) | 2,360 (72%) |
- Note: Continuous variables are expressed as mean (SD) and analyzed by Kruskal-Wallis test. Categorical variables are expressed as n (%) and analyzed by chi-square test.
- * KPSC population refers the general KPSC population without NAFLD after applying study inclusion and exclusion criteria.
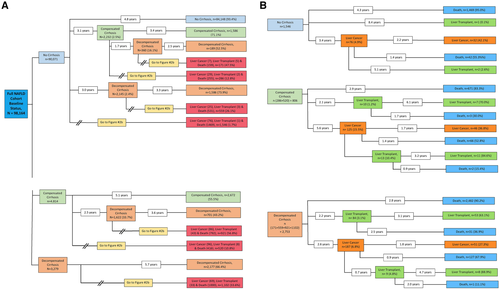
Progression of NAFLD from the three prevalent baseline states is depicted in Fig. 2A. Figure 2B shows the continued progression of disease for the full NAFLD cohort to the final outcomes of liver cancer, liver transplant, and death for the subpopulations that reached one of the final outcomes during the study period. The mean time (in years) and conditional probabilities of reaching one disease state to the next are shown. During the study period, most (93.4%) of those without cirrhosis at baseline remained without cirrhosis (Fig. 2A). A subset of patients was identified as developing cirrhosis, as shown (2.5% over an average period of 3.1 years). Among this group, 360 (16.1% over 1.7 years) developed a complication qualifying as a decompensation event, and a subset of this group (n = 171) reached one of the outcomes of liver cancer, liver transplant, or death during the study period (Fig. 2B). An additional subset of patients was identified whose members developed a decompensation event as their first manifestation of cirrhosis (n = 2,145 [2.4%] over an average period of 3.0 years). A subset of this group remained in the state of decompensated cirrhosis (n = 1,586 [73.9%]), and a second subset progressed to one of the outcomes of liver cancer, liver transplant, or death (n = 559 [26.1%]). A similar progression is shown for the two groups with prevalent compensated cirrhosis (n = 4,814) and prevalent decompensated cirrhosis (n = 3,279). Please see Fig. 2A,B for details.
To determine whether diabetes may be a risk factor for more rapid progression of disease in NAFLD, we performed a subanalysis of this population (Fig. 3A,B). Patients with diabetes at baseline progressed from a noncirrhotic state to compensated cirrhosis (4.1% over 3.0 years) and then to decompensated cirrhosis (19.2% over 1.9 years) at a higher rate than the total population with NAFLD. In fact, the percentage of individuals with diabetes who reached a subsequent more advanced disease state is approximately 1.5-2-fold higher than in the total population with NAFLD. Similarly, more rapid progression is seen in the prevalent compensated cirrhosis groups and decompensated cirrhosis groups in the population with diabetes compared to the total population with NAFLD (Fig. 3A,B).
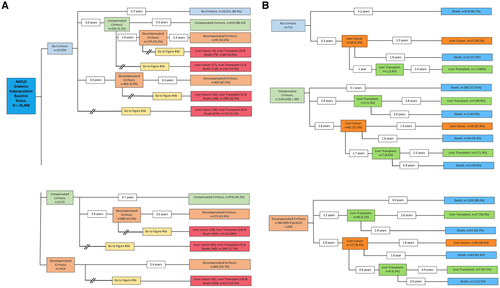
Table 2 provides the results of a multivariable Cox proportional hazards regression analysis to investigate the effects of baseline diseases on liver cancer and all-cause death. Compensated cirrhosis and decompensated cirrhosis significantly increased the hazard of liver cancer compared to those with no cirrhosis at baseline (hazard ratio [HR] and 95% confidence interval [CI] equal to 14.07 [11.08-17.86] and 8.12 [5.99-11.01], respectively). For death, the HR and 95% CI was 3.63 (3.38-3.90) and 3.86 (3.57-4.17) for those with baseline compensated cirrhosis or decompensated cirrhosis, respectively. Age and male sex significantly increased the hazard of both liver cancer and death. Among the comorbidities, diabetes was associated with a significant increase in the hazard of both liver cancer and all-cause death (2.56 [2.04-3.20] and 1.43 [1.35-1.52], respectively). None of the other comorbidities were associated with an increased hazard of liver cancer, whereas CHF, CKD, and hypertension were all associated with an increased hazard of all-cause death.
| Liver Cancer | All-Cause Death | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| No cirrhosis (reference) | ||||
| Compensated cirrhosis | 14.07 (11.08-17.86) | <0.0001 | 3.63 (3.38-3.90) | <0.0001 |
| Decompensated cirrhosis | 8.12 (5.99-11.01) | <0.0001 | 3.86 (3.57-4.17) | <0.0001 |
| Age (years) | 1.05 (1.04-1.06) | <0.0001 | 1.06 (1.06-1.07) | <0.0001 |
| Male sex | 1.50 (1.22-1.83) | <0.0001 | 1.17 (1.10-1.24) | <0.0001 |
| Race/ethnicity (White, reference) | ||||
| African American | 1.00 (0.63-1.61) | 0.99 | 1.01 (0.92-1.12) | 0.803 |
| Hispanic | 2.03 (1.61-2.56) | <0.0001 | 0.74 (0.69-0.80) | <0.0001 |
| Native American | 2.64 (1.29-5.40) | 0.008 | 0.82 (0.63-1.08) | 0.164 |
| Asian/Pacific Islander | 1.80 (1.27-2.56) | 0.001 | 0.67 (0.60-0.75) | <0.0001 |
| Other | 1.10 (0.54-2.26) | 0.790 | 0.97 (0.82-1.16) | 0.750 |
| CHF | 0.53 (0.36-0.79) | 0.002 | 2.12 (1.97-2.28) | <0.0001 |
| CKD | 0.91 (0.72-1.17) | 0.471 | 1.55 (1.45-1.66) | <0.0001 |
| Diabetes | 2.56 (2.04-3.20) | <0.0001 | 1.43 (1.35-1.52) | <0.0001 |
| Hypertension | 1.04 (0.78-1.38) | 0.796 | 1.21 (1.12-1.32) | <0.0001 |
| Obesity | 1.14 (0.92-1.41) | 0.235 | 0.90 (0.84-0.95) | 0.0004 |
Note:
- Data are expressed as HRs with 95% CIs. P values <0.05 were considered significant.
Tables 3 and 4 provide the incidence of the outcomes of liver cancer, liver transplant, and all-cause death according to disease category at baseline for the full NAFLD cohort and the diabetes subpopulation. The incidence of death is increased nearly 10-fold in patients with compensated cirrhosis at baseline (5,515/100,000 patient-years) compared with no cirrhosis at baseline (576/100,000 patient-years), and the incidence of liver cancer was increased over 20-fold (761/100,000 patient-years vs. 32/100,000 patient-years). A decrease in liver cancer rate and liver transplant rate are seen in those with decompensated cirrhosis versus compensated cirrhosis at baseline (406/100,000 patient-years for liver cancer and 206/100,000 patient-years for liver transplant), which may be the result of competing risks (all-cause death is higher in decompensated cirrhosis than in compensated cirrhosis). The incidence rates of all-cause death, liver cancer, and liver transplant for the three baseline disease categories in the diabetes subpopulation are also displayed in Table 3. There is an increased rate of all three outcomes up to 2-fold in the diabetes population compared with the full NAFLD cohort.
| Outcome, NAFLD Full Cohort | No Cirrhosis (n = 90,071) | Compensated Cirrhosis (n = 4,814) | Decompensated Cirrhosis (n = 3,279) | P Value* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Total | Incidence/100,000 Person-Years | n | Total | Incidence/100,000 Person-Years | n | Total | Incidence/100,000 Person-Years | ||
| Liver cancer | 137 | 432,781 | 32 | 182 | 23,930 | 761 | 69 | 16,976 | 406 | <0.0001 |
| Liver transplant | 15 | 432,933 | 3 | 69 | 24,036 | 287 | 35 | 16,968 | 206 | <0.0001 |
| All-cause death | 2,496 | 432,987 | 576 | 1,337 | 24,243 | 5,515 | 1,061 | 170,83 | 6,211 | <0.0001 |
- * P values were generated by robust Poisson regression; P < 0.05 was considered significant.
| Outcome, Diabetes Population | No Cirrhosis (n = 22,959) | Compensated Cirrhosis (n = 2,115) | Decompensated Cirrhosis (n = 1,414) | P Value* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Total | Incidence/100,000 Person-Years | n | Total | Incidence/100,000 Person-Years | n | Total | Incidence/100,000 Person Years | ||
| Liver cancer | 79 | 110,426 | 72 | 122 | 10,356 | 1,178 | 45 | 6,895 | 653 | <0.0001 |
| Liver transplant | 7 | 110,523 | 6 | 41 | 10,454 | 392 | 21 | 6,895 | 305 | <0.0001 |
| All-cause death | 1,192 | 110,546 | 1,078 | 698 | 10,572 | 6,602 | 586 | 6,968 | 8,409 | <0.0001 |
- * P values were generated by robust Poisson regression; P < 0.05 was considered significant.
Figure 4 shows the final disposition of the full NAFLD cohort (i.e., mean time to event and annual rates of progression for compensated cirrhosis, decompensated cirrhosis, liver cancer, liver transplant, and death, starting from the three baseline prevalent health states: no cirrhosis, compensated cirrhosis, and decompensated cirrhosis). Among those without cirrhosis at baseline, 0.59% per year developed compensated cirrhosis or decompensated cirrhosis. Among those who had compensated cirrhosis at baseline, 2.4% per year progressed to decompensated cirrhosis.
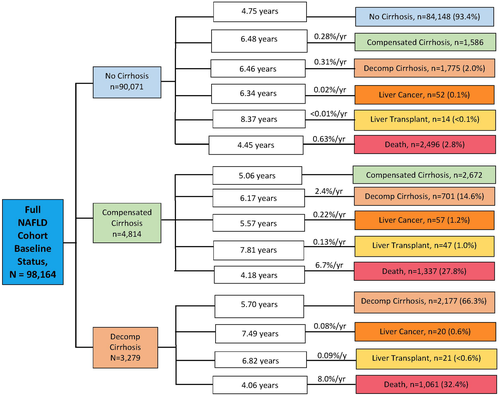
Additionally, we calculated rates of progression over 2 years from cirrhosis to decompensated cirrhosis and from decompensated cirrhosis to the outcomes of liver cancer, liver transplant, and death. The rate of progression over 2 years from no cirrhosis to compensated or decompensated cirrhosis was 2.1%. The rate of progression over 2 years from cirrhosis to decompensated cirrhosis was 15.4%. The rate of progression over 2 years from decompensated cirrhosis to liver cancer was 1.3%, to liver transplant was 0.6%, and to all-cause death was 17.6%.
Discussion
In this retrospective cohort study, using data from a large community-based health care delivery system, we describe the rates of progression from coded diagnoses for patients with NAFLD without cirrhosis and with cirrhosis the outcomes of liver cancer, liver transplant, and all-cause death. Furthermore, we determined the rates of progression to intermediate stages of disease for a period of up to 10 years. Additionally, we conducted a natural history disease progression analysis restricted to patients with NAFLD identified as having diabetes at baseline. We identified a study population (n = 98,164) with an ICD code for NAFLD/NASH from a source population of 1,827,167 (5.4%). This suggests considerable underdiagnosis of this condition, as published epidemiological studies estimate a prevalence from 19% to as high as 46%.(25, 26) A meta-analytic study by Younossi et al. estimated a prevalence of NAFLD in North America of 13%-24%, depending on diagnostic methods used.(1) This may be, at least in part, because no specific ICD-9 code for NASH existed. ICD-10 codes were implemented on October 1, 2015, in the United States; thus, NASH diagnosis codes were not available for almost 8 years during this 10-year study. Furthermore, because most (93.4%) of the study population remained noncirrhotic, they would not have been expected to develop liver-related problems leading to medical care. Additionally, NAFLD is underrecognized in the primary care setting, and patients at risk are often not evaluated for the condition.(27) Our study population consists of a slightly higher percentage of females. This is in contrast to the study by Hashimoto et al,(28) but is consistent with more recently published studies.(29, 30) Consistent with previous reports,(31, 32) noncirrhotic NAFLD is more prevalent in Hispanics and less prevalent in African Americans. This population also includes individuals with simple steatosis to steatohepatitis, as liver biopsies were not evaluated in this large database study. Also consistent with prior studies,(13, 33) obesity (BMI >30) was present in all three baseline NAFLD groups, and the comorbidities analyzed (CHF, CKD, diabetes, and hypertension) all increased with progressive liver disease. For the current study, CHF was analyzed as a surrogate for cardiovascular disease because a robust CHF clinic exists at KPSC, ensuring a reliable capture of this condition. Notably, CHF was seen in nearly 20% of those with decompensated cirrhosis at baseline. An increased risk of heart failure with increasing stage of fibrosis due to NAFLD has also been reported in other recent studies.(22, 34) Progression from no cirrhosis to compensated cirrhosis over a time period of about 3 years in the total population with NAFLD and in the subpopulation with diabetes is 2.5% and 4.1%, respectively. This is in line with the findings of Alexander et al.(20) in their large matched cohort study, which showed that 0.6% of patients with a coded diagnosis of NAFLD/NASH acquire a diagnosis of cirrhosis and/or liver cancer within 3 years, and that diabetes at baseline was the strongest association with incident liver outcomes. Sanyal et al.(35) reported that 3%-4% per year progressed from stage 3 fibrosis to cirrhosis and that liver fibrosis was the strongest determinant of fibrosis progression and of liver-related complications. Thus, we speculate that a large proportion of those without cirrhosis at baseline in the present study had relatively advanced fibrosis. We found that in those with decompensated cirrhosis at baseline, the death rate was 8.0 %/year, which is consistent with the death rate reported in chronic hepatitis C by Fattovich et al.(36) Note that, according to our study design, patients moved only forward in the algorithm (i.e., once a decompensation event occurred, they did not revert to compensated cirrhosis). Thus, those who responded well to medical and procedural management of portal hypertensive bleeding, ascites, and hepatic encephalopathy remained in the decompensated group when stable.
Multivariable Cox regression analysis showed that Hispanics, Asians, and Native Americans had a significantly increased hazard of liver cancer, but that Hispanics and Asians demonstrated a decreased hazard of all-cause death. As this study is an analysis of a population in Southern California, there is a large proportion of Hispanics. The U.S. Census Bureau reports that 39.4% of California’s population is Hispanic-Latino (of any race) as of July 1, 2019 (www.census.gov › quickfacts › CA). Accordingly, California has a higher proportion of Hispanics than the overall U.S. population, which was estimated at approximately 18% in 2019 in the overall U.S. population.(37) However, two states (New Mexico and Texas) have a higher proportion of Hispanics than California, at approximately 49% and 40%, respectively. Because of the large proportion of Hispanics with no cirrhosis at baseline (43%) compared with that of Whites (34%), there is likely an enrichment of associated comorbidities in this population.(38, 39) Specifically diabetes is present in a relatively high proportion of the study population, possibly due to the high number of Hispanics. Of interest is that there are lower proportions of Hispanics with compensated cirrhosis and decompensated cirrhosis at baseline compared with Whites. Hispanics with compensated cirrhosis and decompensated cirrhosis were 32.8% and 29.2%, respectively, versus 49.2% and 47.2% for Whites. This interesting finding may reflect the relatively large proportion of Mexican American ethnicity in Southern California. Previous studies have suggested that Mexican American ethnicity is associated with a higher risk for NAFLD but not necessarily a higher risk of advanced hepatic fibrosis and cirrhosis.(40-42) However, a recent study(43) showed increasing rates of NAFLD-related deaths among Mexican Hispanics and Asian Indians. Future studies are needed to clarify possible underlying genetic and environmental factors to explain these findings, and further study of the current data set is ongoing to investigate liver-related versus non-liver-related causes of death. Consistent with prior studies,(30, 44, 45) diabetes was significantly associated with an increased hazard of both liver cancer and all-cause death, supporting the notion that individuals with diabetes are more likely to suffer worse outcomes of NAFLD as well as be at increased risk for the disease.(8, 33) Notably, the presence of obesity was associated with a decreased hazard of all-cause death (HR = 0.90 [0.84-0.95]). One may speculate that obesity alone (i.e., without other risk factors such as type 2 diabetes) may not confer increased risk, and/or one may speculate that with advancing disease, protection from cachexia may play a role in this finding. We found that the incidence of liver cancer, liver transplant, and all-cause death is up to 2-fold-increased in the subpopulation with diabetes compared with the full NAFLD cohort. An interesting finding is the decrease in rate of liver cancer and liver transplant in those with baseline decompensated cirrhosis compared with compensated cirrhosis for both the subpopulation with diabetes and the full NAFLD cohort. We speculate that a proportion of individuals in these groups died (competing risks) before developing liver cancer or receiving a liver transplant, as all-cause mortality was increased in both groups.
Because of a recent study by Sanyal et al.(35) suggesting that, in a biopsy-proven cohort of patients who participated in two phase 2b clinical trials, the rate of progression from F3 fibrosis to cirrhosis and from cirrhosis to liver-related clinical events was approximately 20% over 2 years, we performed additional calculations to determine the rate of progression over 2 years in our study cohort. Our study showed a rate of progression over 2 years from cirrhosis to decompensated cirrhosis of 15.4% and a rate of progression from decompensated cirrhosis to liver cancer, liver transplant, or death of 19.5%. Although the present study includes patients with a coded diagnosis for NAFLD (i.e., not biopsy-proven staging) and the populations are quite different (community-based vs. tertiary centers), these figures are in line with those reported by Sanyal et al.(35)
One should consider several limitations when interpreting our results. First, because of the retrospective study design, misclassification and underdiagnosis of NAFLD can occur. Contributing to probable underdiagnosis of NASH is the fact that a specific ICD code for NASH did not exist before October 2015. Second, although significant effort was made to exclude alcohol overuse/abuse from the population, coding for alcohol overuse/abuse may have been incomplete. Third, diagnoses of NAFLD were made using clinical information instead of liver biopsy; thus, natural history according to histological progression cannot be assessed. Fourth, many patients came to clinical attention due to complications of NAFLD, which may result in overestimation morbidity and mortality due to NAFLD.
The large data set for analysis is a strength of this study. Extensive chart review was performed to validate the algorithms for NAFLD, cirrhosis, and decompensation, and to maximize data validity. Furthermore, the study cohort is drawn from a large community-based, multispecialty, integrated health care delivery system; thus, it is more generalizable than previously published studies that report data from specific populations or from tertiary care centers (although our study population consists of a higher proportion of Mexican Americans than the general U.S. population).
In summary, our findings contribute to the understanding of the natural history of NAFLD. We were able to identify and quantitate progression pathways for a large population of patients diagnosed with NAFLD. In particular, we found that CHF, CKD, and hypertension were all associated with an increased hazard of all-cause death in this population. Furthermore, diabetes was associated with a significant increase in both the hazard of liver cancer and all-cause death as well as progression of disease. Our results highlight the importance of recognizing associated comorbidities and the need to institute consistent interventions to reduce the public health burden of NAFLD.
Acknowledgment
This publication was made possible by the support of the Southern California Permanente Medical Group and the Department of Research and Evaluation. The authors thank Melanie Balasanian for the manuscript submission and editorial support.