Endoscopic Ultrasound/Fine Needle Aspiration Is Effective for Lymph Node Staging in Patients With Cholangiocarcinoma
Abstract
Background and Aims
Presence of malignant regional lymph nodes (MRLNs) precludes curative oncological resection or liver transplantation for cholangiocarcinoma (CCA). Limited data support the utility of endoscopic ultrasound (EUS)/fine needle aspiration (FNA) for detection of MRLNs in extrahepatic CCA, but there are no data for its role in intrahepatic CCA (iCCA). The aim of this study is to evaluate the staging impact of EUS for CCA, including analysis by subtype.
Approach and Results
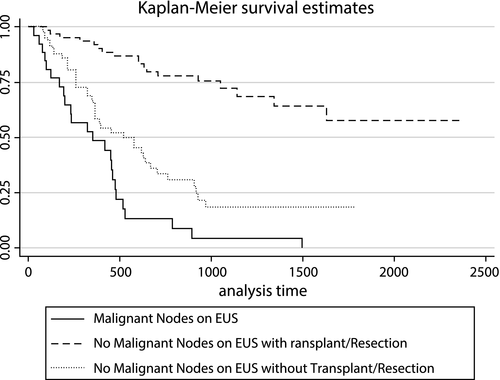
We identified consecutive patients with CCA who underwent EUS staging at a single tertiary care center from October 2014 to April 2018. Among this cohort, we abstracted clinical demographic, radiographical, procedural, cytopathological, and surgical data. STATA 15 software was used for comparative analysis calculations (StataCorp LP, College Station, TX). The study cohort included 157 patients; 24 (15%), 124 (79%), and 9 (6%) with intrahepatic, perihilar, and distal CCA, respectively. EUS was able to identify regional lymph nodes (RLNs) in a higher percentage of patients compared to cross-sectional imaging (86% vs. 47%; P < 0.001). FNA was performed in 133 (98.5%) patients with RLNs, with a median of three passes per node. EUS-FNA identified MRLN in 27 of 31 (87.1%) patients ultimately found to have MRLNs. For iCCA, EUS detected a higher percentage of RLN compared to cross-sectional imaging (83% vs. 50%; P = 0.048), with MRLNs identified in 4 (17%) patients. Among the entire cohort, identification of at least one MRLN by EUS was associated with lower median survival (353 vs. 1,050 days; P < 0.001) and increased risk of death (hazard ratio = 4.1; P < 0.001).
Conclusions
EUS-FNA is effective for identifying MRLN in patients with CCA, and should be routinely incorporated into staging of all CCA subtypes given the impact of MRLN on prognosis and management decisions.
Abbreviations
-
- CCA
-
- cholangiocarcinoma
-
- CT
-
- computed tomography
-
- dCCA
-
- distal CCA
-
- eCCA
-
- extrahepatic CCA
-
- EUS
-
- endoscopic ultrasound
-
- FNA
-
- fine needle aspiration
-
- iCCA
-
- intrahepatic CCA
-
- IQR
-
- interquartile range
-
- MRI
-
- magnetic resonance imaging
-
- MRLN
-
- malignant regional lymph node
-
- pCCA
-
- perihilar CCA
-
- PET
-
- positron emission tomography
-
- PSC
-
- primary sclerosing cholangitis
-
- RLN
-
- regional lymph node
-
- ROSE
-
- rapid on-site evaluation
Cholangiocarcinoma (CCA) is a malignancy arising from epithelial cells of the biliary tree. Annual incidence in the United States ranges from 0.6 per 100,000 to 1.0 per 100,000.1-6 Although relatively uncommon, CCA carries a very poor prognosis. Oncological surgical resection and liver transplantation remain the only curative therapies for CCA.6 As our understanding of CCA has evolved, a great deal has changed with regard to the management of CCA in recent years. CCA is now classified into three subtypes: intrahepatic, perihilar, and distal, with unique American Joint Committee on Cancer (AJCC) criteria among each subtype. Liver transplantation has become an accepted treatment option for select patients. Regional lymph node (RLN) metastasis and margin status are the most important determinants of postsurgical outcomes. Identification of malignant regional lymph nodes (MRLNs) in surgical pathology specimens is a strong predictor of poor postsurgical outcomes, with 3- and 5-year survival of 9% and 0%, respectively, regardless of the site of involvement.7-10 Thus, although staging criteria differ across the three subtypes of CCA, malignant lymph node involvement largely prohibits curative surgical resection or transplantation.6, 11 Computed tomography (CT) and magnetic resonance imaging (MRI) are routinely obtained as part of the initial staging of CCA and can readily identify overt advanced disease or distant metastasis. These modalities may also identify regional lymphadenopathy and can provide information regarding likelihood for malignant involvement, using characteristics such as size, morphology, and anatomical location.12, 13 Unfortunately, with accuracy ranging from 24% to 84% for CT and estimated to be 66% for MRI, neither CT nor MRI alone can be relied upon to definitively assess for regional lymphadenopathy.14-16 Positron emission tomography (PET) imaging may be used in addition to CT and MRI and is particularly useful for identifying unsuspected distant metastasis not observed on CT or MRI. However, in limited studies, PET imaging has been shown to have a very poor sensitivity for identifying MRLNs in patients found to have abdominal lymphadenopathy on initial CT scanning.17-20
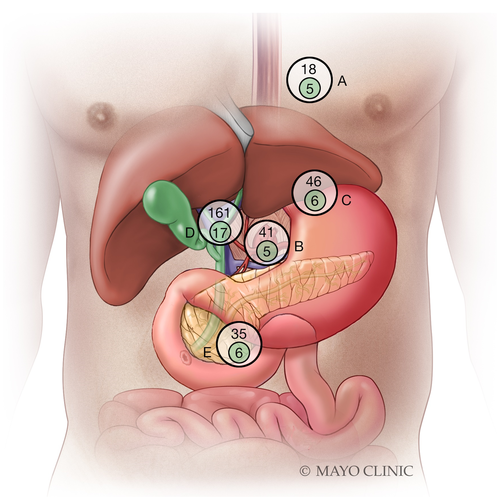
Endoscopic ultrasound (EUS) is a highly effective and minimally invasive means by which to identify and sample RLNs with fine needle aspiration (FNA) before committing patients to the morbidity and cost of neoadjuvant chemoradiation and staging laparotomy (Fig. 1).20 Previous limited data have supported the utility of EUS-FNA in identifying MRLNs for improved staging of extrahepatic CCA (eCCA).21-25 In an earlier study of 47 patients with unresectable CCA being considered for liver transplantation at our institution, 17% were found to have MRLNs by EUS with FNA, thereby precluding them from transplantation without the need for staging laparotomy.21

Although available data support the use of preoperative EUS-FNA in staging of eCCA, additional confirmatory data are needed. Furthermore, there are no published data evaluating the impact of EUS lymph node staging on patient outcomes. In addition, data for the role of EUS for staging intrahepatic CCA (iCCA) are lacking, with previous studies being conducted before changes in CCA classification and AJCC criteria. The objective of this study is to explore the staging impact of EUS-FNA for CCA, including patient outcomes, and to compare its role for staging different subtypes.
Patients and Methods
This single-tertiary-care-center retrospective study was approved by the Institutional Review Board (IRB) at Mayo Clinic (IRB 18-004166). Consecutive patients with CCA who underwent EUS staging from October 2014 to April 2018 were included in the study cohort. Clinical demographic information, CT, MRI, PET, and EUS imaging, and cytopathological and surgical data were abstracted from the electronic medical record. Subtypes of CCA were categorized as intrahepatic, perihilar, and distal, with the term eCCA encompassing both pCCA and dCCA.
RLNs identified on CT, MRI, PET, and EUS imaging were noted. CT and MRI imaging data obtained within 30 days preceding EUS were included. PET imaging data obtained within 30 days before or 30 days after EUS were included. Data regarding identification of RLNs on CT, MRI, and PET imaging were based on the official finalized radiology report.
EUS Procedure
Lymph node staging was performed using curvilinear EUS (GF-UCT180 or GF-UC160P-AT8; Pentax EG-3870UTK; PENTAX of America, Montvale, NJ). Specimens were obtained using a standard 22-gauge FNA needle (EUSN-3; Cook Medical Inc., Winston-Salem, NC). A previous study from our group showed that EUS imaging features of RLNs are not predictive of malignant involvement.21 Therefore, it is standard practice at our institution to sample visualized lymph nodes at different stations. Lymph node sampling was performed for all identified lymph nodes only if the needle trajectory avoided traversing of any aspect of the primary tumor or other sites of possible metastasis. Rapid on-site evaluation (ROSE) was utilized in conjunction with cytopathologist telecytology review, when requested. Data regarding identification of RLNs on EUS imaging were based on the official finalized EUS report. All lymph nodes documented in the EUS report were included regardless of size.
Cytopathology
The official cytopathology report was considered the reference standard in determining whether an RLN was positive or negative for malignant metastasis. A diagnosis of MRLNs was made if cancer cells were identified in the presence of a polymorphic population of lymphocytes. Cytopathology interpretations of positive or suspicious for malignancy were deemed malignant. Other interpretations were regarded as benign provided that sufficient lymphocytes were identified to confirm lymph node sampling. Cases with insufficient lymphocytes to confirm lymph node sampling were separately classified as “inadequate.”
Statistical Analysis
Continuous variables are reported as means with SD or median with interquartile range (IQR). The proportion of malignant lymph nodes detected by EUS or imaging were compared with the chi-square test. When the expected counts were <5, Fisher's exact test was used. Survival curves were compared using the log-rank test for survival curve equality and illustrated by the Kaplan-Meier method. Cox regression was used to model the effect of malignant lymph nodes detected. The proportional hazards assumption was assessed. All tests were two-sided with a P value <0.05. STATA 15 software was used for the analysis (StataCorp LP, College Station, TX).
Results
Patient Demographics
From October 2014 to April 2018, a total of 767 patients with CCA were evaluated at our institution. Among this cohort, 157 patients (median age, 60 [IQR, 47-68]; 70% male) underwent EUS staging for CCA. Among this cohort, 24 (15%), 124 (79%), and 9 (6%) patients had intrahepatic (iCCA), perihilar (pCCA), and distal CCA (dCCA). Fifty-one patients (33%) had primary sclerosing cholangitis (PSC). Patients with pCCA had the highest proportion of PSC (35%; Table 1).
| Characteristic | All CCA | pCCA | iCCA | dCCA |
|---|---|---|---|---|
| No. patients | 157 | 124 | 24 | 9 |
| Body mass index | 25.9 ± 5.4 | 26.0 ± 5.5 | 25.3 ± 5.4 | 24 ± 4.0 |
| Hepatitis C | 3 (2%) | 2 (2%) | 1 (4%) | 0 (0%) |
| PSC | 51 (33%) | 44 (35%) | 5 (21%) | 2 (22%) |
| RLNs identified (EUS) | 135 (86%) | 110 (89%) | 20 (83%) | 5 (56%) |
| RLNs identified (CT/MRI) | 74 (47%) | 59 (48%) | 12 (50%) | 3 (33%) |
| Detection of MRLNs, EUS-FNA | 27 (17%) | 20 (16%) | 4 (17%) | 3 (33%) |
| No. of FNAs per patient | 6 [4-14] | 6 [3-12] | 5 [3-11] | 4 [3-7] |
| No. of FNAs per node | 3 [2-4] | 3 [2-5] | 3 [1-4] | 3 [2-5] |
| Mean long axis (mm), EUS* | 20 [15-27] | 20 [14-26] | 21 [15-28] | 21 [20-22] |
- * Dimension of largest node.
Radiographical Studies
Of the 157 patients in the study cohort, 155 (98.7%) underwent CT or MRI within 30 days preceding EUS. CT imaging alone was performed in 41 patients (26%), MRI imaging alone performed in 43 patients (27%), and both CT and MRI performed in 71 patients (45%). Among the entire cohort, cross-sectional imaging identified RLNs in 74 patients (47%). CT identified RLNs in 51 of 112 patients (46%), MRI identified RLNs in 68 of 114 patients (60%), and in those who underwent both modalities, RLNs were identified in 29 of 74 (41%) of patients. In the 29 patients with RLNs identified who had both CT and MRI, 17 had RLNs identified on both modalities, 11 had RLNs identified on only MRI, and 1 had RLNs identified on only CT.
With regard to PET imaging, 14 patients (4 iCCA, 9 pCCA, and 1 dCCA) underwent PET imaging, of which 6 (2 iCCA, 3 pCCA, and 1 dCCA) were found to have hypermetabolic RLNs. Of the 6 patients with hypermetabolic RLNs who underwent EUS-FNA, 2 had confirmed MRLNs, 3 had a negative cytology, and 1 had an inadequate cytology. Two of the 6 patients (both with negative EUS-FNA) proceeded to staging laparotomy and neither were found to have intra-abdominal metastases or MRLNs. Of the 8 patients who underwent PET without findings of hypermetabolic RLNs, 1 had confirmed MRLNs by EUS-FNA, and 1 had confirmed intra-abdominal metastases at the time of staging laparotomy.
EUS Findings
Overall
EUS identified a total of 301 RLNs in 135 patients. FNA was performed in 133 of the 135 patients (98.5%) with RLNs identified by EUS, with a median of three needle passes per node (IQR, 2-4). In 1 patient, a hilar lymph node was not sampled because of the common bile duct in the needle path for FNA. In another patient, the identified RLN was not sampled at the discretion of the endosonographer. Median length of the long axis of the largest node per patient was 20 mm (IQR, 14-26). ROSE was used in 103 (77.4%) cases in which FNA was performed. Median needle passes per node in patients with ROSE was 3 (IQR, 2-4), which was similar to the entire overall cohort. Cytology interpretation was deemed inadequate in 8 patients, 7 of which utilized ROSE. The most common location for visualized lymph nodes was the periportal region (Table 2; Fig. 2). EUS identified a significantly higher percentage of RLNs compared to cross-sectional imaging (86% vs. 47%; P < 0.001). In iCCA alone, EUS also detected a higher percentage of RLNs compared to cross-sectional imaging (83% vs. 50%; P ≤ 0.001).
| EUS-Identified Lymph Node Location | eCCA | iCCA | Total | |
|---|---|---|---|---|
| Perihilar (n = 243) | Distal (n = 9) | Intrahepatic (n = 49) | (n = 301) | |
| Periesophageal/mediastinal | 16 (6.6%) | 1 (11.1%) | 1 (2.0%) | 18 (6.0%) |
| Celiac | 30 (12.3%) | 3 (33.3%) | 8 (16.3%) | 41 (13.6%) |
| Gastrohepatic | 40 (16.5%) | 0 (0.0%) | 6 (12.2%) | 46 (15.3%) |
| Periportal | 127 (52.3%) | 4 (44.4%) | 30 (61.2%) | 161 (53.5%) |
| Aortocaval | 30 (12.3%) | 1 (11.1%) | 4 (8.2%) | 35 (11.6%) |
| EUS-Identified MRLN | eCCA | iCCA | Total | |
|---|---|---|---|---|
| Perihilar (n = 30) | Distal (n = 3) | Intrahepatic (n = 6) | (n = 39) | |
| Periesophageal/mediastinal | 5 (16.7%) | 0 (0.0%) | 0 (0.0%) | 5 (12.8%) |
| Celiac | 2 (6.7%) | 2 (66.7%) | 1 (16.7%) | 5 (12.8%) |
| Gastrohepatic | 5 (16.7%) | 0 (0.0%) | 1 (16.7%) | 6 (15.4%) |
| Periportal | 13 (43.3%) | 0 (0.0%) | 4 (66.7%) | 17 (43.6%) |
| Aortocaval | 5 (16.7%) | 1 (33.3%) | 0 (0.0%) | 6 (15.4%) |
EUS-FNA Positive for MRLN
In total, EUS-FNA identified 39 MRLNs in 27 (17%) patients. Of these 27 patients, 26 had positive cytology whereas 1 patient had suspicious cytology. The periportal region was most commonly involved for MRLNs detected by EUS (43.6%; Table 2; Fig. 2). In 124 patients with pCCA, 44 had PSC and 80 did not (Table 1). EUS identified MLRNs in 4 of 44 (9%) patients with PSC and 16 of 80 (20%) without PSC (P = 0.11). Regional lymphadenopathy was not identified by initial cross-sectional imaging in 9 (33%) patients with MRLNs detected by EUS-FNA. None of the 27 patients with MRLNs identified by EUS-FNA proceeded to staging laparotomy (Fig. 3). In iCCA alone, EUS-FNA identified MRLNs in 4 (17%) patients. None of these 4 patients proceeded to staging laparotomy (Table 1).
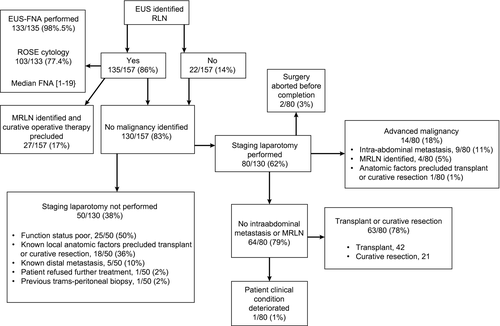
EUS-FNA Negative for MRLN
Of the 130 patients without MRLNs detected by EUS-FNA, 80 (62%) proceeded to staging laparotomy after a median of 124 days (IQR, 58-262). Of these 80 patients evaluated by staging laparotomy, 14 (18%) were found to have pathology that precluded curative resection or transplant; 9 patients were found to have intra-abdominal metastasis, 4 patients with MRLNs, and 1 patient with hepatic artery encasement (Fig. 3). Of the 9 patients with intra-abdominal metastasis, 5 had peritoneal, 2 had omental, and 2 had small bowel metastasis. All 4 patients with MRLNs had metastases in the periportal lymph nodes. Of these 4 patients, 2 had FNA with negative cytology, 1 had FNA with inadequate cytology, and 1 had no RLNs identified at the time of EUS. One patient with negative cytology had FNA of a single periportal node, and the other had FNA of the multiple periportal and aortocaval nodes. The patient with inadequate cytology underwent FNA of a single mediastinal node.
EUS-FNA in Patients With and Without Lymphadenopathy on Cross-Sectional Imaging
EUS identified lymphadenopathy in 70 of 74 patients (95%) with lymphadenopathy on cross-sectional imaging and in 65 of 83 (78%) without lymphadenopathy on cross-sectional imaging (P = 0.002). In the 74 patients with lymphadenopathy on cross-sectional imaging, 18 (24%) were confirmed to have MRLNs by EUS-FNA, whereas in the 83 without lymphadenopathy on cross-sectional imaging, 9 (11%) were confirmed to have MRLNs by EUS-FNA (P = 0.03).
All Patients With MRLNs
Overall, 31 patients in this series were found to have MRLNs, of whom EUS detected MRLN in 27 (87%). In these 31 patients, 12 (39%) had no lymphadenopathy identified with cross-sectional imaging.
Survival Based on EUS Nodal Staging
Identification of at least one MRLN by EUS was associated with a statistically significant reduction in long-term survival (Fig. 4). Median survival time in patients with and without MRLNs was 353 and 1,050 days (P < 0.001). Mortality risk was 4 times greater among patients with MRLNs identified at time of EUS (hazard ratio = 4.1; P < 0.001).
Discussion
Presence of MRLN predicts very poor survival in CCA patients and precludes curative oncological resection or liver transplantation. It is imperative to recognize MRLNs early in the staging process to avoid the morbidity and cost of neoadjuvant chemoradiation and staging laparotomy that patients will undergo before definitive surgical intervention. Cross-sectional imaging alone cannot reliably identify RLNs.14-16 In this study, regional lymphadenopathy was described in only 47% of patients who underwent cross-sectional (CT/MRI) imaging. Furthermore, of the 31 patients found to have MRLN, 12 (39%) had no lymphadenopathy identified on initial imaging. It is also important to note that although certain morphological characteristics of lymphadenopathy detected by cross-sectional imaging can inform the likelihood of malignancy, malignant status cannot be confirmed without tissue sampling. Of the 6 patients with hypermetabolic RLNs on PET imaging, 2 had both negative cytology on EUS-FNA as well as no evidence of MRLNs or metastatic disease at the time of staging laparotomy. Of the 8 patients without hypermetabolic RLNs on PET imaging, 1 had confirmed MRLNs by EUS-FNA and 1 had confirmed intra-abdominal metastases at time of staging laparotomy.
In this study, RLNs were identified by EUS imaging in 86% of patients. A previous study from our group showed that EUS imaging features of RLNs are not predictive of malignant involvement.21 Therefore, in our study, 98.5% of those with RLNs identified underwent FNA. MRLNs were detected in 17% of the study cohort. A total of 4 (17%), 20 (16%), and 3 (33%) patients with iCCA, pCCA, and dCCA were identified to have MRLNs following EUS-FNA and cytopathological examination. This suggests that EUS-FNA identification of MRLNs is similar across all types of CCA. Identification of at least one MRLN by EUS was associated with significantly worse long-term survival. This is consistent with recent literature and the assertion that regional lymph metastasis diagnosed at the time of surgery is one of the most important determinants of postsurgical outcomes.6 In all cases, identification of MRLNs immediately precluded patients from neoadjuvant chemoradiation and further unnecessary surgical interventions, including staging laparotomy.
These data could lead to different strategies utilizing EUS for lymph node staging in patients without widely metastatic CCA. In this study, EUS had a much higher yield in patients with regional lymphadenopathy on initial cross-sectional imaging, identifying a significantly higher proportion of lymphadenopathy as well as MRLNs when compared to patients without regional lymphadenopathy on initial cross-sectional imaging. Given that MRLNs were identified even in patients without regional lymphadenopathy on initial cross-sectional imaging, one approach would be to perform EUS for nodal staging in all patients without obvious metastatic disease, which is our institutional practice. An alternate approach would be to consider EUS only in patients who are found to have regional lymphadenopathy on cross-sectional imaging and consider proceeding directly to staging laparoscopy in those patients without lymphadenopathy on cross-sectional imaging. Prospective studies are needed to determine the ideal approach.
Overall duration between EUS and staging laparotomy was 124 days, which reflects the amount of time required to administer neoadjuvant chemotherapy, delays related to disease complications, and other efforts to improve patient functional status. As such, not only did EUS-FNA identification of MRLNs spare patients from the morbidity of unnecessary treatments and investigations, but it also provided important prognostic information earlier in the disease course. This may have allowed patients to shift focus from a curative mindset to palliative treatment much earlier and enabled them to appropriately focus on quality of life and personal goals.
Of the 130 patients without MRLNs detected by EUS ± FNA, 4 (3.1%) were subsequently identified to have periportal MRLNs at time of staging laparotomy at an average of 144 days (range, 40-369) after the EUS procedure. These patients may have been missed by previous EUS evaluation. Three of the patients underwent FNA sampling. These 3 patients had FNA of a single mediastinal node, a single periportal node, and multiple periportal and aortocaval nodes.
A major limitation of this study is its retrospective single-center nature. Patients with widely metastatic disease noted with cross-sectional imaging are not referred for EUS, and such patients with CCA who only had imaging were not systematically reviewed. Exclusion of these patients may have resulted in an underestimation of CT, MRI, and PET performance characteristics. In addition, although this is the largest study of preoperative EUS-FNA staging of CCA to date, the overall sample size and number of patients with MRLN remains small. Patients with multiple lymph nodes identified may have been subject to random sampling error. Given that no patients with MRLNs identified by EUS-FNA underwent subsequent staging laparotomy, the performance characteristics of EUS-FNA could not be directly compared to staging laparotomy. It is possible that disease progression may explain the small proportion of patients found to have MLRNs at the time of staging laparotomy that were under-recognized on previous EUS. Conversely, it is also possible that MRLNs were missed by EUS, but were ablated by neoadjuvant chemoradiation treatments administered before staging laparotomy. It is also uncertain whether specific MRLN locations confer higher risk than others. A multidisciplinary approach with shared patient decision making should therefore be used when making difficult clinical decisions.
In summary, EUS evaluation should be considered in patients undergoing preoperative staging for all subtypes of CCA, with FNA performed for identified RLNs. Endosonographers should pay careful attention to periportal lymph nodes when staging patients with CCA given that this is the most common location for MRLNs. EUS-FNA identifies a significant proportion of patients with previously unrecognized MRLN, thus avoiding the unnecessary cost and morbidity of neoadjuvant chemoradiation and staging laparotomy. EUS nodal staging correlates with survival outcomes, thereby providing prognostic information earlier in their disease course, affording more time for patients to act in accord with their personal goals.
Author Contributions
T.M., G.J.G., E.J.V. and V.C., substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; T.M., M.J.L., F.C.G., A.C.S., E.J.V., M.D.T., B.K.A.D., P.G.I., E.R., G.J.G., L.R.R. and V.C., drafting the article or revising it critically for important intellectual content and final approval of the version to be published.




