TMPRSS4 Drives Angiogenesis in Hepatocellular Carcinoma by Promoting HB-EGF Expression and Proteolytic Cleavage
Abstract
Background and Aims
Heparin-binding epidermal growth factor (HB-EGF), a member of the epidermal growth factor family, plays a pivotal role in the progression of several malignancies, but its role and regulatory mechanisms in hepatocellular carcinoma (HCC) remain obscure. Here, we report that transmembrane protease serine 4 (TMPRSS4) significantly enhanced the expression and proteolytic cleavage of HB-EGF to promote angiogenesis and HCC progression.
Approach and Results
A mechanistic analysis revealed that TMPRSS4 not only increased the transcriptional and translational levels of HB-EGF precursor, but also promoted its proteolytic cleavage by enhancing matrix metallopeptidase 9 expression through the EGF receptor/Akt/mammalian target of rapamycin/ hypoxia-inducible factor 1 α signaling pathway. In addition, HB-EGF promoted HCC proliferation and invasion by the EGF receptor/phosphoinositide 3-kinase/Akt signaling pathway. The level of HB-EGF in clinical samples of serum or HCC tissues from patients with HCC was positively correlated with the expression of TMPRSS4 and the microvessel density, and was identified as a prognostic factor for overall survival and recurrence-free survival, which suggests that HB-EGF can serve as a potential therapeutic target for HCC. More importantly, we provide a demonstration that treatment with the HB-EGF inhibitor cross-reacting material 197 alone or in combination with sorafenib can significantly suppress angiogenesis and HCC progression.
Conclusions
HB-EGF can be regulated by TMPRSS4 to promote HCC proliferation, invasion, and angiogenesis, and the combination of the HB-EGF inhibitor cross-reacting material 197 with sorafenib might be used for individualized treatment of HCC.
Abbreviations
-
- ChIP
-
- chromatin immunoprecipitation
-
- Con
-
- control
-
- CRM197
-
- cross-reacting material 197
-
- DEG
-
- differentially expressed gene
-
- EGF
-
- epidermal growth factor
-
- EGFR
-
- EGF receptor
-
- ERK
-
- extracellular signal-regulated kinase
-
- GO
-
- Gene Ontology
-
- HB-EGF
-
- heparin-binding EGF-like growth factor
-
- HCC
-
- hepatocellular carcinoma
-
- HIF-1α
-
- hypoxia-inducible factor 1 α
-
- HUVEC
-
- human umbilical vein endothelial cell
-
- IF
-
- immunofluorescence
-
- IHC
-
- immunohistochemistry
-
- KEGG
-
- Kyoto Encyclopedia of Genes and Genomes
-
- LY294002
-
- 2-morpholin-4-yl-8-phenylchromen-4-one
-
- MMP
-
- matrix metallopeptidase
-
- mTOR
-
- mammalian target of rapamycin
-
- MVD
-
- microvessel density
-
- OE
-
- overexpressing
-
- p
-
- phosphorylated
-
- PI3K
-
- phosphoinositide 3-kinase
-
- RFS
-
- recurrence-free survival
-
- RNA-seq
-
- RNA-sequencing
-
- sHB-EGF
-
- soluble HB-EGF
-
- TCGA
-
- The Cancer Genome Atlas
-
- TMA
-
- tissue microarray
-
- TMPRSS4
-
- transmembrane protease serine 4
-
- WB
-
- western blot
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and is associated with high mortality rates worldwide.1 Over the past decade, the treatment model for HCC has evolved considerably, and patients with HCC can now benefit from multiple effective options that improve their overall survival (OS) regardless of the evolutionary stage of the disease at diagnosis.1 However, the overall prognosis of HCC remains unsatisfactory. Recent clinical trials have led to the regulatory approval of several molecular targeted agents for HCC treatment, including the multikinase inhibitors, such as sorafenib, lenvatinib, and regorafenib, and immune checkpoint inhibitors, such as nivolumab and pembrolizumab. However, the objective response rates to these agents is 20% at most, and these agents increase the survival of patients with HCC by no more than a few months.2-4 The identification of potential responders is critical for avoiding the prescribing of ineffective drugs to patients who will not benefit from these treatments.
As one of the hallmarks of malignancies, aberrant angiogenesis represents a form of niche construction in which cancer cells promote a local vascular ecology by delivering nutrients and growth factors, removing potentially toxic metabolites and thereby promoting cancer cell proliferation and metastasis. HCC is associated with a hyperangiogenic state due to the overproduction of various growth factors. Over the last 10 years, clinical and preclinical trials have tested the benefit of a variety of anti-angiogenic drugs combined with antiblastic chemotherapy, radiotherapy, or other targeted drugs for the treatment of advanced HCC, but these therapies have exhibited only modest clinical success.5, 6 The elucidation of the molecular mechanisms of angiogenesis in HCC might provide insights for the identification of molecular targets for anti-angiogenic therapy and might contribute to the development of targeted drugs.
As a heparin-binding member of the epidermal growth factor (EGF) family, heparin-binding EGF-like growth factor (HB-EGF) is synthesized as a transmembrane precursor molecule called HB-EGF precursor (pro-HB-EGF) and undergoes extracellular proteolytic cleavage by matrix metalloproteinases to yield the mature secreted form of the growth factor called soluble HB-EGF (sHB-EGF), which promotes tumor angiogenesis and progression.7 A previous study has suggested that HB-EGF plays a critical role in improving hepatocyte proliferation and liver functions by acting as a potent mitogen and chemotactic factor.8 As a major cause of hepatocarcinogenesis, the core protein of hepatitis C virus (HCV) can activate the Akt pathway through the Ras/phosphoinositide 3-kinase (PI3K) pathway by the autocrine secretion of HB-EGF to promote hepatocarcinogenesis and tumor progression.9 In addition, HB-EGF is potentially involved in ovarian carcinoma resistance against the chemotherapeutic agent paclitaxel.10 However, the role of HB-EGF in HCC angiogenesis and the regulatory mechanisms of HB-EGF remain unclear.
As a type 2 transmembrane serine protease, transmembrane protease serine 4 (TMPRSS4) is highly expressed in several solid malignancies, including HCC. TMPRSS4, which is localized on the cell surface, mediates signal transduction between the cell and its surrounding environment and is a proteolytic modifier of particular targets, such as growth factors and protease-activated receptors, which are critical for the activation of oncogenic signaling pathways. Despite tumorigenesis, dysregulated expression of TMPRSS4 is also associated with infection and cardiovascular diseases in which angiogenesis is part of the pathology. Knockdown of TMPRSS4 can cause severe defects in embryonic development, including a degenerated vascular system.11 These findings indicate that TMPRSS4 may be involved in regulating the physiological and pathological process of angiogenesis. Clear evidence of TMPRSS4 involved in HCC angiogenesis has not been provided until our preliminary study showed a causal relationship in HCC-bearing mice.12 However, the mechanism underlying this process remains unclear and deserves to be elucidated.
In this study, we found that TMPRSS4 significantly enhanced the expression and secretion of HB-EGF to promote HCC angiogenesis. TMPRSS4 not only increased the transcriptional and translational levels of pro-HB-EGF, but also promoted its proteolytic cleavage by increasing matrix metallopeptidase (MMP)-9 expression through the EGFR receptor (EGFR)/Akt/mammalian target of rapamycin (mTOR)/hypoxia-inducible factor 1 α (HIF-1α) pathway. Moreover, the results showed that HB-EGF promoted HCC proliferation and invasion by the EGFR/PI3K/Akt pathway. The levels of HB-EGF in clinical samples of serum or HCC tissues from patients with HCC were significantly positively correlated with the expression of TMPRSS4 and the microvessel density (MVD), and is a prognostic factor for OS and recurrence-free survival (RFS). More importantly, we also found that treatment with the specific HB-EGF inhibitor cross-reacting material 197 (CRM197) alone or combined with sorafenib can significantly suppress angiogenesis and HCC progression.
Methods and Materials
Cell Lines
The human HCC cell lines MHCC97H (established at and obtained from the Liver Cancer Institute, Zhongshan Hospital, Fudan University, Shanghai, China),13, 14 Huh-7, PLC/PRF/5, and Hep3B were used in this study.
Patients, Follow-up, and Tissue Microarrays
These experiments were approved by the Ethics Committee of Qilu Hospital, Shandong University, and were performed in accordance with the approved guidelines. Informed consent was obtained from all the subjects. A tissue microarray (TMA) was constructed by Shanghai Outdo Biotech Company using archival specimens from 398 anonymous patients with HCC. The clinicopathological information and follow-up procedures are described in the Supporting Materials and Methods.
Immunohistochemistry, Immunofluorescence, and Western Blot Analyses
The immunohistochemistry (IHC), immunofluorescence (IF), and western blot (WB) analyses were performed as described,15 and the primary antibodies used in these analyses are listed in Supporting Table S1.
IHC staining was evaluated according to the German semiquantitative scoring system as described.12
Transfection Experiment
The processes of transfection experiment are described in the Supporting Materials and Methods.
In Vitro Cell Migration, Cell Proliferation, and Tube Formation Assays
The cell migration, cell proliferation, and tube formation assays in vitro were performed as described.16
In Vivo Tumor Growth Assay
All animal studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals established by the National Institutes of Health. HCC cells were used to establish subcutaneous xenograft tumor models as described.16 Seven days after tumor implantation, the mice received intraperitoneal injections of CRM197 (2.5 μg/kg), sorafenib (30 mg/kg), or phosphate-buffered saline every 2 days.
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation (ChIP) was conducted using an EZ-Magna ChIP A/G kit (Millipore, Burlington, MA) according to the manufacturer’s instructions. ChIP quantitative real-time PCR was conducted as described.16 The sequences of the primers used for PCR are listed in Supporting Table S2.
RNA Isolation, RNA Sequencing, and RNA-Sequencing Data Analysis
Total mRNA of HCC cell lines and samples was isolated using the TRIzol reagent (Invitrogen, Carlsbad, CA). The RNA concentration was quantified using a NanoDrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA), and the RNA integrity was evaluated with a 2100 Bioanalyzer and an RNA 6000 kit (Agilent Technologies, Santa Clara, CA). Libraries were prepared with a TruSeq RNA library preparation kit (Illumina, San Diego, CA), quantified by quantitative real-time PCR, normalized, pooled, and sequenced using an Illumina HiSeq 4000 instrument.
Quantitative Real-Time PCR
Quantitative real-time PCR was performed as described.15 The sequences of the primers used for PCR are listed in Supporting Table S2.
Statistical Analyses
The statistical analyses were performed using the SPSS statistical software package (version 21.0; SPSS, Inc., Chicago, IL). The values were expressed as the mean ± SD. Student t test was used for comparisons between groups, and the chi-square test or the Fisher exact probability test was used for the evaluation of categoric variables. The OS rate was calculated using the Kaplan-Meier method and compared using the log-rank test. Multivariate Cox proportional hazards regression analyses were performed to analyze the independent prognostic factors. Two-tailed P values less than 0.05 were considered statistically significant.
Results
TMPRSS4 regulates tumor angiogenesis in HCC tissues
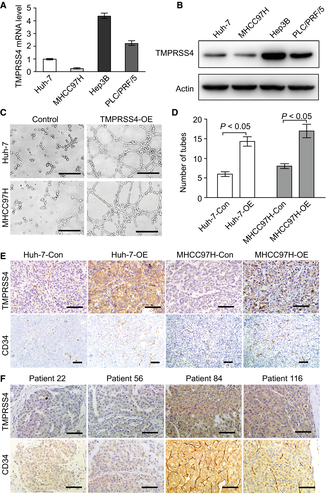
To investigate the roles of TMPRSS4 in regulating the angiogenic process in HCC, we first detected the mRNA and protein levels of TMPRSS4 in different human HCC cell lines and found relatively lower TMPRSS4 expression levels in the Huh-7 and MHCC97H cells compared with the Hep3B and PLC/PRF/5 cells (Fig. 1A,B). We then overexpressed TMPRSS4 in the Huh-7 and MHCC97H cell lines, and found that the tumor-conditioned medium (TCM) from TMPRSS4-overexpressing (TMPRSS4-OE) HCC cells induced significantly greater stimulation of human umbilical vein endothelial cells (HUVECs) to generate capillary-like structures compared with the medium from the TMPRSS4-control (TMPRSS4-Con) group (Fig. 1C,D). We subcutaneously injected Huh-7-Con, Huh-7 TMPRSS4-OE (Huh-7-OE), MHCC97H-Con, and MHCC97H TMPRSS4-OE (MHCC97H-OE) cells into nude mice. Our results revealed that all groups successfully formed tumors and that the overexpression of TMPRSS4 could promote the abdominal metastasis of HCC in vivo (Supporting Fig. S1A,B). The expression of CD34 molecule (CD34), an angiogenesis marker, was detected through IHC examinations. We also demonstrated that the MVD was significantly increased in TMPRSS4-OE tumors compared with the MVD in TMPRSS4-Con tumors (Fig. 1E). Subsequent IHC analysis of the expression of TMPRSS4 and CD34 in human HCC samples using a human TMA revealed that the MVD in TMPRSS4-high patients was significantly higher than the MVD in TMPRSS4-low patients, regardless of the status of hepatitis B virus (HBV) infection (Fig. 1F; Supporting Fig. S1C-E). These results indicated that the overexpression of TMPRSS4 could induce HCC angiogenesis independent of HBV infection.
Oncogenic Role of TMPRSS4 Might Mainly Depend on Activation of the PI3K/Akt//HIF-1α Signaling Pathway
To delineate the crucial molecules and mechanisms triggered by TMPRSS4 that mediate the effects of TMPRSS4 on HCC angiogenesis, mechanistic insights regarding the TMPRSS4-OE and TMPRSS4-Con HCC cells were obtained through RNA-sequencing (RNA-seq). The raw data obtained from the RNA-seq have been submitted successfully to the Sequence Read Archive database (Accession No. PRJNA561563).
A total of 6,324 and 769 differentially expressed genes (DEGs) (fold change ≥2 and P < 0.05) were found from comparisons between the Huh-7-OE and Huh-7-Con cells and between the MHCC97H-OE and MHCC97H-Con cells, respectively (Fig. 2A; Supporting Tables S3 and S4). For the Huh-7 and MHCC97H cells, we identified 54 commonly up-regulated genes and 97 commonly down-regulated genes in the comparison of the TMPRSS4-OE group with the TMPRSS4-Con group, respectively (Fig. 2B,C). We then randomly selected several genes to assess the consistency of the results from those two groups, and the levels of six cancer-related genes that exhibited various fold changes in expression among the pairs of cell lines were validated by quantitative real-time PCR (Supporting Fig. S2A). A gene set enrichment analysis revealed that the overexpression of TMPRSS4 in HCC cells was associated with positive regulation of angiogenesis, calcium-dependent cell–cell adhesion by plasma membrane cell adhesion molecules, signal transduction, and endothelial cell–cell adhesion (Supporting Fig. S2B; Supporting Tables S5 and S6).
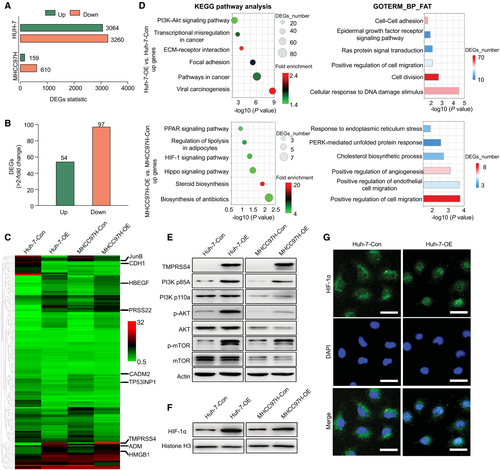
To further explore the crucial mechanism through which TMPRSS4 regulates HCC angiogenesis, we performed Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis and Gene Ontology (GO) functional analysis of DEGs in Huh-7 and MHCC97H cells. KEGG pathway analysis showed that the up-regulated DEGs in Huh-7 cells were enriched in signaling pathways such as the PI3K/Akt signaling pathway and viral carcinogenesis, whereas the up-regulated DEGs in MHCC97H cells were enriched in the HIF-1 signaling pathway. In addition, GO functional classifications showed that the DEGs in the Huh-7 cell line were related to the positive regulation of cell migration, Ras protein signal transduction, and the EGFR signaling pathway, whereas the DEGs in the MHCC97H cell line were related to the positive regulation of cell migration and positive regulation of angiogenesis (Fig. 2D; Supporting Tables S7-S10). The results from the KEGG and GO functional analyses of the down-regulated DEGs in the pairs of Huh-7 and MHCC97H cells are presented in Supporting Fig. S2C,D and Supporting Tables S11-S14.
The PI3K/Akt pathway plays prominent roles in cell survival, cellular physiology, and pathological alterations. Perturbations in this pathway can elicit the tumorigenesis of several solid malignancies and confer resistance to targeted drugs. HIF-1α can translocate to the nucleus after activation of the PI3K/Akt/mTOR pathway, and once located in the nucleus, this molecule can bind to relevant elements and thereby promote the expression of genes involved in multiple biological functions, such as cell proliferation, apoptosis, migration, invasion, and angiogenesis.17 Compared with the TMPRSS4-Con cells, several potential HIF-1α target genes, such as heme oxygenase 1, were activated in both Huh-7-OE cells and MHCC97H-OE cells (Supporting Tables S3 and S4). Therefore, we hypothesized that TMPRSS4 might play an oncogenic role through activation of the PI3K/Akt/mTOR/HIF-1α pathway.
To confirm our hypothesis, we examined the changes in the PI3K/Akt/mTOR/HIF-1α pathway. A WB analysis revealed that TMPRSS4 overexpression induced marked activation of PI3K, phosphorylated (p-)Akt, and p-mTOR (Fig. 2E). In addition, TMPRSS4 overexpression induced HIF-1α nuclear translocation, which might enhance its activity in HCC cells (Fig. 2F,G). These results suggest that TMPRSS4 may exert its carcinogenic effects by disrupting the PI3K/Akt/mTOR/HIF-1α pathway.
TMPRSS4 Promotes HCC Angiogenesis by Increasing the Expression and Proteolytic Cleavage of pro-HB-EGF
Ectopic growth factors produced by the tumor microenvironment play important roles in cancer angiogenesis, progression, and aggressiveness.18 Based on the RNA-seq results, we found that the HB-EGF mRNA level was up-regulated in the TMPRSS4-OE group compared with the TMPRSS4-Con group, and this finding was obtained with both the Huh-7 and MHCC97H cell lines (Supporting Tables S3 and S4). As a member of the EGF family, HB-EGF is synthesized as a transmembrane precursor molecule (pro-HB-EGF) and undergoes extracellular proteolytic cleavage by MMPs, such as MMP9, to yield sHB-EGF, and the mature growth factor can promote endothelial cell migration and tumor angiogenesis.7, 19-21 Previous studies have shown that MMP9 can be regulated by TMPRSS4 to promote gastric cancer progression.22 In a variety of malignancies, MMP9 was demonstrated to be a target of HIF-1α,23, 24 which could be activated by TMPRSS4 in our HCC cells. Therefore, we hypothesized that TMPRSS4 might act as a positive regulator of HB-EGF expression and proteolytic cleavage by activating MMP9 and thus promote HCC angiogenesis and progression.
We first detected the mRNA expression of pro-HB-EGF in HCC cells with different TMPRSS4 levels, and our results demonstrated that the overexpression of TMPRSS4 in HCC cells increased the mRNA level of pro-HB-EGF in these cells (Fig. 3A). Because a low level of MMP9 was found in Huh-7 and MHCC97H cells (fragments per kilobase of exon per million mapped reads <1 in both the TMPRSS4-Con and TMPRSS4-OE cells), the change in MMP9 mRNA expression was also confirmed by quantitative real-time PCR (Fig. 3B). Our results revealed that TMPRSS4 could increase the expression of MMP9 mRNA in both Huh-7-OE and MHCC97H-OE cells compared with that in the TMPRSS4-Con cell lines. We subsequently detected the changes in HB-EGF and MMP9 protein expressions in TMPRSS4-OE HCC cells and found that the overexpression of TMPRSS4 could also increase the protein levels of pro-HB-EGF, pro-MMP9, and active MMP9 in Huh-7 and MHCC97H cells (Fig. 3C). Tumor tissues derived from Huh-7-OE and MHCC97H-OE cells also expressed high levels of pro-HB-EGF and MMP9 in vivo (Fig. 3D). In addition, the sHB-EGF concentration in the supernatant of TMPRSSE-OE HCC cells was significantly higher than that of the TMPRSS4-Con cells (Supporting Fig. S3A).
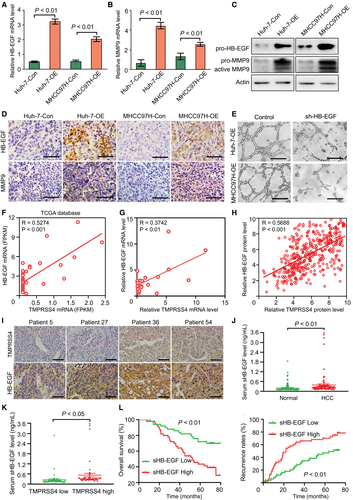
To determine the critical role of HB-EGF in TMPRSS4-induced HCC angiogenesis, we knocked down the HB-EGF expression in TMPRSS4-OE HCC cells. Our results demonstrated that the knockdown of HB-EGF in TMPRSS4-OE cells attenuated the TMPRSS4-induced HUVEC tube formation (Fig. 3E).
The correlation between TMPRSS4 and HB-EGF in HCC samples was further investigated. First, we analyzed the data of 369 HCC samples from The Cancer Genome Atlas (TCGA) database and found a positive correlation between the TMPRSS4 and HB-EGF mRNA levels in HCC tissues (Fig. 3F). In addition, the quantitative real-time PCR results of TMPRSS4 and HB-EGF mRNA profiles in 21 cases of HCC tissues also supported a positive correlation between TMPRSS4 and HB-EGF (Fig. 3G). We subsequently detected the protein expression of TMPRSS4 and HB-EGF in HCC tissues by IHC using a TMA. The results demonstrated that the HB-EGF protein level was positively correlated with the TMPRSS4 protein level in HCC tissues (Fig. 3H,I). These data suggested that TMPRSS4 could promote the expression and proteolytic cleavage of HB-EGF and thus promote HCC angiogenesis.
To further determine the role of HB-EGF in HCC progression and the relevance of HB-EGF and TMPRSS4 in patients with HCC, we detected the sHB-EGF levels in the serum of 100 healthy volunteers and 100 patients with HCC (including 50 patients with high TMPRSS4 levels and 50 patients with low TMPRSS4 levels, determined on our TMA by IHC). Our results revealed that the serum level of sHB-EGF was significantly higher in patients with HCC than in the healthy population (Fig. 3J), and was significantly higher in patients with HCC with high TMPRSS4 expression than in patients with HCC patients with low TMPRSS4 expression (Fig. 3K). In addition, the MVD in patients with HCC with high serum sHB-EGF was significantly higher than the MVD of patients with low serum sHB-EGF (Supporting Fig. S3B), and the patients with high-serum sHB-EGF levels exhibited worse OS and RFS than patients with low-serum sHB-EGF levels (Fig. 3L).
TMPRSS4 Regulates HB-EGF Expression in HCC by the PI3K/Akt/mTOR/HIF-1α Pathway
To elucidate the role of the PI3K/Akt/mTOR pathway in TMPRSS4-induced pro-HB-EGF and MMP9 activation, we knocked down the expression of TMPRSS4 in TMPRSS4-OE HCC cells. As expected, TMPRSS4 knockdown in our HCC cells attenuated the activity of PI3K, p-Akt and p-mTOR, and thereby reduced HB-EGF and MMP9 expressions (Fig. 4A). The TMPRSS4-OE HCC cells were then pretreated with the PI3K inhibitor 2-morpholin-4-yl-8-phenylchromen-4-one (LY294002) (20 µg/mL) for 48 hours. Compared with the control group, LY294002 significantly reduced the activity or expression of p-mTOR, pro-HB-EGF, and MMP9 (Fig. 4B,C; Supporting Fig. S4A,B). In addition, we knocked down the expression of Akt or mTOR in TMPRSS4-OE HCC cells or treated TMPRSS4-OE cells with the mTOR inhibitor rapamycin (10 μM) for 48 hours. Our results revealed that in Huh-7-OE and MHCC97H-OE cells, knockdown of Akt significantly reduced the TMPRSS4-induced activation of p-mTOR and subsequent expression or activation of pro-HB-EGF and MMP9, whereas the knockdown or inhibition of mTOR significantly inhibited the expression or activation of pro-HB-EGF and MMP9 (Fig. 4D,E; Supporting Fig. S4A-D).
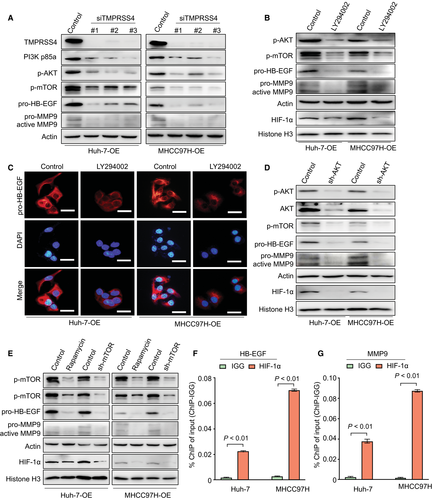
HIF-1α, one of the main HIFs activated in cells during adaption to hypoxic conditions, also exerts oncogenic biological functions through a hypoxia-independent mechanism.25-27 To further determine the role of HIF-1α in regulating TMPRSS4-induced HB-EGF expression, we detected the levels of HIF-1α in the nucleus of cells in which the activation of the PI3K/Akt/mTOR/HIF-1α pathway was inhibited. Our results revealed that the nuclear translation and activation of HIF-1α was significantly reduced in TMPRSS4-OE cells treated with LY294002 or rapamycin, or in conditions when the Akt/mTOR was knocked down in TMPRSS4-OE cells (Fig. 4B,D,E). The JASPAR bioinformatics software (http://jaspar.genereg.net/) was used to analyze the human HB-EGF and MMP9 promoter region, which has been suggested to contain potential HIF-1α-binding regions (Supporting Tables S15 and S16). ChIP quantitative real-time PCR revealed that the levels of HIF-1α bound to the HB-EGF or MMP9 promoters were statistically significant in HCC cells (Fig. 4F,G).
A previous study reported that TMPRSS4 functions as a positive regulator of the Raf/mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK)1/2 pathway and promotes HCC progression.12 To further determine whether the activation of the Raf/MEK/ERK1/2 pathway is involved in TMPRSS4-induced HCC angiogenesis, we knocked down the ERK expression in TMPRSS4-OE HCC cells, and found that ERK knockdown in TMPRSS4-OE HCC cells not only did not inhibit the expression of HB-EGF, but also did not attenuate the TMPRSS4-induced HUVEC tube formation (Supporting Fig. S4E,F).
HB-EGF Promotes HCC Progression by the EGFR/PI3K/Akt Signaling Pathway and Can Serve as a Potential Therapeutic Target
Similar to other EGF family members, HB-EGF plays a critical role in improving the proliferation of hepatocytes and embryonic stem cells (ESCs) to promote liver function recovery and angiogenesis by activating the EGFR/PI3K/Akt pathway.8, 28 However, the role of HB-EGF in HCC progression and whether it can serve as a potential therapeutic target remains unclear.
First, our analysis of HCC data from the TCGA database revealed that the expression of HB-EGF mRNA in HCC tissues was higher than that in normal liver tissues (Fig. 5A). Second, the mRNA and protein levels of HB-EGF in HCC tissues were significantly higher than those in para-tumor tissues (Fig. 5B,C). More importantly, patients with high HB-EGF levels exhibited significantly worse OS and RFS than those with low HB-EGF levels (Fig. 5D,E). In addition, the HB-EGF level in clinical HCC samples was positively correlated with MVD (Fig. 5F). A multivariate analysis revealed that the HB-EGF level was an independent predictor of OS and RFS (Supporting Tables S17 and S18). These results indicated that HB-EGF is a promising predictive factor for angiogenesis and prognosis of HCC.
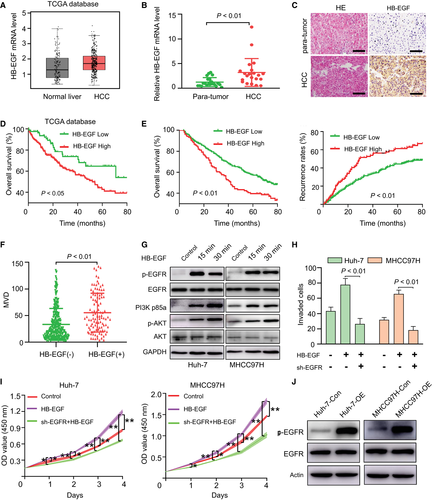
To address the functional significance of HB-EGF in HCC progression, we investigated the influence of HB-EGF on the EGFR pathway and the biological characteristics of control HCC cells. Consistent with its role in ESCs, HB-EGF can activate the EGFR/PI3K/Akt signaling pathway (Fig. 5G) and then promote the cell proliferation and invasion of HCC cells. The silencing of EGFR by short hairpin RNAs significantly decreased HB-EGF-induced HCC viability and invasion (Fig. 5H,I). In contrast to the control HCC cells, the modulation of HB-EGF did not alter the cellular viability or the EGFR/PI3K/Akt signaling pathway in TMPRSS4-OE cells (Supporting Fig. S5). We further detected the p-EGFR level in TMPRSS4-OE cells and TMPRSS4-Con cells. Our results revealed that TMPRSS4 can activate the EGFR signaling pathway in HCC cells (Fig. 5J). Collectively, our results demonstrated that the elevation of HB-EGF likely affects HCC aggressiveness through the EGFR signaling pathway, and this molecule can be regarded as a potential therapeutic target.
The HB-EGF Inhibitor CRM197 Synergistically Enhances the Antitumor Activity of Sorafenib Treatment for HCC
Sorafenib can improve the survival of patients with advanced HCC by only a few months,29 and activated EGFR, which can be induced by several growth factors, including HB-EGF, has been identified as a parameter that promotes the resistance of HCC cells to sorafenib.30, 31 As CRM197 can block the mitogenic activity of HB-EGF by preventing EGFR binding,32 we attempted to determine the effect of CRM197 and its synergistic effect with sorafenib on the tube-forming ability of HUVECs. Our results revealed that CRM197 and sorafenib could synergistically inhibit the TMPRSS4-induced tube-forming ability of HUVECs in vitro (Fig. 6A).
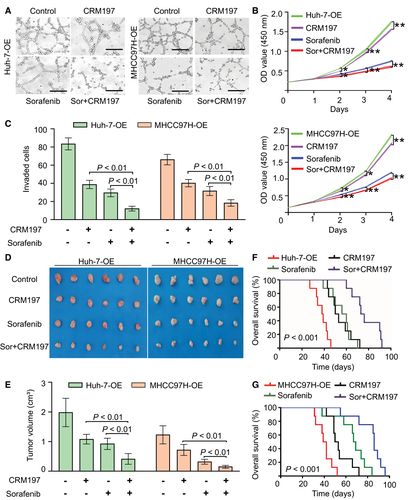
To further examine whether CRM197 could serve as an effective drug to synergistically enhance the antitumor activity of sorafenib for HCC treatment by inhibiting tumor progression and angiogenesis, we performed a series of in vitro and in vivo experiments using the TMPRSS4-OE HCC cell lines. Our results confirmed that CRM197 could inhibit HCC cell proliferation, and the combination therapy of CRM197 and sorafenib induced an additive effect on inhibition of the HCC proliferation rate (Fig. 6B). Conspicuously, CRM197 combined with sorafenib exerted significant synergistic inhibitory effects on HCC cell invasion (Fig. 6C).
Furthermore, we confirmed the synergistic antitumor effect of CRM197 and sorafenib in mice bearing HCC xenografts. Our results revealed that the tumor volumes in the combined treatment group were markedly smaller than those in either individual treatment group (Fig. 6D,E), and the OS was significantly improved by combination therapy than by individual treatment (Fig. 6F,G).
Discussion
In this study, we demonstrated that HB-EGF could be positively regulated by TMPRSS4 to promote HCC angiogenesis and that HB-EGF might serve as a promising therapeutic target in HCC. First, the TCM from TMPRSS4-OE HCC cells stimulated HUVECs to generate significantly more capillary-like structures than the conditioned media from the control cells. A mechanistic analysis revealed that TMPRSS4-OE HCC cells exhibited significantly higher levels of HB-EGF expression and secretion than the control cells, and this increased expression and secretion of HB-EGF in TMPRSS4-OE HCC cells promoted HCC angiogenesis and progression through the PI3K/Akt/mTOR pathway (Fig. 7). Second, the HB-EGF level, which was related to the OS and RFS of patients with HCC, was significantly positively associated with the TMPRSS4 levels in HCC samples. Third, HB-EGF can promote HCC proliferation and invasion in vitro through the EGFR/PI3K/Akt pathway. More importantly, the HB-EGF inhibitor CRM197 synergistically enhanced the antitumor activity of sorafenib in the treatment of HCC. These findings will provide a more in-depth understanding of tumor angiogenic mechanisms and will aid in the development of individualized treatment for patients with HCC.
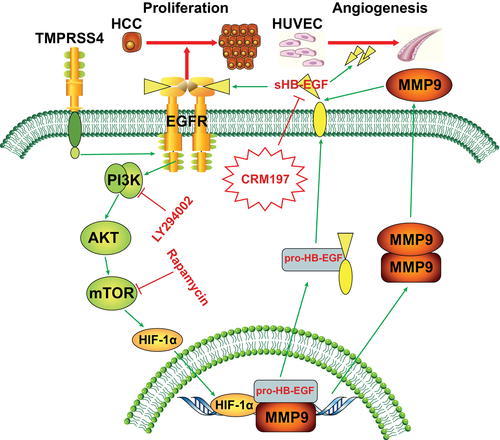
Angiogenesis is a dynamic and indispensable process with a critical function in tumor growth and metastasis. The abnormal angiogenic process that occurs in tumors is induced in part by the aberrant levels of growth factors secreted by tumor and stromal cells. Growth factors, including vascular endothelial growth factor, platelet-derived growth factor subunit B, and transforming growth factor β families, have been implicated in tumor angiogenesis.16, 18 Our study identified HB-EGF as an important angiogenic signal in HCC progression. As a mitogen for hepatocytes, HB-EGF is expressed primarily in Kupffer cells and the sinusoidal endothelium. Previous studies have revealed that HB-EGF is not only implicated in the physiological processes of tissue injury, wound healing and reproduction, but is also involved in tumor progression.20, 21, 33, 34 However, the regulatory mechanisms of HB-EGF and its role in HCC progression have scarcely been investigated. Our study demonstrates that HB-EGF is mediated by TMPRSS4 to promote HCC angiogenesis and progression. The HB-EGF level is up-regulated in the serum and HCC tissues of the HCC patient, is positively correlated with the MVD and tumor grade and size, and is an independent risk factor for postoperative OS and RFS. In addition, in vitro studies revealed that HB-EGF can promote the proliferation and invasion of HCC cells by the EGFR/PI3K/Akt pathway. These results suggest that HB-EGF can be regarded as an effective target for the inhibition of HCC angiogenesis and progression.
The signaling pathways involved in TMPRSS4-induced cancer progression consist of the down-regulation of E-cadherin at the plasma membrane, the activation of integrin-α5 and urokinase plasminogen activator, and the phosphorylation of focal adhesion kinase, Src, and ERK1/2.35 Our present study elucidates the role and molecular mechanisms of TMPRSS4 in regulating the process of HCC angiogenesis. We found that TMPRSS4 can not only promote the transcription and translation of HB-EGF, and subsequently increase HB-EGF expression, but also promote the proteolytic cleavage of HB-EGF by increasing the expression and activity of MMP9 through the EGFR/Akt/mTOR/HIF-1α pathway. Specifically, the knockdown of HB-EGF in HCC cells can inhibit the TMPRSS4-induced stimulation of HUVEC tube formation, whereas disruption of the EGFR/Akt/mTOR/HIF-1α pathway can also regulate HB-EGF expression. These results provide a more in-depth understanding of the oncogenic molecular mechanism through which TMPRSS4 promotes HCC progression and can provide clues for the anti-angiogenic treatment of HCC.
Angiogenesis inhibitors represent an effective therapeutic approach for HCC. At present, clinical trials are examining the benefit of anti-angiogenic drugs with antiblastic chemotherapy in several malignancies, including HCC.5, 6 As a naturally nontoxic inhibitor of HB-EGF, CRM197 can bind to human HB-EGF and block its activity by preventing EGFR binding.34 The EGFR pathway has been implicated in the pathogenesis of HCC, and EGFR inhibitors hinder HCC growth and development36 and enhance the response of HCC to sorafenib.30, 31 Based on the results from clinical trials, whether the addition of an EGFR inhibitor improves the efficacy of sorafenib or other targeted drugs remains controversial, and further clinical studies are needed to determine the role of CRM197, which is a naturally nontoxic, safe, and immunogenic agent, in combination with sorafenib in the treatment of HCC.
In fact, the synergistic antitumor activities of CRM197 with paclitaxel in ovarian cancer have been confirmed, and CRM197 is currently being evaluated in a phase 1 clinical trial for patients with ovarian cancer.32 In this study, we found that CRM197 can significantly inhibit TMPRSS4-induced angiogenesis in HCC. Sorafenib is a critical drug used for the therapeutic inhibition of HCC angiogenesis and proliferation and can therefore be used to improve the prognosis of patients with HCC. Our results demonstrate that CRM197 can synergize with sorafenib to inhibit HCC growth, invasion and angiogenesis, and improve the OS of tumor-bearing mice. These data further support the notion that CRM197 might be a promising candidate for combination therapy with other anti-angiogenic agents to treat HCC.
In conclusion, we demonstrate that HB-EGF, which is involved in HCC angiogenesis and development, can be regulated by TMPRSS4 through activation of the EGFR/Akt/mTOR/HIF-1α pathway. More importantly, our study also presents an anti-angiogenic strategy for individualized HCC treatment using the HB-EGF inhibitor CRM197 alone or in combination with sorafenib.
Author Contributions
L.T., D.Z.R., S.D. and Y.Y.F., Conceptualization; L.T., D.Z.R., S.D., Y.Y.F., Z.W. and W.X.W., Data curation; L.T., D.Z.R., W.R., S.K., Y.L.J., Y.Y.C., Y.C.Y., C.Z.Q. and Z.X.T., Formal analysis; L.T. and D.Z.R., Funding acquisition; L.T., D.Z.R., S.D., Y.Y.F., Z.W., S.K., Y.Y.C., W.X.W., C.Z.Q. and Z.X.T., Investigation; L.T., D.Z.R., S.D., Y.Y.F., Z.W., W.R., Y.Y.C., Y.L.J. and Y.C.Y., Methodology; L.T., D.Z.R., S.D., Y.Y.F., Z.W., W.R., W.X.W. and Y.Y.C., Project administration; L.T., D.Z.R., S.D., Y.Y.F., Z.W., W.R., S.K. and Z.X.T., Resources; L.T., D.Z.R., Y.L.J., Y.Y.C. and C.Z.Q., Software; L.T., Supervision; L.T., D.Z.R., S.D., Y.Y.F., Z.W., W.X.W., Y.L.J. and Y.C.Y., Validation; L.T., D.Z.R. and Y.Y.F., Visualization; L.T. and D.Z.R., Writing-original draft; L.T., D.Z.R., S.D., Y.Y.F., Z.W., W.R., W.X.W., S.K., Y.Y.C., Y.L.J., Y.C.Y., C.Z.Q. and Z.X.T., Writing-review & editing.




