Nonfasted Liver Stiffness Correlates with Liver Disease Parameters and Portal Hypertension in Pediatric Cholestatic Liver Disease
Supported by the National Institute of Diabetes and Digestive and Kidney Diseases (grants DK62436 to Ann and Robert H. Lurie Children's Hospital of Chicago, DK62497 to Cincinnati Children's Hospital Medical Center, DK62453 to Children's Hospital Colorado, DK62481 to Children's Hospital of Philadelphia, DK62466 to Children's Hospital of Pittsburgh, DK62500 to University of California San Francisco (UCSF) Children's Hospital, DK62453 to St. Louis University School of Medicine, DK84536 to Riley Hospital for Children of Indiana University School of Medicine, DK84575 to Seattle Children's Hospital, DK103135 to The Hospital for Sick Children, DK103140 to University of Utah, DK84538 to Children's Hospital Los Angeles, DK062470 to Children's Healthcare of Atlanta, DK103149 to Texas Children's Hospital, DK62456 to Scientific Data Coordinating Center), the National Center for Advancing Translational Sciences (grants UL1TR001422 to Ann and Robert H. Lurie Children's Hospital of Chicago, UL1TR000077 to Cincinnati Children's Hospital Medical Center, UL1TR002535 to Children's Hospital Colorado, UL1TR000005 to Children's Hospital of Pittsburgh, UL1TR000004 to UCSF Children's Hospital, UL1TR001108 to Riley Hospital for Children at Indiana University School of Medicine, UL1TR000423 to Seattle Children's Hospital, UL1TR000130 to Children's Hospital Los Angeles, UL1TR000454 to Children's Healthcare of Atlanta), and King's College Hospital, London (grant XXX).
The study was registered at ClinicalTrials.gov NCT 02922751.
Potential conflict of interest: Dr. Kamath received grants from and consults for Albireo and Mirum. Dr. Karpen consults for Albireo, Intercept, Mirum, Retrophin, and Spruce Bioscience. Dr. Leung received grants from and consults for Gilead; he received grants from AbbVie and consults for Merck. Dr. Loomes received grants from and consults for Albireo and Mirum. Dr. Molleston received grants from AbbVie, Gilead, and Mirum. Dr. Murray consults for Albireo and Gilead. Dr. Rosenthal received grants from and consults for Gilead, AbbVie, Retrophin, Albireo, and Mirum; he received grants from Merck and Arrowhead and consults for Audentes and Dicerna. Dr. Romero received grants from Gilead and Merck. Dr. Sokol consults for Albireo, Retrophin, and Mirum. Dr. Sundaram advises Intercept. The other authors have nothing to report.
Abstract
Elastographic measurement of liver stiffness is of growing importance in the assessment of liver disease. Pediatric experiences with this technique are primarily single center and limited in scope. The Childhood Liver Disease Research Network provided a unique opportunity to assess elastography in a well-characterized multi-institutional cohort. Children with biliary atresia (BA), alpha-1 antitrypsin deficiency (A1ATD), or Alagille syndrome (ALGS) followed in a prospective longitudinal network study were eligible for enrollment in a prospective investigation of transient elastography (FibroScan). Studies were performed in participants who were nonfasted and nonsedated. Liver stiffness measurements (LSMs) were correlated with standard clinical and biochemical parameters of liver disease along with a research definition of clinically evident portal hypertension (CEPH) graded as absent, possible, or definite. Between November 2016 and August 2019, 550 participants with a mean age of 8.8 years were enrolled, 458 of whom had valid LSMs (BA, n = 254; A1ATD, n = 104; ALGS, n = 100). Invalid scans were more common in participants <2 years old. There was a positive correlation between LSM and total bilirubin, international normalized ratio (INR), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT), GGT to platelet ratio (GPR), pediatric end-stage liver disease score, AST to platelet ratio index, and spleen size, and a negative correlation with albumin and platelet count in BA, with similar correlations for A1ATD (except AST, ALT, and albumin) and ALGS (except for INR, GGT, GPR, and ALT). Possible or definite CEPH was more common in BA compared to ALGS and A1ATD. LSM was greater in definite versus absent CEPH in all three diseases. Disease-specific clinical and biochemical characteristics of the different CEPH grades were observed. Conclusion: It is feasible to obtain LSMs in children, especially over the age of 2 years. LSM correlates with liver parameters and portal hypertension, although disease-specific patterns exist.
Abbreviations
-
- A1ATD
-
- alpha-1 antitrypsin deficiency
-
- aCEPH
-
- absent clinically evident portal hypertension
-
- ALGS
-
- Alagille syndrome
-
- APRI
-
- aspartate aminotransferase to platelet ratio index
-
- AST
-
- aspartate aminotransferase
-
- BA
-
- biliary atresia
-
- CEPH
-
- clinically evident portal hypertension
-
- ChiLDReN
-
- Childhood Liver Disease Research Network
-
- CI
-
- confidence interval
-
- dCEPH
-
- definite clinically evident portal hypertension
-
- FORCE
-
- FibroScan in Pediatric Cholestatic Liver Disease
-
- GGT
-
- gamma-glutamyl transpeptidase
-
- GPR
-
- gamma-glutamyl transpeptidase to platelet ratio
-
- INR
-
- international normalized ratio
-
- IQR
-
- interquartile range
-
- LOGIC
-
- Longitudinal Observational Study of Genetic Causes of Intrahepatic Cholestasis
-
- LSM
-
- liver stiffness measurement
-
- pCEPH
-
- possible clinically evident portal hypertension
-
- PELD
-
- pediatric end-stage liver disease
-
- PROBE
-
- Prospective Database of Infants with Cholestasis
-
- TB
-
- total bilirubin
-
- TE
-
- transient elastography
In the last decade, transient elastography (TE) has significantly enhanced clinical monitoring of adults with chronic liver disease, changing the role of and need for liver biopsy. This noninvasive technique, which measures liver stiffness, is particularly useful in differentiating advanced fibrosis on liver biopsy from no or minimal fibrosis in adults.(1) TE is not as well studied in pediatrics, although experience is growing. A meta-analysis of transient and shear wave elastography reports in children with chronic liver disease published before 2017 revealed sensitivity 90%, specificity 79%, and receiver operating characteristic 0.92 for varying definitions of portal hypertension.(2) One of these studies assessed 249 children with cystic fibrosis, while the pediatric studies in biliary atresia (BA) were single center, including up to 73 children.(3, 4) However, there is a pressing need for high-quality multicenter data for TE in children of varying ages. Specific attention should be accorded to the wide spectrum of liver conditions seen in childhood, which are remarkably different from those seen in adulthood.
While the most common liver conditions in adults are fatty liver disease and chronic hepatitis C infection, infants and children often suffer from a range of congenital cholestatic disorders, including BA, alpha-1 antitrypsin deficiency (A1ATD), and Alagille syndrome (ALGS). BA occurs in one in 8,000 to 18,000 live births and is manifest by uniquely rapid progression to advanced fibrosis and subsequent cirrhosis in the first months to years of life.(5) BA is also the most common indication for liver transplantation during early childhood. Although most individuals with A1ATD are asymptomatic, 15% may present with neonatal cholestasis or cirrhosis in childhood or adulthood.(6, 7) ALGS is an autosomal-dominant multisystem disorder with paucity of bile ducts and progressive liver disease; 40% to 75% or more require liver transplantation before adulthood.(8, 9) Noninvasive measurements, such as TE, are an unmet need to assess the progression of liver disease in each of these distinct disorders.
Hepatic fibrosis, cirrhosis, and portal hypertension are common final pathways of a number of pediatric liver diseases. Portal hypertension may occur in the first months of life in BA and presents during early childhood in patients with ALGS and A1ATD. While splenomegaly on physical examination and thrombocytopenia are crude measures of portal hypertension, more precise data would be helpful in assessing the progression of pediatric liver disease over time and in predicting the risk of complications of portal hypertension, including variceal hemorrhage and ascites.(10)
The National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health-sponsored Childhood Liver Disease Research Network (ChiLDReN) is a multicenter consortium that longitudinally follows infants and children with cholestatic liver diseases, including BA, A1ATD, ALGS, progressive familial intrahepatic cholestasis, bile acid synthetic defects, and mitochondrial disorders. Clinical history, physical examination, and laboratory findings are collected annually on participants in ChiLDReN research protocols. Since 2016, the FibroScan in Pediatric Cholestatic Liver Disease (FORCE) study has investigated the use of TE to assess liver stiffness in children with BA, A1ATD, and ALGS. This study provides a unique opportunity to assess liver stiffness in the context of clinical and laboratory markers of liver disease and portal hypertension in a prospective, longitudinal, multicenter approach in both a variety of cholestatic diseases and in a wide range of pediatric age groups.
Participants and Methods
Study Population
Children and young adults 21 years of age or younger were approached and enrolled in FORCE if they had a diagnosis of BA and were an active participant in one of two longitudinal observational studies (Prospective Database of Infants with Cholestasis [PROBE; NCT00061828] or Biliary Atresia Study in Infants and Children [BASIC; NCT00345553]), or had ALGS or A1ATD and were an active participant in the Longitudinal Observational Study of Genetic Causes of Intrahepatic Cholestasis (LOGIC; NCT00571272). Children with known polysplenia/asplenia, situs inversus, clinically significant ascites, an implantable active medical device (such as a pacemaker or defibrillator), an open wound near the FibroScan site, current pregnancy, or who had undergone liver transplantation were not eligible for FORCE.
Written informed consent was obtained from caregivers or the participant, and assent was obtained from the child when appropriate according to local Institutional Review Board (IRB) rules. This study was approved by local IRBs and complied with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Study Design
Liver stiffness (reported in kPa) was measured by vibration-controlled TE in nonfasted and nonsedated participants, using FibroScan according to the manufacturer’s instructions (Echosens, Waltham, MA). The time since last food or nonclear liquid intake was recorded. A valid scan included at least 10 valid measurements using the appropriate probe (S or M) and examination type (S1 or S2) (according to the manufacturer’s instructions) with an interquartile kPa range (IQR)/median value of <30%. The XL probe was not used for FORCE, and as such, participants with a skin to capsule distance >2.5 cm were excluded from continued participation in the study. Spleen excursion below the left costal margin was assessed and measured by physical examination at the time of scanning. Laboratory studies were obtained as part of routine clinical care. Thrombocytopenia was defined as a platelet count <150,000 μL. Aspartate aminotransferase (AST) to platelet ratio index (APRI) and gamma-glutamyl transpeptidase (GGT) to platelet ratio were calculated.(11) Clinically evident portal hypertension (CEPH) was categorized as definite (dCEPH), possible (pCEPH), or absent (aCEPH), using a described research definition.(10)
Statistical Methods
Sample Size Calculation
Enrollment in FORCE was powered to achieve two specific aims. The first aim was to detect a significant difference in liver stiffness in children with BA and CEPH versus those with aCEPH. It was estimated that 49% of participants with BA would have dCEPH while 34% would have aCEPH. Data from Chongrisawat et al.(4) were used to estimate liver stiffness measurements (LSMs) for dCEPH and aCEPH; BA with splenomegaly was equated with dCEPH, while BA without splenomegaly was equated with aCEPH. A sample size of 192 participants with BA was estimated to have >99% power to detect a 12-kPa difference and >80% power to detect a 6.2-kPa difference in LSMs between dCEPH and aCEPH. An additional consideration for sample size determination was based on future investigations directed at detecting an increase between 4 and 5.3 kPa over 2 years in children with BA. Given potential attrition over time, a decision was made to enroll 250 participants with BA who had undergone valid baseline TE. The number of participants with A1ATD and ALGS was a convenience sample based on enrollees in LOGIC.
Analytic Methods
Summary statistics for demographics and conventional laboratory determinants of liver disease were reported for the three diseases. To evaluate the feasibility of performing TE in children with BA, ALGS, and A1ATD, we calculated the proportion and 95% confidence intervals (CIs) of participants with valid (as defined above) LSMs among all participants for whom FibroScan was attempted. Feasibility analyses were based on all enrolled participants. Subsequent analyses were performed only on participants with valid baseline LSMs.
The association of conventional laboratory determinants of liver disease at enrollment was assessed using Spearman correlation coefficients. We then used scatter plots to graphically explore the relationships between LSM and these laboratory values with penalized splines. Multiple linear regression models, including conventional laboratory tests, were further used to assess how much variance in LSM can be explained by these conventional laboratory tests. LSM, GGT, AST, and total bilirubin (TB) were modeled log transformed due to non-normality of the distributions. All analyses were conducted in the three diseases separately.
Additional linear regression models using all combined data were used to study differences in associations between conventional laboratory determinants and LSM among the three different disease types. We studied these conventional laboratory determinants individually. In each linear regression model, the conventional laboratory determinant under investigation, disease types, and the interaction terms between disease types and the conventional laboratory determinant were included as covariates. The differences in associations between conventional laboratory determinants and LSM among disease types were assessed by testing these interaction terms.
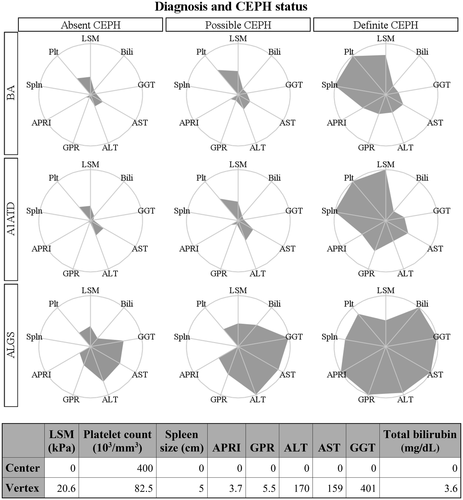
We compared the distribution of LSMs at enrollment between participants with dCEPH and aCEPH in each of the three disease groups. Summary statistics (mean, SD, median, quartiles) and boxplots of LSMs were calculated to inspect the relationships between the distribution of LSMs of the CEPH status groups. The t test was employed for testing the difference in log-transformed LSMs at enrollment between subjects with dCEPHT and those with aCEPHT, as described in our a priori-developed statistical analysis plan. If the primary comparison described above was significant, we compared dCEPH versus pCEPH and pCEPH versus aCEPH using post-hoc comparison in the analysis of variance (ANOVA) test. The intent of this analysis sequence was to reduce type I error. Furthermore, we compared conventional laboratory tests of liver disease among the three diseases by CEPH status using ANOVA and post-hoc comparisons. We produced radar plots (median values of the parameters for each group along the spokes of the circles) to provide a visual representation of the distribution of LSMs and other clinical parameters by diagnosis and CEPH status. Values were scaled so that the center of each radar plot represents more favorable values for the clinical parameters observed among all the groups. The specific values used are shown in a table below the radar plots.
Results
Participants
Between November 2016 and August 2019, 811 of 1,010 active participants with BA in PROBE or BASIC BA, and with ALGS and A1ATD in LOGIC were potentially eligible for FORCE (Fig. 1). A total of 550 participants consented to the study, 458 of whom had a valid baseline TE measurement (BA, 254; A1ATD, 104; ALGS, 100). Demographics of potentially eligible but not enrolled participants were generally similar to those enrolled in FORCE, although enrollment of participants less than 1 year of age was diminished (Supporting Table S1).
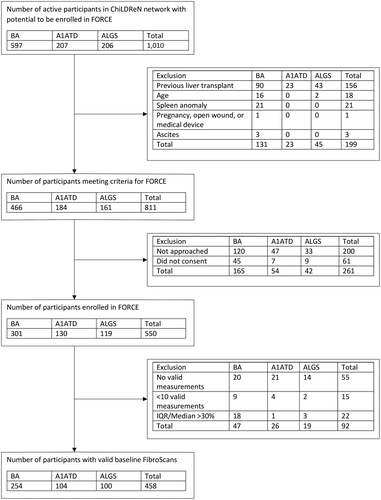
Feasibility of FibroScan
A total of 458 (83.3%; 95% CI, 80.2%-86.4%) of the 550 participants who enrolled in FORCE had a valid TE measurement. Among the 92 participants without a valid scan (Supporting Table S2), 45 studies were invalid due to incorrect probe or examination type selection while 22 were invalid due to an IQR/median >30%. Behavioral issues accounted for 27% of the invalid studies, and these were more frequent in younger participants. The proportion of valid scans by age was 62.3% (95% CI, 49.2%-75.3%), 80.5% (95% CI, 73.4%-87.7%), 85.8% (95% CI, 80.6%-91.0%), and 88.2% (95% CI, 83.7%-92.6%) for <2, 2-5, 5-10, and 10+ years of age, respectively. All subsequent analyses were performed on participants with valid baseline LSMs.
Clinical Characteristics
The mean age of participants with a valid LSM was 8.8 (SD, 4.9) years, 48% were female participants, 67% were white, and 18% were Hispanic (Supporting Table S3). Biochemical testing as part of routine clinical practice was performed in >90% of participants (Table 1). The cohort overall had preserved hepatic synthetic function with only a small portion with serum albumin <3.0 g/dL; international normalized ratio (INR) was primarily normal, and pediatric end-stage liver disease (PELD) scores were typically <0. TB, GGT, ALT, and AST were highest in ALGS participants, consistent with the more profound cholestasis in this group. There were greater numbers of participants with thrombocytopenia in the BA cohort. APRI distribution was similar in ALGS and BA, while it was less remarkable in A1ATD. GGT to platelet ratio was highest in ALGS.
| Characteristic | BA (n = 254) | A1ATD (n = 104) | ALGS (n = 100) | All diagnoses (n = 458) |
|---|---|---|---|---|
| TB, n | 240 | 103 | 97 | 440 |
| Mean, mg/dL (SD) | 1.0 (1.3) | 0.6 (0.7) | 3.0 (4.7) | 1.3 (2.6) |
| Median, mg/dL (Q1, Q3) | 0.6 (0.4, 1.0) | 0.4 (0.3, 0.6) | 1.1 (0.6, 3.0) | 0.6 (0.4, 1.1) |
| GGT, n | 223 | 90 | 84 | 397 |
| Mean, mg/dL (SD) | 140.5 (190.5) | 65.5 (102.0) | 451.0 (400.7) | 189.6 (275.1) |
| Median, mg/dL (Q1, Q3) | 74.0 (26.0, 172.0) | 27.0 (17.0, 53.0) | 326.5 (171.5, 609.0) | 77.0 (26.0, 234.0) |
| AST, n | 241 | 103 | 97 | 441 |
| Mean, mg/dL (SD) | 78 (71) | 60 (42) | 155 (120) | 91 (87) |
| Median, mg/dL (Q1, Q3) | 51 (34, 92) | 48 (31, 76) | 111 (74, 189) | 59 (36, 109) |
| ALT, n | 241 | 103 | 96 | 440 |
| Mean, mg/dL (SD) | 81 (84) | 73 (57) | 174 (125) | 99 (98) |
| Median, mg/dL (Q1, Q3) | 51 (30, 94) | 59 (36, 85) | 143 (89, 248) | 68 (36, 122) |
| Albumin, n | 236 | 99 | 96 | 431 |
| Mean, g/dL (SD) | 4.2 (0.5) | 4.4 (0.4) | 4.2 (0.5) | 4.2 (0.5) |
| n (%) <3.0 | 4 (2%) | 1 (1%) | 3 (3%) | 8 (2%) |
| n (%) <2.5 | 0 (0%) | 0 (0%) | 1 (1%) | 1 (0%) |
| n (%) <2.0 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| INR, n | 204 | 77 | 84 | 365 |
| Mean, g/dL (SD) | 1.1 (0.1) | 1.1 (0.1) | 1.1 (0.1) | 1.1 (0.1) |
| GPR, n | 222 | 87 | 82 | 391 |
| Mean, g/dL (SD) | 2.6 (4.4) | 0.9 (1.9) | 4.6 (7.9) | 2.6 (5.2) |
| Median, g/dL (Q1, Q3) | 1.0 (0.3, 2.9) | 0.2 (0.1, 0.5) | 2.7 (1.3, 5.6) | 0.9 (0.2, 3.0) |
| Platelet count, n | 247 | 97 | 95 | 439 |
| Mean (SD) | 173 (104) | 273 (102) | 262 (100) | 214 (113) |
| n (%) <150 | 118 (48%) | 10 (10%) | 12 (13%) | 140 (32%) |
| n (%) <100 | 84 (34%) | 6 (6%) | 5 (5%) | 95 (22%) |
| n (%) <50 | 18 (7%) | 3 (3%) | 1 (1%) | 22 (5%) |
| APRI, n | 239 | 97 | 95 | 431 |
| Mean (SD) | 1.9 (2.5) | 0.8 (1.1) | 2.2 (4.4) | 1.7 (2.9) |
| Median (Q1, Q3) | 0.9 (0.4, 2.3) | 0.4 (0.3, 0.8) | 1.2 (0.7, 2.6) | 0.8 (0.4, 1.9) |
| n (%) >1.0 | 109 (46%) | 18 (19%) | 54 (57%) | 181 (42%) |
| n (%) >1.5 | 86 (36%) | 13 (13%) | 35 (37%) | 134 (31%) |
| n (%) >2.0 | 68 (29%) | 8 (8%) | 27 (28%) | 103 (24%) |
| PELD score, n | 193 | 75 | 83 | 351 |
| Mean (SD) | −9.3 (5.7) | −12.1 (5.4) | −4.3 (8.4) | −8.7 (6.9) |
| Median (Q1, Q3) | −10.5 (−13.0, −5.7) | −13.1 (−15.8, −10.3) | −6.6 (−11.1, 0.8) | −10.4 (−13.4, −5.2) |
| n (%) >0 | 13 (7%) | 3 (4%) | 24 (29%) | 40 (11%) |
| n (%) >10 | 1 (1%) | 0 (0%) | 6 (7%) | 7 (2%) |
| n (%) >20 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Correlation of LSM with Biochemical Characteristics of Liver Disease
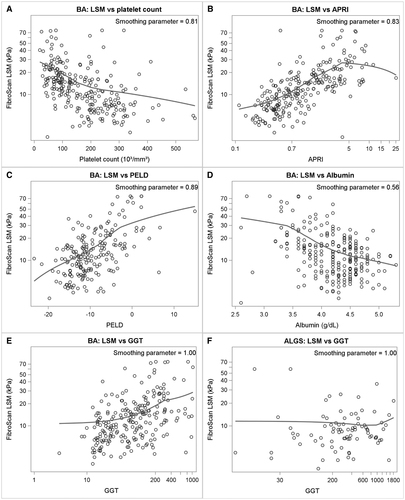
Each parameter was examined for its correlation with LSM in the context of the three diseases. In BA, there was a positive correlation between LSM and TB, INR, AST, ALT, GGT, GPR, PELD score, APRI, and spleen size and a negative correlation with albumin and platelet count (Fig. 2; Supporting Table S4). The negative relationship between platelet count and LSM in BA persisted even with platelet counts in the “normal” range (i.e., >150,000; P = 0.025) (Supporting Fig. S1). Similar correlations existed for A1ATD (except AST, ALT, and albumin) and for ALGS (except for INR, GGT, GPR, and ALT).
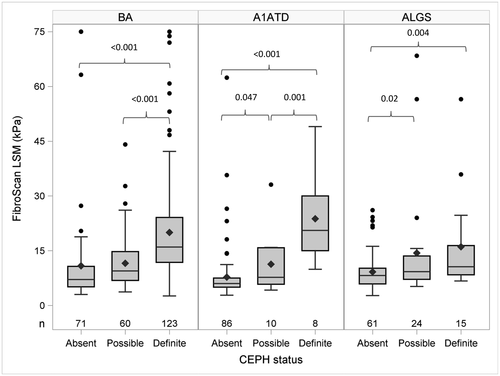
Association of LSM with CEPH
The relationship between LSM and portal hypertension was investigated using the recently developed research definition of CEPH.(10) dCEPH was more common in BA (48%) than in A1ATD (8%) or ALGS (15%) (Fig. 3; Supporting Tables S5 and S6). LSM was greater in dCEPH compared to aCEPH for all three diseases (dCEPH vs. aCEPH LSM kPa: mean (+SD) BA, 20.0 (13.6) vs. 10.8 (13.5), P < 0.001; mean A1ATD, 23.8 (12.5) vs. 7.8 (7.7), P < 0.001; mean ALGS, 16.1 (13.6) vs. 9.2 (5.3), P = 0.003). In BA LSM, pCEPH was lower than in dCEPH but not different from aCEPH. In contrast, in ALGS LSM, pCEPH was higher than aCEPH but not different from dCEPH. In A1ATD LSM, pCEPH was lower than dCEPH and higher than aCEPH. Among participants with the same CEPH status, the clinical, biochemical, and LSM varied by disease type. In aCEPH, LSM was higher in BA (Table 2). Participants with ALGS with aCEPH had higher TB, GGT, ALT, and AST levels. Participants with A1ATD with aCEPH had the lowest LSM, GGT, AST, and PELD score. These parameters were similar for BA and A1ATD with pCEPH (Supporting Table S7). Measurements of TB, GGT, AST, ALT, APRI, and PELD score were also higher in participants with ALGS with pCEPH. A complex picture emerged in comparing these parameters for dCEPH (Table 3). Spleen excursion below the costal margin was similar for all three diseases. LSM was higher and platelet count lower in BA and A1ATD compared to ALGS. However, TB, GGT, AST, ALT, and PELD score in ALGS were higher than in BA and A1ATD. The differences in parameters by disease type and CEPH status are summarized in the radar plot in Fig. 4. The number of hours of fasting did not impact the LSM among participants with the same disease type and CEPH status (Supporting Fig. S2; Supporting Table S8).
| Parameter | Mean (SD) | P Value for Differences in Means | |||||
|---|---|---|---|---|---|---|---|
| BA (n = 71) | A1ATD (n = 86) | ALGS (n = 61) | Overall Difference | BA vs. A1ATD | BA vs. ALGS | A1ATD vs. ALGS | |
| FibroScan LSM, kPa | 10.8 (13.5) | 7.8 (7.7) | 9.2 (5.3) | 0.02 | 0.01 | 0.95 | 0.02 |
| TB, mg/dL | 0.6 (0.6) | 0.6 (0.7) | 1.8 (2.5) | <0.001 | 0.66 | <0.001 | <0.001 |
| GGT, mg/dL | 149 (250) | 57 (105) | 418 (423) | <0.001 | 0.001 | <0.001 | <0.001 |
| AST, mg/dL | 66 (60) | 59 (44) | 142 (132) | <0.001 | 0.64 | <0.001 | <0.001 |
| ALT, mg/dL | 72 (89) | 73 (59) | 161 (140) | <0.001 | 0.21 | <0.001 | <0.001 |
| Albumin, g/dL | 4.3 (0.5) | 4.4 (0.4) | 4.3 (0.5) | 0.33 | |||
| INR, g/dL | 1.1 (0.1) | 1.1 (0.1) | 1.0 (0.1) | 0.05 | |||
| GPR, g/dL | 1.2 (2.2) | 0.4 (0.9) | 3.0 (3.1) | <0.001 | <0.001 | <0.001 | <0.001 |
| Platelet count | 279 (90) | 298 (84) | 296 (85) | 0.34 | |||
| APRI | 0.6 (0.6) | 0.6 (0.6) | 1.2 (1.0) | <0.001 | 0.33 | <0.001 | <0.001 |
| PELD score | −12.1 (4.8) | −13.1 (4.8) | −7.0 (7.0) | <0.001 | 0.36 | <0.001 | <0.001 |
| Spleen size (cm below costal margin) | 0.1 (0.3) | 0.1 (0.4) | 0.0 (0.2) | 0.49 | |||
| Parameter | Mean (SD) | P Value for Differences in Means | |||||
|---|---|---|---|---|---|---|---|
| BA (n = 123) | A1ATD (n = 8) | ALGS (n = 15) | Overall Difference | BA vs. A1ATD | BA vs. ALGS | A1ATD vs. ALGS | |
| FibroScan LSM, kPa | 20.0 (13.6) | 23.8 (12.5) | 16.1 (13.6) | 0.14 | |||
| TB, mg/dL | 1.3 (1.5) | 0.8 (0.4) | 6.4 (8.6) | <0.001 | 0.59 | <0.001 | <0.001 |
| GGT, mg/dL | 150 (176) | 149 (72) | 458 (311) | <0.001 | 0.29 | <0.001 | 0.049 |
| AST, mg/dL | 87 (76) | 78 (33) | 181 (122) | 0.001 | 0.80 | <0.001 | 0.03 |
| ALT, mg/dL | 84 (81) | 86 (60) | 178 (91) | <0.001 | 0.59 | <0.001 | 0.02 |
| Albumin, g/dL | 4.1 (0.5) | 4.0 (0.6) | 3.9 (0.8) | 0.63 | |||
| Platelet count | 96 (55) | 88 (42) | 149 (70) | 0.004 | 0.67 | 0.001 | 0.01 |
| APRI | 3.0 (3.2) | 2.7 (2.0) | 4.0 (3.1) | 0.50 | |||
| PELD score | −6.8 (5.8) | −7.0 (6.0) | 0.6 (10.0) | <0.001 | 0.93 | <0.001 | 0.01 |
| Spleen size (cm below costal margin) | 5.3 (4.0) | 5.6 (4.5) | 5.0 (4.7) | 0.93 | |||
Modeling of LSM by Disease Type
Multiple linear regression analysis showed that conventional determinants of liver disease, including TB, platelet count, spleen excursion below the costal margin, GGT, AST, and albumin could explain 55%, 60%, and 47% of the variability of LSM in BA, A1ATD, and ALGS, respectively. The impact of these parameters on LSM varied by disease (Table 4). The relationship between these parameters and LSM in BA and A1ATD is largely similar, whereas there are significant differences for TB, platelet count, and GGT in ALGS. Albumin had a consistent impact on LSM for all three diseases. As albumin decreased, there was a significant increase in LSM.
| Factor | Multiplicative Estimate for LSM (95% CI) | Tests for Differences Between Estimates | ||||
|---|---|---|---|---|---|---|
| BA | A1ATD | ALGS | BA vs. A1ATD | BA vs. ALGS | A1ATD vs. ALGS | |
| For every doubling of bilirubin | 1.33 (1.25, 1.41) | 1.31 (1.17, 1.46) | 1.18 (1.10, 1.26) | 0.75 | 0.007 | 0.11 |
| For every 50,000 decrease in platelet count | 1.17 (1.13, 1.22) | 1.14 (1.08, 1.21) | 1.12 (1.06, 1.19) | 0.39 | 0.17 | 0.66 |
| For every 1-cm increase in spleen size below costal margin | 1.08 (1.06, 1.11) | 1.11 (1.06, 1.16) | 1.06 (1.02, 1.11) | 0.06 | 0.21 | 0.01 |
| For every doubling of GGT | 1.21 (1.16, 1.27) | 1.34 (1.24, 1.46) | 1.01 (0.94, 1.09) | 0.04 | <0.001 | <0.001 |
| For every doubling of AST | 1.42 (1.32, 1.52) | 1.24 (1.10, 1.41) | 1.14 (1.03, 1.27) | 0.08 | 0.001 | 0.32 |
| For every 1-unit decrease in albumin | 1.74 (1.48, 2.03) | 1.48 (1.11, 1.96) | 1.49 (1.19, 1.86) | 0.33 | 0.27 | 0.96 |
Discussion
The baseline results of FORCE represent one of the largest prospective studies of TE in children with chronic liver disease. The vast majority of published analyses of TE in children are single-center studies. The largest experiences focus on healthy children, providing key information on technical details of the application of this important technique in pediatrics.(12-14) The largest TE studies in children with liver disease have examined LSMs in liver transplant recipients (n = 117), cystic fibrosis (n = 249), hepatitis C (n = 223), fatty liver disease (n = 106), and BA (n = 100).(3, 15-18) These studies correlate LSM with a variety of disease parameters, including clinical features of liver disease, manifestations of portal hypertension, and histologic assessment of fibrosis. The FORCE protocol is unique in that it has been developed in the context of three large-scale, prospective, multicenter, observational studies that collected comprehensive and high-quality clinical metadata in three major etiologies of neonatal cholestasis: BA, ALGS, and A1ATD.
One key purpose of FORCE was to confirm the feasibility of measuring LSM in children with chronic liver disease of all ages. Requirements for fasting and sedation were eliminated in FORCE so the findings could be generalized to routine clinical practice. LSM may be higher in the nonfasted state and under general anesthesia.(13) The difference noted for fasting, while statistically significant, may not be clinically significant especially at the levels generally observed in children with chronic cholestasis. In this study, there did not appear to be a strong effect of fasting on LSM. Valid scans are obtained in ~90% of other TE studies performed in children. It has been consistently reported that these studies are more difficult to perform in children under the age of 2 years, which is not unexpected given behavioral challenges and lack of cooperation with the examination in children at this age. Our findings corroborate this observation. During the conduct of FORCE, two technical issues were identified and corrected; this has led to an increase in the rate of successful scans as the study has moved forward. In real-time, the IQR/median is estimated but may be higher once the examination is finalized. This may lead to studies in which borderline IQR/median levels (25%-30%) during the examination result in final IQR/median levels greater than 30%, leading to an invalid scan. In FORCE, we developed IQR/median targets of <27% to avoid this issue. Care also needs to be taken to select the correct probe (M vs. S) and examination type (S1 vs. S2) based on the thoracic perimeter measurement. The S1 examination is typically only applicable to children <2 years of age. Within our study, FibroScan-based TE measurements are feasible in most children.
The cohort of children who participated in FORCE is representative of patients with liver disease seen in the pediatric outpatient setting. This is not an inception cohort and as such does not necessarily reflect the full range of disease severity associated with these disorders. With an inception cohort, patients with BA and A1ATD who have persistent cholestasis in infancy or complications of advanced liver disease are likely to undergo liver transplantation in the first 2 years of life and will be underrepresented in this type of cohort. Significant ascites precludes an accurate TE examination. The clinical characteristics of participants with BA, A1ATD, and ALGS are distinct (Table 1; Fig. 1). Most had compensated liver disease with very few having significant hypoalbuminemia or coagulopathy. Not surprisingly, PELD scores were generally negative. Importantly, features of portal hypertension were more commonly found in children with BA, of whom nearly 50% had thrombocytopenia and/or CEPH. In contrast, only a minority of participants with ALGS or A1ATD had thrombocytopenia. Interestingly, CEPH was more common in ALGS, even without thrombocytopenia, which raises questions about either the utility of CEPH or the sensitivity of thrombocytopenia as a feature of portal hypertension in this disease. ALGS frequently manifests profound cholestasis, a fact that is supported by higher TB and GGT in enrolled participants with ALGS. It appears that hepatocellular injury or inflammation was commensurately increased in ALGS as both ALT and AST levels were higher in these children.
The purpose of this study was not to use LSM to predict the presence or absence of portal hypertension, which can potentially be assessed using simpler techniques. Rather, the primary goal in FORCE was to determine if LSM was different in children with portal hypertension relative to those without portal hypertension. No attempt was made to correlate LSM with histologic findings of fibrosis, primarily because liver biopsy was not a feasible study procedure and in part due to inherent issues with needle-based biopsy approaches to histologic quantification of fibrosis.(19) Based on prior studies, we postulated that LSMs would be significantly higher in those with portal hypertension.(4) Portal hypertension is rarely quantified in pediatric liver disease. Some publications have used esophageal varices as a marker of portal hypertension; however, the clinical sites in ChiLDReN do not routinely screen for varices, so this marker of portal hypertension could not be used as has been done in other pediatric studies.(20) In light of these issues, the described research definition of CEPH was used as a validation for the potential utility of LSM in pediatric cholestasis.(10)
We found statistically and clinically relevant differences in LSMs in all three diseases in children with dCEPH versus aCEPH. In A1ATD, there were clear step-wise increases in LSMs based on progressive CEPH. Outside of the neonatal period, A1ATD may act more like a hepatocellular disease rather than one that is primarily cholestatic, like ALGS. In addition, there are likely a significant number of children with A1ATD-related liver disease who have minimal hepatic fibrosis, distinct from BA and ALGS. Thus, TE in children with A1ATD who had aCEPH resembled normal children, more than the children with BA and ALGS. BA is a profoundly fibrotic disease even in those individuals who are doing well and who are not apparently cholestatic. It is likely that children with BA and aCEPH may still have advanced fibrosis or compensated cirrhosis. This is demonstrated by minimal differences in LSMs in aCEPH versus pCEPH in BA. In contrast, there was a significant increase in LSM in children with dCEPH compared to pCEPH, suggesting that dCEPH is indicative of a more advanced stage of disease in BA. The performance of elastography related to CEPH in ALGS is less certain and more complicated. While significant differences were observed in LSMs between aCEPH and dCEPH among those with ALGS, the magnitude of the difference was less. There is limited longitudinal assessment of hepatic fibrosis in ALGS, and as such, continued investigations of fibrosis in ALGS are warranted.
This is the first study to formally apply CEPH definitions in a rigorous research protocol. The radar plots in Fig. 4 depict the impact of various clinical parameters among the different liver diseases and categories of CEPH. There are many similarities in the clinical parameters between BA and A1ATD, but ALGS is uniquely different. Specific comparisons of liver diseases among the same CEPH status are instructive. In dCEPH, spleen excursion below the costal margin is similar for the three diseases as are LSMs, although LSM values are highest in A1ATD. The clinical characteristics of the ALGS participants with dCEPH were clearly distinctive with higher TB, GGT, ALT, AST, and platelet counts. The latter finding is intriguing in the context of splenomegaly and increased LSMs. The comparisons in aCEPH are also enlightening among ALGS. By definition, splenomegaly and thrombocytopenia are not present in this group. LSM differs amongst the diseases, with the lowest value in A1ATD and similar values for BA and ALGS. This may suggest increased normality of the liver in A1ATD and raises the important issue of the impact of degree of cholestasis on LSM relative to the other two diseases. As with dCEPH, liver tests are more elevated in ALGS.
The correlation of liver biochemistries and LSM demonstrate many consistent relationships but also highlight notable differences among the diseases. There was a strong inverse correlation between TB and LSM in all three diseases. Features of portal hypertension, including spleen excursion below the costal margin and platelet count, also correlated with LSM. Despite obvious limitations of physical examination as an assessment of spleen size, positive correlation with LSM was still present. It is not surprising that LSM was inversely correlated with platelet count. It is, however, surprising that this correlation extended into the normal range of platelet counts. This suggests that the normal range is more statistical rather than biological. Platelet counts in the low-normal range may be significant in chronic liver disease, and trends in platelet counts over time may be relevant in staging disease. There were some inconsistencies in relationships between laboratory parameters and LSM in different diseases. Most notably, GGT and ALT levels did not correlate with LSM in ALGS. This may be a reflection of the highly cholestatic nature of ALGS. The difference of disease type is further reflected in the modeling done in the current analysis. The impacts of TB, GGT, and AST on LSM were each significantly greater in BA compared to ALGS. Differences in impact of these parameters in BA versus A1ATD were infrequent or subtle. Decreased serum albumin levels also had a significant effect on LSM in all three diseases; thus, albumin may be a very important parameter to follow in pediatric chronic liver disease.
The current analysis of FORCE assesses baseline measurements. FORCE is a prospective longitudinal study with proposed follow-up LSM at 1 and 2 years. Future investigations will assess change in LSM over time and the correlation of baseline and change in LSM with clinical events identified in the three on-going prospective longitudinal ChiLDReN studies.
LSM can be performed using a variety of techniques, including TE. The larger area of liver assessed by these techniques relative to needle biopsy enhances the potential accuracy of this approach relative to biopsy. FibroScan-based LSM in children is noninvasive and feasible, although there is a critical need to attend to technical details of the measurements. Its use in unsedated children under the age of 2 years may be limited. These studies have confirmed in a large cohort of children with three distinct types of liver disease that LSM correlates with features of portal hypertension and routine clinical laboratory assessments of liver disease in children. LSM may be influenced by the type of liver disease in ways that are not clear at present, warranting careful interpretation of results and further investigation.




