Rat Organic Anion Transport Protein 1A1 Interacts Directly With Organic Anion Transport Protein 1A4 Facilitating Its Maturation and Trafficking to the Hepatocyte Plasma Membrane
Abstract
Organic anion transport proteins (OATPs) on the basolateral surface of hepatocytes mediate uptake of a number of drugs and endogenous compounds. Previous studies showed that rat OATP1A1 (rOATP1A1) has a postsynaptic density protein, drosophila disc large tumor suppressor, zonula occludens-1 protein (PDZ) consensus binding motif at its C-terminus and binds to PDZ domain containing 1 (PDZK1), which is required for its cell-surface localization. PDZK1 associates with rOATP1A1-containing endocytic vesicles within cells, mediating recruitment of motor proteins required for microtubule-based trafficking to the plasma membrane. rOATP1A4 also traffics to the plasma membrane, although it lacks a PDZ binding consensus sequence. The current study was designed to test the hypothesis that trafficking of rOATP1A4 to the plasma membrane requires its direct interaction with rOATP1A1 resulting in a complex that traffics through the cell in common subcellular vesicles in which the cytosolic tail of rOATP1A1 is bound to PDZK1. We found that 74% of rOATP1A4-containing rat liver endocytic vesicles (n = 12,044) also contained rOATP1A1. Studies in transfected HEK293 cells showed surface localization of rOATP1A1 only when coexpressed with PDZK1 whereas rOATP1A4 required coexpression with rOATP1A1 and PDZK1. Studies in stably transfected HeLa cells that constitutively expressed PDZK1 showed that coexpression of rOATP1A4 with rOATP1A1 resulted in more rapid appearance of rOATP1A4 on the plasma membrane and faster maturation to its fully glycosylated form. Similar results were observed on immunofluorescence analysis of single cells. Immunoprecipitation of rat liver or transfected HeLa cell lysates with rOATP1A1 antibody specifically co-immunoprecipitated rOATP1A4 as determined by western blotting. Conclusion: These studies indicate that optimal rOATP1A4 trafficking to the cell surface is dependent upon coexpression and interaction with rOATP1A1. As rOATP1A1 binds to the chaperone protein, PDZK1, rOATP1A4 functionally hitchhikes through the cell with this complex.
Abbreviations
-
- BCA
-
- bicinchoninic acid
-
- BSA
-
- bovine serum albumin
-
- BSP
-
- bromosulfophthalein
-
- cDNA
-
- complementary DNA
-
- CHAPS
-
- 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate
-
- DTT
-
- dithiothreitol
-
- EDTA
-
- ethylenediaminetetraacetic acid
-
- Endo H
-
- endoglycosidase H
-
- ER
-
- endoplasmic reticulum
-
- GAPDH
-
- glyceraldehyde 3-phosphate dehydrogenase
-
- IgG
-
- immunoglobulin G
-
- KLH
-
- keyhole limpet hemocyanin
-
- mEGFP
-
- monomeric enhanced green fluorescent protein
-
- mRFP
-
- monomeric red fluorescent protein
-
- OATP
-
- organic anion transport protein
-
- PBS
-
- phosphate buffered saline
-
- PDZ
-
- postsynaptic density protein, drosophila disc large tumor suppressor, zonula occludens-1 protein
-
- PDZK1
-
- PDZ domain containing 1
-
- PNGase F
-
- peptide N-glycosidase F
-
- rOATP1A1
-
- rat OATP1A1
-
- 35S-BSP
-
- 35S-bromosulfophthalein
-
- sfGFP
-
- superfolder green fluorescent protein
Previous studies showed that the hepatocyte efficiently eliminates organic anions from the circulation with single-pass extraction as high as ≥50%.1 Pharmacokinetic studies were consistent with carrier mediation,2, 3 and subsequent functional cloning experiments identified a transport protein, initially named organic anion transporting polypeptide (OATP).4, 5 This 75-kDa glycoprotein has 12 transmembrane domains6 and is sufficient to mediate ligand transport when transfected into various cell lines.7, 8 Since its initial discovery, over 20 additional members of the OATP family have been described.1 All are 12 transmembrane domain glycoproteins with overlapping substrate specificities and tissue distributions. The original OATP is now known as rat OATP1A1 (rOATP1A1) and is a member of the solute carrier organic anion transporter (SLCO) family9 with the gene name Slco1a1.
Our previous studies revealed that the C-terminal 4 amino acids of rat and mouse OATP1A1 (KTKL) encode a postsynaptic density protein, drosophila disc large tumor suppressor, zonula occludens-1 protein (PDZ) consensus binding motif that binds to PDZ domain containing 1 (PDZK1).10 This interaction with PDZK1 is required for efficient trafficking of rOATP1A1 to the basolateral plasma membrane of hepatocytes.10 Studies in PDZK1 knockout mice showed that surface localization of mOATP1A1, but not total cell content, was reduced as was uptake of bromosulfophthalein (BSP).10 Experiments performed in transfected cell lines confirmed that interaction with PDZK1 enhances cell-surface expression of OATP1A1,11 suggesting trafficking between an intracellular endocytic vesicular pool and the cell surface.11 Further studies showed that these OATP1A1-associated endocytic vesicles could bind to and move along microtubules.12, 13 In vitro motility studies of endocytic vesicles associated with OATP1A1 and PDZK1 prepared from wild-type mice showed selective recruitment of kinesin-1, a plus end directed motor molecule that traffics its cargo along microtubules toward the cell surface.12 Vesicles prepared from PDZK1 knockout mice are largely associated with dynein, a minus end directed motor molecule that traffics its cargo away from the cell surface.12
Although many members of the OATP family possess PDZ consensus binding motifs at their C-termini, an almost equal number do not.1 In particular, rat OATP1A4 does not have a PDZ binding motif whereas its very close mouse homolog does (KTKL). Despite lack of interaction with a PDZ protein, rOATP1A4 can still traffic to the basolateral plasma membrane of hepatocytes.14 We hypothesized that rOATP1A4 can interact with rOATP1A1 and traffic through the cell in common endocytic vesicles. This was examined in the present study.
Materials and Methods
Plasmids
pMEP4-rOATP1A4
rOATP1A4 complementary DNA (cDNA) cloned in the plasmid pCR2.1 was a gift from Dr. Richard Kim (University of Western Ontario, Canada).15 The rOATP1A4 cDNA was recloned into pMEP4, a vector in which expression is under the zinc-inducible metallothionein IIa promoter.7 In brief, rOATP1A4-PCR2.1 and pMEP4 were linearized with KpnI and BamHI respectively and filled in to generate blunt ends. The resulting DNAs were digested with XhoI, and the rOATP1A4 cDNA was ligated into the pMEP4 plasmid.
pCDNA3.1/Zeo (-)-mRFP-rOATP1A4
This plasmid encodes a monomeric red fluorescent protein (mRFP) fused to the N-terminus of rOATP1A4. rOATP1A4 cDNA was amplified by PCR from a previously described pCDNA3.1/Zeo(-)-rOATP1A4 plasmid16 using as sense primer 5′-GGGCAGATGGAGGGAAAATGGGAAAATCTGAGAAAAG-3′ and antisense primer 5′AACGGTACCAACTCAGTC CTC CGTCACTTT-3′ that contains a KpnI restriction site. mRFP was amplified by PCR from an mRFP plasmid kindly provided by Dr. Erik Snapp17 with a sense primer 5′-GTTCTCGAGGTTATGGTGTCCGAGCTGATTAAG-3′ containing an XhoI restriction site and antisense primer 5′-CTTTTCTCAGATTTTCCCATTTTCCCTCCATCGCCTGCCC-3′. PCR products were purified using the QIAquickPCR purification kit from Qiagen (Limburg, Netherlands). A 1:1 ratio of purified DNA product was allowed to run for three cycles of denaturation, annealing, and extension before the addition of primers containing restriction sites KpnI and XhoI for another 25 cycles.18 The PCR ligated mRFP-rOATP1A4 was gel purified and subjected to another round of PCR amplification using the two restriction site containing primers. The final PCR product was inserted into the XhoI and KpnI restriction sites of pCDNA3.1/Zeo (-).
GFP-rOATP1A1
The superfolder green fluorescent protein (sfGFP) plasmid was a kind gift of Dr. Erik Snapp. rOATP1A1 cDNA was prepared following digestion of a previously described monomeric enhanced green fluorescent protein (mEGFP)-rOATP1A1 plasmid11 with Bgl II and XbaI and inserted into the sfGFP plasmid at these sites. An mEGFP-rOATP1A1 was prepared as described.11
pFLAG-CMV-5c-PDZK1
This FLAG-tagged murine PDZK1 plasmid was prepared as described.16
All plasmids were confirmed by full-length sequencing using appropriate primers in the Einstein sequencing facility.
Cell Culture
HEK293 and HeLa cells were obtained from the Cell Culture and Genetic Engineering Core of the Marion Bessin Liver Research Center at Einstein, which also validated their identities. Cells were grown in high-glucose Dulbecco’s modified Eagle’s growth medium (DMEM; Life Technologies, Inc., Carlsba, CA) supplemented with 10% fetal bovine serum (FBS; Bio-West, Midland, TX), 100 units/mL of penicillin, and 0.1 mg/mL of streptomycin (Life Technologies, Inc.).
Cell Transfection
Transient transfection of plasmid constructs was performed in HEK293 cells with PolyFect Transfection Reagent (Qiagen), using the manufacturer’s conditions for HeLa cells. HeLa cell lines stably expressing rOATP1A1 under regulation of a zinc-inducible promoter were prepared by transfection of cells with pMEP4-rOATP1A1 as we described.7 Similar methods were used to prepare a pMEP4-rOATP1A4 HeLa cell line stably expressing rOATP1A4. To prepare a HeLa cell line expressing both transporters, pMEP4-rOATP1A1 HeLa cells were transfected simultaneously with pMEP4-rOATP1A4 and pCDNA3.1/Neo using PolyFect. Stable transfectants were grown in the presence of hygromycin (200 µg/mL) and neomycin G418 (1.2 mg/mL) and were screened for rOATP1A1 and rOATP1A4 expression by western blotting analysis after incubation in 150 µM of zinc sulfate for 48 hours.
Antibodies
Antibodies to C-terminal regions of rOATP1A1 and rOATP1A4 used for immunofluorescence and western blotting studies were prepared as described.16, 19 N-terminal antibody to rOATP1A1 was prepared similarly as described.16 Mouse monoclonal anti-FLAG antibody was obtained from Sigma-Aldrich (St, Louis, MO). Horseradish peroxidase–conjugated affinity-purified goat antirabbit immunoglobulin G (IgG) and goat antimouse IgG were obtained from GE Healthcare Biosciences (Piscataway, NJ). Fluorescent secondary cyanine 5 (Cy5) affinity-purified goat antirabbit IgG (product #111-175-144), cyanine 3 affinity-purified goat antirabbit IgG (product #111-165-144), and Alexa Fluor 647 affinity-purified goat antimouse IgG (product #115-605-166) were purchased from Jackson ImmunoResearch (West Grove, PA). Mouse monoclonal antibody to human PDZK1 (product #NBP2-22568) was obtained from Novus Biologicals (Littleton, CO).
Preparation and Immunofluorescence Labeling of Endocytic Vesicles From Rat Liver
Endocytic vesicles were prepared from rat liver as described.20 All procedures utilizing animals were approved by the Animal Use Committee of the Albert Einstein College of Medicine. Immunofluorescence labeling of vesicles was performed in optical chambers with a capacity of approximately 5 µL as we have described.12, 21 In some experiments coassociation of rOATP1A1 and rOATP1A4 in vesicles was examined by immunofluorescence using antibodies that were prepared to each protein in rabbits. In these studies, endocytic vesicles were mixed in PMEE buffer (35 mM of K2-PIPES [piperazine-N,N′-bis(2-ethane sulfonic acid)], 2 mg/mL of bovine serum albumin [BSA], 5 mM of MgCl2, 1 mM of ethylene glycol tetraacetic acid [EGTA], 0.5 mM of ethylenediaminetetraacetic acid [EDTA], 4 mM of dithiothreitol [DTT], and 2 mg/mL of ascorbic acid; pH 7.4) and flowed into an uncoated optical chamber as described.22 Wash buffer (35 mM of K2-PIPES, 2 mg/mL of BSA, 5 mM of MgCl2, 1 mM of EGTA, 0.5 mM of EDTA, 4 mM of DTT, and 5 mg/mL of casein; pH 7.4) was used to dilute reagents and for washes. Vesicles in chambers were sequentially labeled with rabbit antibodies to rOATP1A4 and rOATP1A1 following the Multiple Immunofluorescent Labeling Protocol from the Multiple Antigen Labeling Guide from Vector Laboratories, which utilizes biotin-labeled secondary antibodies and detection by fluorescent streptavidin. We followed blocking steps in the protocol including an incubation with high-concentration unlabeled antirabbit antibody between primary antibody binding steps, according to the manufacturer’s instructions. In brief, vesicles were washed two times and incubated for 2 minutes with StrepA block, from the streptavidin/biotin blocking kit (#SP2002; Vector Laboratories), diluted 1:10 in wash buffer. Subsequently, affinity-purified rOATP1A4 antibody (1:200 dilution) was added to the chamber and incubated for 6 minutes. Vesicles were then washed four times and incubated for 6 minutes with biotinylated horse antirabbit IgG (H+L; Vector Laboratories) at a 1:250 dilution. After four additional washes, DyLight 647 streptavidin (Vector Laboratories) was added (1:300) to the chamber and incubated for 6 minutes, then washed two times. Vesicles were incubated for 6 minutes with unconjugated horse antirabbit IgG (1:75) to block any unbound rabbit IgG. The StrepA block step was then repeated, followed by a 2-minute biotin block at 1:10 dilution to prevent interaction with the second streptavidin addition. These procedures were repeated for binding of primary rOATP1A1 antibody at a 1:1,000 dilution and secondary antibody and DyLight 488 streptavidin. Stained vesicles were kept in PMEE buffer containing ascorbic acid without casein until microscopy was performed. Appropriate controls without one or both primary and/or secondary antibodies were performed to check for cross-reaction during immunostaining.
Immunofluorescence Studies in Cells
HEK293 cells were transfected in 35-mm culture dishes, replated after 24 hours in eight-well Glass Chambers (Nunc Lab-Tek Chambered Coverglass #155411) at 70%-80% confluence, and grown in DMEM growth medium for an additional 24 hours. HeLa cells were seeded directly in these chambers, and expression of rOATP1A1 or/and rOATP1A4 could be induced by addition of 150 µM of ZnSO4. Cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in phosphate-buffered saline (PBS) for 15 minutes and permeabilized with 0.01% saponin in PBS for an additional 10 minutes at room temperature. Cells were washed three times with PBS for 3 minutes each and were blocked in 10% FBS in PBS for 30 minutes. They were then incubated in primary antibodies diluted in blocking buffer for 1 hour. Antibody was removed with two quick PBS washes and three additional 5-minute washes. Appropriate fluorescent secondary antibodies diluted in blocking buffer were added and incubated for 1 hour. After two quick washes, 10 µM of Hoechst 33342 (Sigma-Aldrich) nuclear dye was added to the cells for 15 minutes. After washing five times with PBS for 5 minutes per wash, stacked confocal images were taken at 60× magnification and merged using ImageJ (NIH, Bethesda, MD) and Photoshop (Adobe Inc.).
Assay of Cell-Surface Expression of rOATP1A1 and rOATP1A4 in HeLa Cell Lines
Cells (1.0-1.5 × 106) were plated in 60-mm dishes (Corning #430166) and induced for 4 or 24 hours with 150 µM of ZnSO4 in antibiotic free medium. Cells were then washed three times with ice-cold PBS/CM buffer (PBS containing 0.9 mM of CaCl2 and 0.33 mM of MgCl2) at pH 7.2 and were biotinylated at 4°C for 1 hour with 0.5 mg/mL of the membrane-impermeant biotinylation reagent, sulfo-NHS-SS-biotin (Pierce), in PBS/CM (pH 8.0) as described.11 Cells were then washed once with ice-cold 50 mM of Tris-HCl in PBS/CM (pH 8.0) and incubated on ice for 5 minutes to quench any unreacted reagent. After two additional washes with PBS (pH 7.4), cell pellets were harvested, frozen on dry ice, and lysed for 30 minutes at 4°C in 350 μL of lysis buffer (PBS containing 1% Triton X-100, 1× protease inhibitor [Sigma# P8340]; pH 7.4). Following centrifugation, supernatants were adjusted to a concentration of 350 µg of protein/500 µL and were rotated overnight at 4°C with 50 μL of prewashed streptavidin-agarose beads (Pierce). Beads were washed with 10 mL of ice-cold lysis buffer, and biotinylated proteins were released by adding sodium dodecyl sulfate (SDS) sample buffer containing 0.1 M of DTT and heating for 5-10 minutes at 95°C. These eluates and starting cell lysates were subjected to western blotting analysis for detection of rOATP1A1 or rOATP1A4.
Assay of the Glycosylation State of rOATP1A1 and rOATP1A4 IN HeLa Cell Lines
OATP-expressing HeLa cells that had been incubated for 4 or 24 hours in 150 µM of ZnSO4 were harvested in PBS containing 1% Nonidet P-40, 1 mM of EDTA, and protease inhibitors. Peptide N-glycosidase F (PNGase F) and endoglycosidase H (Endo H) were obtained from New England Biolabs, Inc (Ipswich, MA). Cell lysates were incubated with these enzymes according to the manufacturer’s directions, except that denaturation was performed at room temperature for 10 minutes rather than at 100°C. Lysates were then incubated with enzyme for 2 hours at 37°C. Controls in which cell lysates were subjected to all procedures without enzyme addition were also performed. Changes in migration of OATPs attributed to deglycosylation were assessed by immunoblotting.
Immunoprecipitation Studies
HeLa cells expressing OATPs were washed with PBS, harvested from culture dishes, and pelleted at low speed (2,000 rpm). Livers from DPPIV (-) male Fisher rats23 obtained from the Einstein Liver Research Center Animal Models, Stem Cells and Cell Therapy Core were Dounce homogenized in PBS containing protease inhibitors and 1 mM of EDTA (pH 7.4) and filtered through cheesecloth. Cells and liver homogenates were incubated for 60 minutes on ice in PBS containing 1% 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), 1 mM of EDTA, and protease inhibitors, then centrifuged at 20,000g for 30 minutes at 4°C, and supernatants were incubated overnight at 4°C with nonimmune rabbit IgG or affinity-purified rOATP1A1 or rOATP1A4 antibody attached to protein A/G agarose beads (Santa Cruz Biotechnology, Dallas, TX) prepared as we described.16 Beads were washed with PBS containing 1% CHAPS and 1 mM of EDTA, incubated with SDS/polyacrylamide gel electrophoresis sample buffer containing 0.1 M of DTT, centrifuged, and the supernatant was subjected to western blotting analysis for detection of rOATP1A1.
Quantification of Uptake of 35S-Bromosulfophthalein and 3H-Digoxin
35S-bromosulfophthalein (35S-BSP), at a specific activity of 4,800 μCi/μmol, was synthesized as described.24 3H-digoxin (26.3 Ci/mmol) was obtained from PerkinElmer (Shelton, CT). Uptake was quantified as described.7 Uptake data was fit, using SigmaPlot (v11.2; Systat Software, Inc., San Jose, CA), to the equation 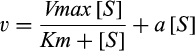 , where
, where 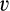 is the initial rate of uptake of 35S-BSP, [S] is the concentration of 35S-BSP, and
is the initial rate of uptake of 35S-BSP, [S] is the concentration of 35S-BSP, and 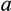 is a linear term representing non-carrier-mediated diffusional uptake.25 Saturation kinetics of 35S-BSP uptake by HEK293 cells expressing mEGFP-OATP1A1 was also determined. Time-dependent uptake of 0.1 µM of 3H-digoxin was assessed over 10-15 minutes using an identical protocol. Because uptake of 3H-digoxin was not observed (see below), saturation kinetics were not assayed. Cell protein was determined in replicate plates by the bicinchoninic acid assay (Pierce), according to the manufacturer's instructions, with BSA as the standard.
is a linear term representing non-carrier-mediated diffusional uptake.25 Saturation kinetics of 35S-BSP uptake by HEK293 cells expressing mEGFP-OATP1A1 was also determined. Time-dependent uptake of 0.1 µM of 3H-digoxin was assessed over 10-15 minutes using an identical protocol. Because uptake of 3H-digoxin was not observed (see below), saturation kinetics were not assayed. Cell protein was determined in replicate plates by the bicinchoninic acid assay (Pierce), according to the manufacturer's instructions, with BSA as the standard.
Results
Colocalization and Interaction of rOATP1A1 and rOATP1A4 in Endocytic Vesicles Prepared From Rat Liver
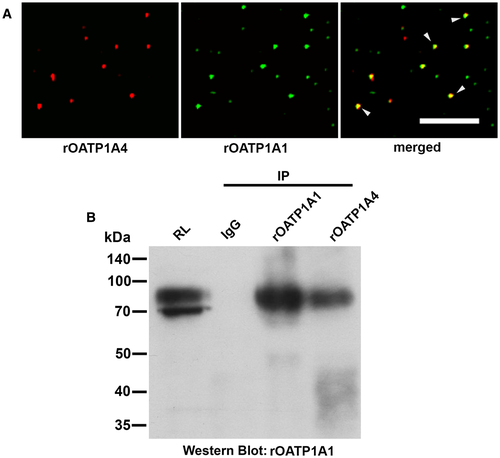
rOATP1A1 and rOATP1A4 were colocalized in 74% of the 12,044 endocytic vesicles that were examined (Fig. 1A). The fact that they are colocalized in common vesicles does not imply that they are bound to each other. This was examined by immunoprecipitation of rat liver lysate with nonimmune rabbit IgG or with rabbit antibodies to rOATP1A1 or rOATP1A4. Immunoprecipitates were analyzed by western blotting with antibody against rOATP1A1. We found that these immunoblottings were optimal when run in the absence of reduction. Antibody to rOATP1A4 immunoprecipitated rOATP1A1 (4th lane) whereas there was no reactivity when nonimmune IgG was used (second lane; representative study in Fig. 1B). rOATP1A1 immunoprecipitated itself as seen in the thirrd lane. We were unable to demonstrate rOATP1A4 in rOATP1A1 immunoprecipitates, although it immunoprecipitated itself (data not shown).
Immunofluorescence Studies in Transfected HEK293 Cells
When expression plasmids encoding sfGFP-rOATP1A1 or mRFP-rOATP1A4 were transfected individually into HEK293 cells, these transporters were distributed throughout the cell, without a clear surface membrane distribution (Fig. 2A). With PDZK1 cotransfection, mRFP-rOATP1A4 was still distributed throughout the cell (Fig. 2B, top panels) whereas sfGFP-rOATP1A1 was largely localized to the plasma membrane (Fig. 2B, bottom panels). When sfGFP-rOATP1A1 and mRFP-rOATP1A4 were cotransfected into HEK293 cells in the absence of PDZK1, they were both distributed primarily throughout cells (Fig. 2C, top panels). In contrast, both sfGFP-rOATP1A1 and mRFP-rOATP1A4 showed strong plasma membrane localization when cotransfected with PDZK1 (Fig. 2C, bottom panels).
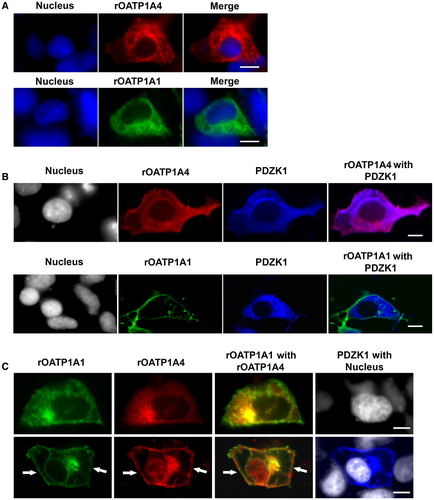
Immunoblotting Studies of OATP Expression in Transfected HeLa Cell Lines
Parental HeLa cells express PDZK1 endogenously (Fig. 3, left panel). Studies were next performed in HeLa cells stably transfected with rOATP1A1, rOATP1A4, or both under control of a zinc-inducible promoter. There was no detectable expression of either transporter in cells grown in the absence of ZnSO4 for 48 hours, contrasting with transporter expression in cells cultured with ZnSO4 (Fig. 3). These results suggested that a pulse-chase type of study could be performed to look at early events in transporter trafficking. Transporter synthesis was initiated with the addition of zinc, and trafficking of transporters to the cell surface was determined 4 and 24 hours later using a surface biotinylation and streptavidin pull-down strategy. After 4 hours of zinc addition, the majority of immunodetectable rOATP1A1 and rOATP1A4 appeared at a lower molecular weight, indicated by the asterisks, as compared to their mature forms as observed in rat liver homogenate (Fig. 4A,B). These low-molecular-weight OATPs suggest altered glycosylation similar to the unglycosylated immature forms that we described.6, 19 Identification of surface proteins by western blotting following surface biotinylation and streptavidin-agarose pulldown revealed only the fully mature forms of these proteins (arrowheads). The lower immunoblottings on each panel are for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), an intracellular protein that is present in the cell lysate, but is not labeled by biotin, validating that only proteins that reside on the cell surface are detected in the streptavidin pulldown. Antibody specificity was confirmed by including a lane of rat liver homogenate that had been extracted with 0.1 M of Na2CO3 (RL Extract) as we have described.19 Soluble proteins such as GAPDH are removed by this carbonate extraction procedure and are not observed in these lanes. rOATP1A1 that had trafficked to the cell surface (arrowhead) was observed in cells that had been singly transfected with rOATP1A1 as well as in cells that had been doubly transfected with rOATP1A1 and rOATP1A4 (Fig. 4A). In contrast, at 4 hours of zinc addition, rOATP1A4 was expressed on the cell surface only when coexpressed with rOATP1A1 (arrowhead, Fig. 4B). By 24 hours after zinc addition, little lower-molecular-weight transporter was observed in any of the cell lines (Fig. 4C,D). At this time, both rOATP1A1 and rOATP1A4 were found on the cell surface whether they were coexpressed or not.
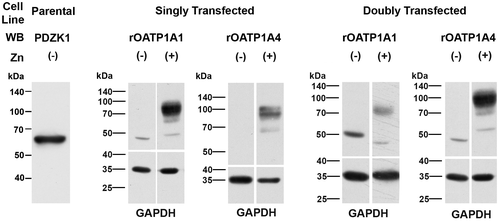
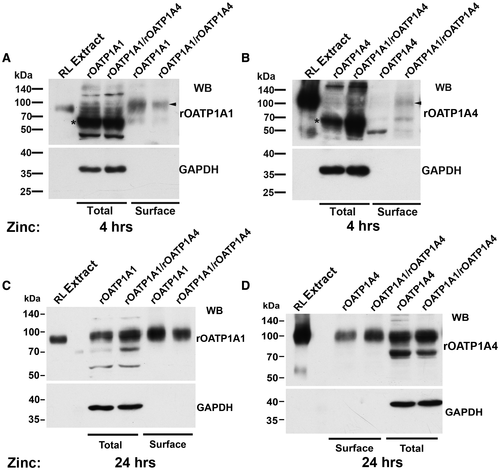
To better characterize the immature forms of the transporters, cell lysates were incubated with the glycosidases, PNGase F or Endo H. PNGase F removes all N-linked carbohydrate from glycoproteins, whereas Endo H removes only high-mannose forms that are typically located in the endoplasmic reticulum (ER) during protein biosynthesis.26, 27 PNGase F incubation resulted in removal of N-linked carbohydrate chains (deglycos) from both the mature and immature forms of rOATP1A1 and rOATP1A4, as observed by reduced molecular weight on immunoblotting (Fig. 5A). This implies that the immature form of these proteins, observed at 4 hours following induction of synthesis by addition of zinc, still contain N-linked carbohydrate. These immature proteins are deglycosylated following incubation with Endo H whereas the mature forms are not (Fig. 5B). These data indicate that the immature transporters contain high-mannose carbohydrate chains and are most likely located in the ER, before final processing in the Golgi.
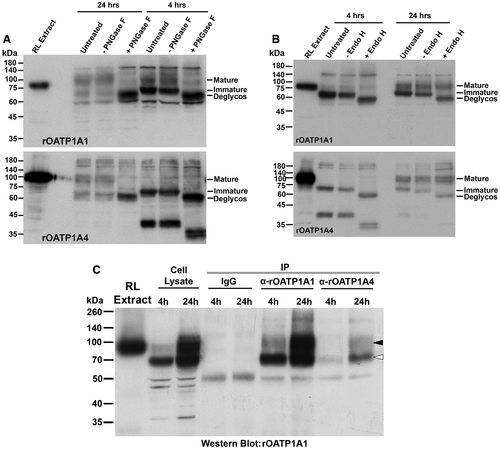
We also asked whether rOATP1A1 and rOATP1A4 interact with each other when coexpressed in the HeLa cell line. rOATP1A1 was not immunoprecipitated from doubly transfected, rOATP1A1/rOATP1A4, HeLa cell lysate with nonimmune IgG (Fig. 5C). When antibody to rOATP1A1 was used, the major band in the immunoprecipitate following a 4-hour exposure of cells to zinc corresponded to its immature form (open arrowhead) with a less-intense band corresponding to its mature fully processed form (black arrowhead). This latter band became the dominant band in the immunoprecipitate from cells following a 24-hour exposure to zinc. When immunoprecipitation was performed with antibody to rOATP1A4, both immature and mature forms of rOATP1A1 were recovered in the immunoprecipitates. This is consistent with their direct interaction during early biosynthesis in the ER, before the final glycosylation processing in the Golgi. We were unable to demonstrate rOATP1A4 in rOATP1A1 immunoprecipitates at either time point (data not shown).
Immunofluorescence Studies of OATP Expression in Transfected HeLa Cell Lines
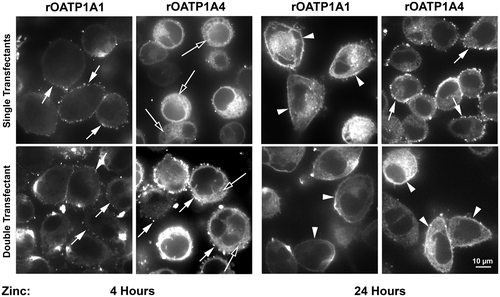
The studies presented above provide information regarding OATP biosynthesis in the entire population of HeLa cells. We next studied subcellular distribution profiles in single cells by immunofluorescence microscopy. Four hours following zinc addition, some rOATP1A1 was distributed as punctae on the cell surface whether or not it was coexpressed with rOATP1A4 (Fig. 6, left panels, short arrows). In contrast, rOATP1A4 expression at 4 hours was predominantly intracellular (long arrows) when expressed alone, but when coexpressed with rOATP1A1 punctate surface expression was observed. At 24 hours, rOATP1A1 was expressed continuously along the plasma membrane (arrowheads) whether expressed alone or together with rOATP1A4. Distribution of rOATP1A4 at 24 hours was punctate on the cell surface when expressed alone and continuous on the cell surface when expressed together with rOATP1A1.
Uptake Kinetics of 35S-BSP by Transfected HeLa Cell Lines and HEK293 Cells
Uptake of 35S-BSP by HeLa cells expressing rOATP1A1 alone or together with rOATP1A4 was determined as in Materials and Methods. Although there was a substantial increase in Vmax between 4 and 24 hours of incubation of cells in zinc, there was no significant difference in parameters in cells expressing only rOATP1A1 versus those coexpressing rOATP1A1 and rOATP1A4 (Table 1). There was little difference in uptake by either group of cells at 4 hours of zinc addition as compared to uninduced cells. Representative uptake curves are presented in Supporting Fig. S1. Uptake of 35S-BSP by HEK293 cells expressing mEGFP-rOATP1A1 was also saturable, indicating that the green fluorescent protein–associated transporter retained its transport activity (Supporting Fig. S2). Although previous studies indicated that rOATP1A4 could mediate uptake of digoxin,28 we were unable to demonstrate uptake of 3H-digoxin by rOATP1A4 expressing HeLa cells whether expressed alone or together with rOATP1A1 (Supporting Fig. S3). Statistical analysis of transport data was performed using Students’ t test and significance was set at P < 0.05.
| Cell line | rOATP1A1 | rOATP1A1 + rOATP1A4 | ||||
|---|---|---|---|---|---|---|
| Time after zinc | 0 hours | 4 hours | 24 hours | 0 hours | 4 hours | 24 hours |
| n | 4 | 6 | 4 | 4 | 6 | 4 |
| Km | 6.31 ± 3.83 | 1.88 ± 0.98 | 1.38 ± 0.73 | 6.14 ± 4.45 | 3.76 ± 1.83 | 1.52 ± 0.34 |
| Vmax | 24.75 ± 12.57 | 24.0 ± 12.0 | 153.3 ± 44.4* | 34.3 ± 20.5 | 38.5 ± 25.0 | 97.6 ± 45.9* |
| a | 0.66 ± 0.99 | 0.68 ± 0.72 | 3.1 e-08 ± 8.5 e-09 | 1.00 ± 1.14 | 0.58 ± 0.53 | 0.19 ± 0.37 |
- All cells were cultured for a total of 24 hours in the presence of 150 µM of ZnSO4 for 4 or 24 hours or in its absence as indicated. Uptake of 35S-BSP was assayed at 24 hours as described in Materials and Methods.
- * P < 0.03 as compared to cells at 0 or 4 hours after Zn addition.
Discussion
The members of the OATP family that are expressed in the liver play an important role in uptake and metabolism of a variety of endogenous compounds and xenobiotics.9, 29 These proteins are localized to the basolateral (sinusoidal) plasma membrane of hepatocytes and also have a substantial intrahepatic pool in endocytic vesicles.12 In previous studies, we found that rat and mouse OATP1A1 has a PDZ consensus sequence (KTKL) at the C-terminus that binds to PDZ domains 1 and 3 of PDZK1.16 In mice in which PDZK1 has been knocked out, trafficking of OATP1A1 to the plasma membrane is reduced,10-12 resulting in decreased transport activity because of accumulation of the transporter in endocytic vesicles within hepatocytes.10 In contrast to rOATP1A1, rOATP1A4 lacks a PDZ consensus binding site, but still traffics to the plasma membrane of hepatocytes. Interestingly, mouse OATP1A4, which is highly homologous to the rat protein, has the KTKL PDZK1 binding consensus sequence at its C-terminus. We hypothesized that rOATP1A1 and rOATP1A4 traffic together within rat hepatocytes where rOATP1A4 acts as a “hitchhiker” utilizing the rOATP1A1-PDZK1 trafficking mechanism. The results in this study are highly supportive of this notion.
Our previous studies showed that hepatocyte basolateral organic anion transporters, including OATP1A1 and sodium taurocholate cotransporting polypeptide, have substantial intracellular pools in endocytic vesicles that can traffic on microtubules toward and away from the cell surface using specific microtubule-based molecular motors.12, 30 Our current studies indicate that rOATP1A4 is also associated with these vesicles (Fig. 1A), where it is likely that it interacts directly with rOATP1A1 (Fig. 1B). Amino acid sequence comparison of the OATP family reveals enrichment in cysteine with rOATP1A1 and rOATP1A4 sharing 25 conserved cysteines.31 Although it is possible that these transporters may interact through formation of disulfide bonds, their migration in western blottings in the absence of reduction did not differ from that in the presence of reduction,19 making this possibility unlikely. Whatever the mechanism, interaction of rOATP1A4 with rOATP1A1 is necessary for its PDZK1-dependent trafficking to the cell surface, as observed in studies performed in HEK293 cells (Fig. 2). Studies in the HeLa cell lines that have endogenous expression of PDZK1 indicate that this interaction is an early event in transporter biosynthesis. Transporter expression in these cells is under regulation of a zinc-inducible promoter, and given that there is essentially no transporter expression until zinc is added to the medium, a wave of de novo synthesized transporter can be followed. Trafficking of these proteins through the cell during biosynthesis can be assessed by their glycosylation state, given that the OATPs are glycoproteins containing N-linked carbohydrate chains that are completed in the Golgi after protein synthesis in the ER.6 At 4 hours of zinc induction, the majority of each of the transporters was immunodetected in a low-molecular-weight form (Fig. 4). These low-molecular-weight forms have N-linked carbohydrate that is removed by PNGase F (Fig. 5A) as well as by Endo H (Fig. 5B), indicating high-mannose N-linked carbohydrate, consistent with localization in the ER.27 Interestingly, the low-molecular-weight form of rOATP1A1 was observed following immunoprecipitation of rOATP1A4 (Fig. 5C), indicating that their association is initiated in the ER. Only the fully glycosylated forms of these transporters are found on the cell surface, as determined by a surface biotinylation procedure. At 4 hours of zinc addition, there was little rOATP1A4 on the cell surface unless it was coexpressed with rOATP1A1 (Fig. 4B), suggesting that they traffic through the cell together. In contrast, at 24 hours of zinc addition, this facilitation of trafficking was not observed and rOATP1A4 was detected on the cell surface even in the absence of coexpression of rOATP1A1 (Fig. 4D). However, although rOATP1A4 was detectable on the cell surface at 24 hours, its distribution in the absence of coexpression with rOATP1A1 was punctate along the cell surface as compared to a smooth surface distribution when coexpressed with rOATP1A1 (Fig. 6). Of potential significance is the fact that rOATP1A1 has a similar punctate distribution on the cell surface at 4 hours after zinc addition, and these cells transport 35S-BSP poorly (Table 1). It is possible that interaction with other proteins on the cell surface is required for a more uniform distribution and fully optimal transporter activity. That these other proteins may be recruited by interaction with PDZK1 can be hypothesized. Previous studies showed that PDZK1 has four independent binding domains (PDZ 1-4) and that rOATP1A1 can bind to domains 1 and 3, leaving 2 and 4 potentially available to bind other ligands.16 In addition, PDZK1 also has a PDZ consensus sequence at its C-terminus that can interact with a PDZ binding domain on ezrin-radixin-moesin–binding phosphoprotein 50, another PDZ protein,32 thus establishing the potential for building a large scaffold of proteins associated with rOATP1A1, although further studies will be needed to clarify this.
These studies indicate that optimal rOATP1A4 trafficking to the cell surface is dependent upon coexpression and interaction with rOATP1A1. Given that rOATP1A1 binds to the chaperone protein, PDZK1, rOATP1A4 functionally hitchhikes through the cell with this complex. There did not appear to be an effect of this transporter interaction on the ability of rOATP1A1 to transport 35S-BSP, although it must be recognized that the stoichiometry of the two transporters in this HeLa cell system is unknown, and it is possible that the majority of rOATP1A1 is not bound to rOATP1A4. We have been unable to perform the corresponding transport study for rOATP1A4, because, in our hands, BSP is not transported by rOATP1A4-expressing HeLa cells (data not shown), and other than sulfolithocholyltaurine (SLCT),14 which is not readily available, we are unaware of specific ligands for this transporter. Digoxin has been shown to be taken up by hepatocytes33 and rOATP1A4 has been the presumed transporter for this process, based largely on data showing modest uptake of digoxin by rOATP1A4-cRNA injected Xenopus oocytes.28, 34 However, we have been unable to show uptake of digoxin in the rOATP1A4-inducible HeLa cell line (Supporting Fig. S3), and digoxin did not inhibit rOATP1A4-mediated uptake of SLCT by these cells.14 Uptake of digoxin by rOATP1A4 knockout mouse hepatocytes did not differ from wild type,35 whereas another pharmacokinetic study showed a subtle reduction in parameters of liver uptake of digoxin.36 Although there is some question about the identity of rOATP1A4-specific substrates, we speculate that enhanced processing and targeting of rOATP1A4 to the cell surface through its interaction with rOATP1A1 should be an important determinant of its transport function.
We suggest that this hitchhiking paradigm will hold for other OATPs, especially those that are expressed in human liver. hOATP2B1 and hOATP1A2, both found in liver, bind to PDZK1, and this regulates their subcellular trafficking and function.37, 38 Although the C-terminal PDZ binding consensus sequence of hOATP1A2 (KTKL) is identical to that of rOATP1A1, hOATP2B1, terminates in the sequence DSRV, but is still a ligand for PDZK1. hOATP1B1, another transporter in human liver, has a C-terminal sequence (ETHC), which is predicted to bind to the first PDZ binding domain of PDZK1 (http://pow.baderlab.org),39 whereas hOATP1B3 has a C-terminal sequence (AAAN), which is predicted not to bind to PDZK1 or other PDZ proteins. Of potential importance, data have been published suggesting that hOATP1B1 and hOATP1B3 may interact with each other when cotransfected into HEK293 cells.40 Although studies of the trafficking of these human OATPs is beyond the scope of the present study, the paradigms developed in this study will facilitate future elucidation of OATP interactions, trafficking, and function in human liver.




