Liver Chemistries in Patients With COVID-19 Who Were Discharged Alive or Died: A Meta-analysis
Supported by the National Natural Science Foundation of China (Grant/Award No. 81871645).
Potential conflict of interest: Nothing to report.
Abstract
Although abnormal liver chemistries are linked to a higher risk of coronavirus disease 2019 (COVID-19)–related death, liver manifestations may be diverse and even confusing. Thus, we performed a meta-analysis of published liver manifestations and described the liver damage in patients with COVID-19 who died or discharged alive. We searched PubMed, Google Scholar, medRxiv, bioRxiv, the Cochrane Library, Embase, and three Chinese electronic databases through April 22, 2020. We analyzed pooled data on liver chemistries stratified by the main clinical outcome of COVID-19, using a fixed or random-effects model. In our meta-analysis of 19 studies, which included a total of 4,103 patients, the pooled mean alanine aminotransferase and aspartate aminotransferase levels were, respectively, 31.7 IU/L and 51.0 IU/L in the patients with COVID-19 who died and 27.7 IU/L and 32.9 IU/L in those discharged alive (both P < 0.0001). Compared with the patients discharged alive, those who died tended to have lower albumin levels but longer prothrombin time and higher international normalized ratio. Conclusion: In this meta-analysis, according to the main clinical outcome of COVID-19, we comprehensively describe three patterns of liver impairment related to COVID-19: hepatocellular injury, cholestasis, and hepatocellular disfunction. The patients who died from COVID-19 tended to have different liver chemistries from those discharged alive. Special caution should be given to the patients with a relatively higher index of liver chemistries.
Abbreviations
-
- ALB
-
- albumin
-
- ALP
-
- alkaline phosphatase
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- CI
-
- confidence intervals
-
- COVID-19
-
- coronavirus disease 2019
-
- GGT
-
- γ-glutamyltransferase
-
- MD
-
- mean difference
-
- MERS
-
- Middle East respiratory syndrome
-
- PT
-
- prothrombin time
-
- SARS
-
- severe acute respiratory syndrome
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2
-
- TBIL
-
- total bilirubin
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a nonsegmented positive-sense RNA virus with an envelope belonging to the family Coronaviridae. The coronavirus is widely distributed in humans and other mammals. In the past 20 years, the coronavirus has caused several localized epidemics and even global pandemics, such as severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and the ongoing COVID-19 pandemic. Worldwide, the spread and upward trend of COVID-19 have accelerated dramatically. According to the situation report released by the World Health Organization (WHO), as of April 25, 2020, 2,719,897 COVID-19 cases were confirmed globally, with a fatality rate of 6.9%.(1) In response to the emerging threat, the WHO declared a Public Health Emergency of International Concern on January 30, 2020, and further labeled the outbreak as a pandemic on March 11, 2020. Compared with the case fatality rates of SARS and MERS, which are 6.8% and 60%, respectively, that of COVID-19 is relatively lower.(2, 3) However, the reproductive number of COVID-19 is even higher than that of SARS.(4) Owing to its huge number of confirmed cases, COVID-19 causes many more deaths than SARS or MERS.
Apart from lung injury, myocardial dysfunction, liver impairment, and acute kidney injury can also result from COVID-19.(5, 6) Chronic hypertension and other cardiovascular comorbidities are frequent risk factors of COVID-19-related death.(6, 7) Other conditions can also contribute to death. According to the observation by Wu et al.,(7) coagulation dysfunction is linked to the likelihood of death. Moreover, univariable logistic regression revealed the association of alanine aminotransferase (ALT) and total bilirubin (TBIL) levels with higher risk of COVID-19-related death.(8) In another study, Fu et al.(9) showed the potential relationship among hypoproteinemia, cholestasis, and higher fatality rate in patients with COVID-19. Our meta-analysis also revealed the different patterns of abnormal liver chemistries between patients with severe and nonsevere COVID-19.(10)
According to the clinical guideline proposed by the American College of Gastroenterology, liver chemistries can be classified into three groups as follows: hepatocellular injury-related indexes, including ALT and aspartate aminotransferase (AST); cholestatic injury-related indexes, including γ-glutamyltransferase (GGT) and alkaline phosphatase (ALP); and hepatocellular function–related indexes such as prothrombin time (PT) and albumin (ALB).(11) In clinical practice, TBIL, direct bilirubin (DBIL), and globulin (GLB) levels, and international normalized ratio (INR) are also assessed. The liver manifestations of COVID-19 are diverse and even confusing. Comprehensive evaluations of liver chemistry abnormalities in patients with COVID-19 are rather few. Hence, the aim of this study was to provide a comprehensive view of liver-test parameters in patients with COVID-19 who died or were discharged alive.
Materials and Methods
Study Selection
The following databases were searched from December 1, 2019, to April 22, 2020: PubMed, Google Scholar, medRxiv, bioRxiv, Embase, the Cochrane Library, and three Chinese electronic databases (Chinese National Knowledge Infrastructure, CQVIP, and Wanfang Data). “COVID-19,” “Coronavirus,” “SARS-CoV-2,” “2019-nCoV-2,” and “novel coronavirus” were used as search keywords. The PRISMA guideline was implemented in the retrieval of potential studies.(12) Details of the search in PubMed are found in the Supporting Information. Endnote X9.3 (Thompson and Reuters, Philadelphia, PA) was used to manage the retrieved articles. Duplicated articles were removed.
Selection Criteria
Two authors (X.D. and Q-Q.X.) independently determined the eligibility of the potential studies. Dissonance was arbitrated by the third author (J-S.P.). The inclusion criteria were as follows: (1) study population: adult patients with COVID-19 who died or were discharged alive; (2) study design: case report, case series, retrospective cohort study, prospective cohort study, randomized controlled trial, and case-control study; and (3) language: studies published in English or Chinese. The exclusion criteria were as follows: (1) pregnant women or pediatric patients; (2) patients who lacked nucleic acid data or serological evidence of SARS-CoV2 infection; (3) asymptomatic patients with SARS-CoV2 infection; (3) study design: commentary, editorial, review article, or meta-analysis; and (4) results: studies that only reported the percentages of the indexes related to liver chemistries rather than the mean or average values, and single-arm studies (e.g., only the data of mortality cases were reported, excluding data from survival cases, and vice versa).
Data Extraction
For the eligible articles, the following items were recorded: first author, study location, sample size, patient age and sex, and liver chemistry–related indexes such as TBIL, DBIL, ALT, AST, GGT, ALP, and ALB levels. The main outcome (either died or discharged alive) of COVID-19 was also recorded.
Data Analysis
R statistical platform version 3.2.3 (R Foundation for Statistical Computing) was used for the statistical analysis. Continuous variables with normal distributions were expressed as mean ± SD, while those with skewed distributions were expressed as median (interquartile range). The method developed by Luo et al.(13) was used to estimate the sample mean and SD of the continuous outcomes from the studies that provided summary data of median, minimum, and maximum values. The related online tool is provided at http://www.math.hkbu.edu.hk/~tongt/papers/median2mean.html. A forest plot was constructed for the 95% confidence intervals (CIs). Heterogeneity among studies was detected using the Cochran Q test, with a P value of <0.10 indicating significant heterogeneity. The proportion of the total variation among the studies, from which heterogeneity was derived, was measured with the I2 statistics. I2 values of <25%, 25%-75%, and >75% represent low, moderate, and high heterogeneity, respectively.(14) A funnel plot was used to evaluate publication bias. A subgroup analysis was performed according to the main clinical outcome: either died or discharged alive.
Results
Characteristics of the Studies Included in the Meta-analysis
The selection process of the potential studies is depicted in Fig. 1. From the 3,033 identified studies, 19 were included in the meta-analysis. The characteristics of the enrolled studies are listed in Table 1.(6-8, 15-29) Information on the study location, sample size, age, sex, clinical outcome, PT, INR, and levels of TBIL, DBIL, ALT, AST, GGT, ALP, ALB, and GLB was recorded. The mean ages of the patients with COVID-19 who were discharged alive and died were 56.4 and 68.7 years, respectively (Supporting Fig. S1). In the enrolled studies, male patients accounted for 55.0% of all patients. Among the studies that reported mortality cases, deaths accounted for 31.6% (range, 11.7%-61.5%) of the cases.
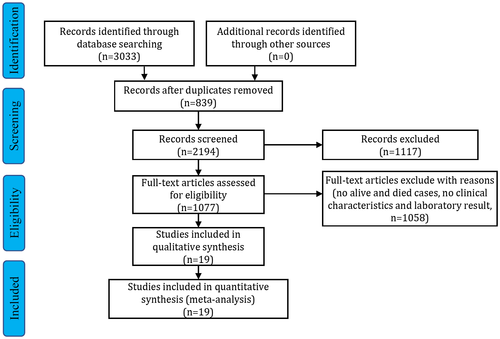
| Study | Location | Number | Age, Mean ± SD or Median (IQR) | Male, n (%) | Died, n (%) | TBIL, Mean ± SD or Median (IQR) | DBIL, Mean ± SD or Median (IQR) | ALT, Mean ± SD or Median (IQR) | AST, Mean ± SD or Median (IQR) |
|---|---|---|---|---|---|---|---|---|---|
| Luo X(21) | Hubei | 403 | 56.0 (39.0-68.0) | 193 (47.9) | 100 (40.3) | 12.8 (10.0-17.5) | NA | NA | NA |
| Chen T(15) | Hubei | 55 | 74.0 (65.0-91.0) | 34 (61.8) | 19 (34.5) | NA | NA | 41.3 (8.0-279.0) | 63.1 (18.0-209.0) |
| Chen T(6) | Hubei | 274 | 62.0 (44.0-70.0) | 171 (62.4) | 113 (41.2) | 9.6 (6.7-13.5) | NA | 23.0 (15.0-38.0) | 30.0 (22.0-46.0) |
| Deng Y(17) | Hubei | 225 | NA | 124 (55.1) | 109 (48.4) | NA | NA | NA | NA |
| Ruan Q(22) | Hubei | 150 | NA | NA | 68 (45.3) | 15.2 ± 9.1 | NA | NA | NA |
| Du RH(18) | Hubei | 179 | 57.6 ± 13.7 | 97 (54.2) | 21 (11.7) | 8.9 (6.6-12.5) | 2.5 (1.8-3.9) | 22.0 (15.0-40.0) | 30.0 (19.0-43.0) |
| Wang L(24) | Hubei | 339 | 69.0 (65.0-76.0) | 166 (49.0) | 65 (19.2) | NA | NA | 27.0 (17.0-44.0) | 32.0 (23.0-46.0) |
| Zhou F(29) | Hubei | 191 | 56.0 (46.0-67.0) | 119 (62.3) | 54 (28.3) | NA | NA | 30.0 (17.0-46.0) | NA |
| Li K(20) | Hubei | 102 | 57.0 (45.0-70.0) | 59 (57.8) | 15 (14.7) | 8.5 (6.6-11.6) | NA | 23.0 (14.0-34.3) | 26.0 (19.0-41.8) |
| Wang Y(25) | Jiangsu | 344 | 64.0 (52.0-72.0) | 179 (52.0) | 133 (38.7) | 10.2 (7.3-14.2) | NA | 24.0 (15.0-38.0) | 31.0 (22.0-47.0) |
| Fu L(8) | Anhui | 200 | 60.0 (48.0-66.0) | 99 (49.5) | 34 (17.0) | 11.4 (9.0-15.6) | 3.3 (2.3-5.0) | 23.5 (16.0-42.0) | 35.0 (23.4-56.8) |
| Zhang F(28) | Hubei | 48 | 70.6 ± 13.4 | 33 (68.8) | 17 (35.4) | NA | NA | 21.0 (12.5-34.0) | 32.0 (22.0-51.8) |
| Yang X(27) | Hubei | 52 | 59.7 ± 13.3 | 35 (67.3) | 32 (61.5) | 17.0 ± 9.9 | NA | NA | NA |
| Wu C(7) | Hubei | 84 | 58.5 (50.0-69.0) | 60 (71.4) | 44 (52.4) | 12.9 (9.5-17.1) | NA | 35.0 (21.5-52.5) | 38.0 (30.5-53.0) |
| Fu YQ(19) | Chongqing | 85 | 64.0 (54.5-70.0) | 49 (57.6) | 14 (16.5) | NA | NA | 29.0 (20.0-55.0) | NA |
| Hu C(16) | Hubei | 183 | NA | 107 (58.5) | 68 (37.2) | 11.3 ± 5.6 | NA | 28.1 ± 20.0 | NA |
| Xu Y(26) | Shanghai | 10 | NA | 6 (60.0) | 2 (20.0) | NA | NA | 24.0 (20.0-39.0 | 26.0 (21.0-33.0) |
| Shi Q(23) | Hubei | 101 | 71.0 (59.0-80.0) | 60 (59.4) | 48 (47.5) | 12.0 (8.4-19.1) | NA | 25.0 (18.0-48.3) | 41.5 (29.0-64.5) |
| Paranjpe I(34) | New York | 1,078 | NA | 627 (51.2) | 310 (28.8) | NA | NA | 34.6 ± 25.8 | 46.2 ± 34.3 |
| Characteristics of the Enrolled Studies for Meta-analysis (Continued) | |||||||||
| Study | Location | Number | GGT, Mean ± SD or Median (IQR) | ALP, Mean ± SD or Median (IQR) | ALB, Mean ± SD or Median (IQR) | GLB, Mean ± SD or Median (IQR) | PT, Mean ± SD or Median (IQR) | INR, Mean ± SD or Median (IQR) |
|---|---|---|---|---|---|---|---|---|
| Luo X(1) | Hubei | 403 | NA | NA | 37.4 (33.3-40.8) | NA | NA | NA |
| Chen T(2) | Hubei | 55 | NA | NA | 33.3 (25.0-43.0) | 30.0 (20.0-48.0) | NA | NA |
| Chen T(3) | Hubei | 274 | 33.0 (21.0-51.0) | 68.0 (55.0-87.0) | 33.9 (30.3-37.6) | NA | 14.3 (13.4-15.4) | 1.1 (1.0-1.2) |
| Deng Y(4) | Hubei | 225 | NA | NA | NA | NA | NA | NA |
| Ruan Q(5) | Hubei | 150 | NA | NA | 30.9 ± 4.3 | NA | 11.1 (10.1-12.4) | NA |
| Du RH(6) | Hubei | 179 | 29.0 (17.0-52.5) | NA | 33.2 (30.7-36.4) | NA | 13.7 (12.4-15.4) | NA |
| Wang L(7) | Hubei | 339 | NA | NA | NA | NA | 12.1 (11.6-12.7) | NA |
| Zhou F(8) | Hubei | 191 | NA | NA | 32.3.0 (29.1-35.8) | NA | 11.6 (10.6-13.0) | NA |
| Li K(9) | Hubei | 102 | NA | NA | 34.8 (31.7-39.5) | NA | 14.2 (13.7-14.8) | 1.08 (1.04-1.15) |
| Wang Y(10) | Jiangsu | 344 | NA | NA | 34.0 (30.0-37.0) | NA | 14.3 (13.5-15.4) | 1.1 (1.0-1.2) |
| Fu L(11) | Anhui | 200 | NA | NA | NA | NA | NA | NA |
| Zhang F(12) | Hubei | 48 | NA | NA | NA | NA | NA | NA |
| Yang X(13) | Hubei | 52 | NA | NA | NA | NA | 12.2 ± 3.0 | NA |
| Wu C(14) | Hubei | 84 | NA | NA | 30.4 (27.2-33.4) | 31.6 (29.4-35.1) | 11.7 (11.1-12.5) | NA |
| Fu YQ(15) | Chongqing | 85 | NA | NA | NA | NA | NA | NA |
| Hu C(16) | Hubei | 183 | 44.1 ± 41.7 | 73.2 ± 30.3 | 33.9 ± 5.6 | NA | 14.5 ± 1.9 | NA |
| Xu Y(17) | Shanghai | 10 | NA | NA | NA | NA | 12.3 (11.7-13.1) | NA |
| Shi Q(18) | Hubei | 101 | NA | NA | 33.0 (30.3-36.4) | NA | 12.8 (12.0-14.0) | NA |
| Paranjpe I(34) | New York | 1,078 | NA | NA | 32.7 ± 5.4 | NA | 14.0 ± 1.5 | NA |
- Abbreviation: NA, not available.
Hepatocellular Injury–Related Liver Chemistry Abnormalities
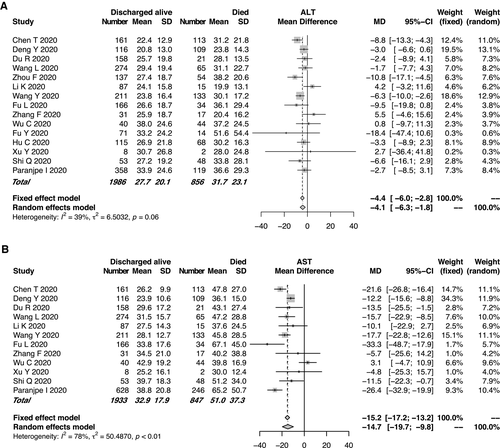
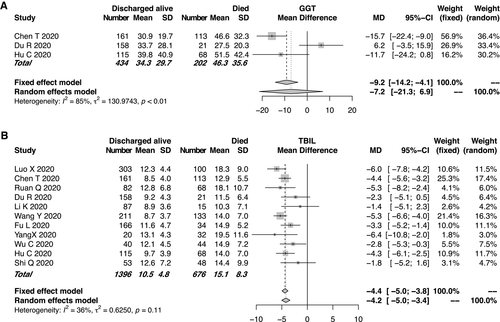
Of the enrolled studies, 15 reported the ALT or AST assays of 2,842 patients with COVID-19. All but one these studies were from China. Of the 19 enrolled studies, 14 (73.7%) were from Hubei Province, where Wuhan is located. The pooled mean ALT level was 31.7 IU/L in the patients with COVID-19 who died and 27.7 IU/L in those discharged alive (95% CI, −6.0 to −2.8, P < 0.0001; Fig. 2A), with moderate heterogeneity among the studies (I2 = 39%, P = 0.06). Similarly, the pooled mean AST level was 51.0 IU/L in the patients who died and 32.9 IU/L in those discharged alive (95% CI: −19.7 to −9.8, P < 0.0001; Fig. 2B). High heterogeneity was observed for the AST levels among the studies (I2 = 78%, P < 0.01), which was significantly higher than that of the ALT levels (I2 = 39%, P = 0.06). Potential publication bias due to ALT/AST levels was evaluated using a funnel plot (Supporting Fig. S2). In the patients with COVID-19, the mean AST level tended to be higher than the mean ALT level in both the mortality and discharged-alive (survival) groups. Moreover, the gaps between the AST and ALT levels (51.0 IU/L and 30.9 IU/L, respectively) were even more significant in the group that died (Fig. 3). The evaluation of potential publication bias is presented in Supporting Fig. S3.
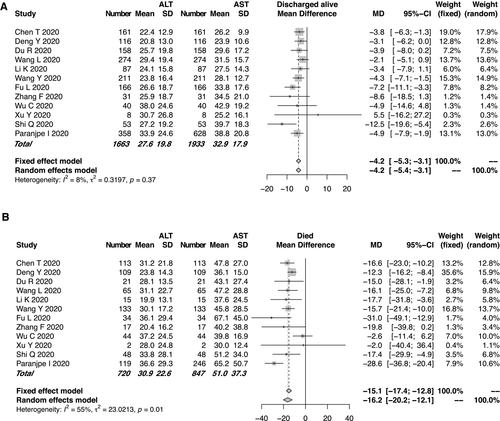
Cholestasis-Related Liver Chemistry Abnormalities
We could not enroll an adequate number of studies for the analyses of the cholestasis-related indexes such as ALP and DBIL levels, as only few studies focused on these indexes. Of the enrolled studies for the meta-analysis, 3 reported GGT assays and 11 reported TBIL measurements. The pooled mean GGT level was 46.3 IU/L in the mortality group and 34.3 IU/L in the group discharged alive (Fig. 4A). The pooled mean TBIL level in the mortality group was slightly higher than that in the group discharged alive. However, the mean TBIL level remained within the normal range in both groups (Fig. 4B). For the TBIL levels, moderate heterogeneity was observed among the studies (I2 = 36%, P = 0.11). The funnel plot of the TBIL levels is shown in Supporting Fig. S4.
Hepatocellular Function–Related Liver Chemistry Abnormalities
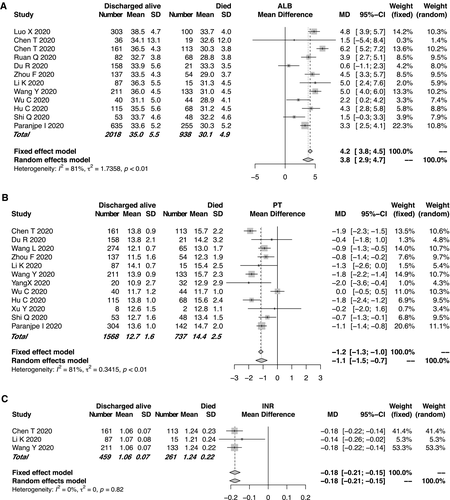
Eleven studies compared the mean ALB levels according to the main clinical outcome of COVID-19, between 938 mortalities and 2,018 cases discharged alive, respectively (Fig. 5A). High heterogeneity was observed among the studies (I2 = 81%, P < 0.01). The mean ALB level in the mortality group was significantly lower than that in the group discharged alive. Significant differences in the coagulation-related indexes such as PT and INR were found between the two groups. The mortality group had longer PT (95% CI: −1.5 to −0.7, P < 0.0001; Fig. 5B) or higher INR (Fig. 5C). The evaluation of publication bias related to ALB level and PT is shown in Supporting Fig. S5. Association between lactate dehydrogenase and clinical outcome is shown in Supporting Fig. S6.
Discussion
In this meta-analysis, all but one of 19 studies from China that consisted of a total of 4,103 patients with COVID-19 were enrolled. Three patterns of liver impairment, namely, hepatocellular injury, cholestasis, and hepatocellular dysfunction, developed in a proportion of patients with COVID-19, especially in those who eventually died. Briefly, the patients who died of COVID-19 tended to have higher baseline ALT/AST levels, TBIL levels, and INR, and prolonged PT but lower ALB levels than those who were discharged alive. The patients with COVID-19 usually had higher AST levels than ALT levels, especially in the group that died. The proportion of deaths tended to be higher in the elderly patients.
The pooled percentage of death cases accounted for up 31.6% of all cases, which was calculated according to the proportion of death cases in each enrolled study. However, this does not mean that the percentage of deaths in the whole population was high, as several reasons may lead to the higher percentages of deaths in the enrolled studies than in the whole population. First, as the patients in the enrolled studies were all hospitalized or even critically ill, the higher percentage of deaths in the enrolled studies than in the whole population is reasonable. Second, the compositions of the enrolled studies differed because of the selection of the reporter. Similarly to our previous study, most studies focused on ALT and AST levels rather than on cholestasis-related indexes such as ALP and GGT levels.(10) The actual prevalence of abnormal liver chemistries could be underestimated in the studies that reported clinical features of COVID-19. Thus, the role of liver chemistries was compromised in disease monitoring and early warning against potential death.
The possible mechanisms of COVID-19-related liver injury include the direct damage caused by SARS-CoV-2 to the liver and the secondary liver injury caused by stress and systemic inflammatory response, liver ischemia or hypoxia, exacerbation of underlying liver disease, or drug-induced liver injury.(30) In hepatitis B surface antigen–positive patients, the following events can also lead to liver injury: sudden cessation of ongoing antiviral treatment in patients with chronic hepatitis B, which may result in sharp deterioration of liver chemistries or acute-on-chronic liver failure, and application of high-dose corticosteroids without antiviral therapy, which may activate the replication of hepatitis B virus. Clinically, secondary liver injury is far more frequent than direct liver injury.(31)
The most frequent complications of COVID-19 in the deceased patients were the following: acute respiratory distress syndrome, sepsis, acute cardiac injury, type I respiratory failure, and heart failure.(6) The percentage of cases that developed acute liver injury was 9% in the deceased patients and only 2% in the recovered patients.(6) An investigation by Deng et al.(17) also indicated high frequencies of developing acute respiratory distress syndrome (89.9%), acute cardiac injury (59.6%), and acute kidney injury (18.3%) in the patients who died. Sepsis and liver ischemia caused by acute respiratory distress syndrome or heart failure can further cause liver chemistry abnormalities or even disordered coagulopathy.
On the whole, the alteration of liver chemistries between the group that died and the group discharged alive is similar to that between the severe and nonsevere groups, as shown in our recent study,(10) although some differences were observed. Also similar was the age difference between the severe and nonsevere groups. In our analysis, the mean age of the group that died was significantly higher than that of the group discharged alive. The mean ALT and AST levels in the mortality group were also higher than those in the group discharged alive. Moreover, both in the group that died and the group discharged alive, the mean AST level was higher than the mean ALT level, similar to our previous study.(10) The GGT level in the group that died was higher than that in the group discharged alive. However, the difference was not statistically significant. This may be due to the underreported GGT levels. The TBIL level in the group that died was also higher than that in the group discharged alive, whereas the mean TBIL levels in both groups were within the normal range. The ALB level in the group that died was significantly lower than that in the group discharged alive, which was in accordance with our study, which compared severe and nonsevere groups.(10) In the group that died, PT and INR were significantly longer and higher, respectively, than in the group discharged alive, with an even more remarkable difference than we previously observed between the severe and nonsevere groups. This suggests that the coagulation function of the group that died was significantly worse than that of the group discharged alive. As almost all coagulation factors are synthesized in the liver, we can speculate that the hepatocellular dysfunction in the group that died was exacerbated under the influence of multiple factors such as hypoxemia, inflammation storm, and infection. In fact, severe sepsis is almost invariably associated with systemic activation of coagulation.(32, 33)
In this study, we found that the liver chemistry abnormalities in the group that died was worse than those in the group discharged alive, which suggests that vigilance is needed to reduce the mortality risk of patients with significantly abnormal liver chemistries on admission. Marked hypoalbuminemia usually developed in the group that died. Thus, solid nutrition support is necessary for this patient group. In addition, for patients with obvious coagulation dysfunction on admission, clinicians must be aware of the risk of progression to severe disease or even death. The coagulation dysfunction must be corrected as early as possible to prevent further deterioration of the patient’s condition.
This study has several substantial merits. First, the enrolled studies focused on COVID-19-related abnormal liver chemistries, which are rapidly evolving and sometimes confusing. This meta-analysis comprehensively summarized the related literature according to the main clinical outcome: either died or discharged alive. The extensive coverage of over 4,000 cases allowed a more precise evaluation of liver chemistry abnormalities in patients with COVID-19. Our analysis revealed that abnormal liver chemistries were associated with worse clinical outcomes and even death, which highlights the importance of a more extensive monitoring of liver chemistries for both diagnostic and prognostic purposes. Second, three patterns of liver impairment, namely, hepatocellular injury, cholestasis, and hepatocellular dysfunction, were all extensively covered in this analysis. However, cholestasis-related indexes such as ALP and GGT levels tended to be inadvertently ignored, even more significantly than in our previous study.(10) We also compared hepatocellular dysfunction between the group that died and the discharged alive group. The alarmingly high prevalence of hypoalbuminemia and coagulation dysfunction in the group that died requires vigilance. Third, we also covered eligible studies preprinted in medRxiv, which ensured that our meta-analysis has a clear leading position.
However, our study has a few limitations. As mentioned previously, cholestasis-related indexes were remarkably underreported, which hindered us from obtaining more precise pooled data. Second, most of the enrolled studies were from mainland China, which restricted a more precise estimation of abnormal liver chemistries in the context of diverse ethnicity. However, this conversely helps to abate the heterogeneity caused by the physiological differences.
In summary, in this analysis we comprehensively described three patterns of liver impairment related to COVID-19, namely, hepatocellular injury, cholestasis, and hepatocellular dysfunction, according to the main clinical outcome of COVID-19. Patients with abnormal liver chemistries are at higher risk of worse outcome. Special caution should be given to the patients with a relatively higher index of liver chemistries.