Urgent Liver Transplantation Soon After Recovery From COVID-19 in a Patient With Decompensated Liver Cirrhosis
Potential conflict of interest: Dr. Romagnoli advises Biotest Italia and consults for Kedrion Biopharma. Dr. Brunetto advises, is on the speakers’ bureau for, and received grants from AbbVie; she is on the speakers’ bureau for and received grants from Gilead; she advises Abbott, Janssen, and Roche; she is on the speakers’ bureau for MSD and BMS. Dr. Lupo received grants from Chiesi; he consults for Astellas and Novartis. The other authors have nothing to report.
TO THE EDITOR:
Italy was the first Western nation to face the corona virus disease of 2019 (COVID-19) outbreak, and all efforts have been taken to preserve liver transplant (LT) activity. Nevertheless, a 25% reduction of procured organs was observed during the first 4 weeks of the epidemic.(1) Little is known about COVID-19 consequences in transplant candidates.
On March 21, 2020, a 39-year-old woman was admitted to our liver unit for decompensated autoimmune cirrhosis (Model for End-Stage Liver Disease [MELD] score 24). She had no comorbidities and was not a smoker. On March 25, she was listed for LT (MELD score 26) with normal chest computed tomography (CT).
On March 30, in the setting of persistent fever, without respiratory symptoms, and with normal chest CT, she tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA on a nasopharyngeal swab (NPS) (DiaSorin Molecular Simplexa COVID-19 Direct Assay System).
She was moved to a COVID-19 ward and was started on hydroxychloroquine for 7 days. In the following days, she developed mild lymphopenia and profound coagulopathy (Fig. 1). On April 2, her oxygen saturation dropped to 85% with an arterial oxygen partial pressure to fractional inspired oxygen ratio of 138 and she required noninvasive ventilation; 24 hours later, her blood gases markedly improved and she was transferred back to the COVID-19 ward with oxygen given by nasal cannula at 2 L/minute. On April 6 and 7, two consecutive SARS-CoV-2 RNA NPSs tested negative, allowing her to be transferred back to our liver unit. On April 7, a negative SARS-CoV-2 real-time polymerase chain reaction (RT-PCR) was confirmed on bronchoalveolar lavage (BAL) fluid and a new chest CT was again negative. Thus, she was reactivated on the LT waiting list (MELD score 36). The day after, an ABO blood type-identical liver from a 46-year-old deceased donor became available and she underwent LT. Immunosuppression consisted of basiliximab, steroids, tacrolimus, and mycophenolate. She made an uneventful recovery and was discharged home on postoperative day (POD) 9. A second SARS-CoV-2 RT-PCR on BAL performed on POD 2 tested negative.
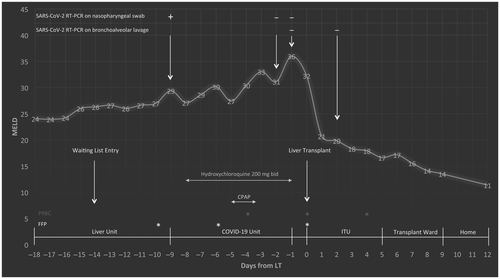
Serology for SARS-CoV-2 infection was performed by testing anti-Spike S1 total antibodies and immunoglobulin M on the day of LT and on POD 7; both samples tested negative, which can be explained by early testing after symptom onset, interference of immunosuppressive treatment on the development of a full humoral response, and a hemodilution effect by multiple transfusions.
In our case, LT appeared as the only way forward and we felt that the expected benefit of a timely LT outweighed the risks linked to the recent COVID-19 infection. The favorable outcome suggests that LT soon after recovery from COVID-19 should be considered as a viable option for candidates with severely compromised liver function.
In conclusion, to the best of our knowledge, this is the first report of an LT candidate recovering from a mild form of COVID-19 and undergoing successful LT shortly after. Aggressive care should be maintained in patients with decompensated cirrhosis who are positive for SARS-CoV-2 in order to overcome viral infection and to proceed as soon as possible with life-saving treatment.