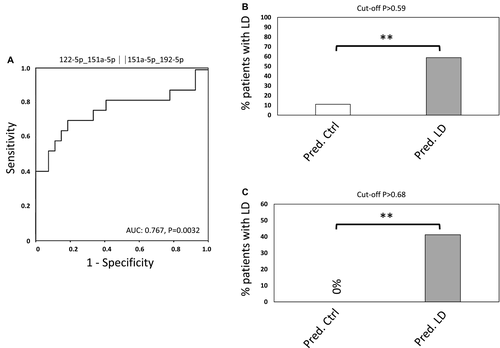
Reply:
We thank Dai et al. for their interest in our study. Indeed, plasma albumin levels significantly differed between patients with and without liver dysfunction (LD) in our exploration cohort.1 As mentioned by the authors, it is already known that decreased plasma albumin levels correlate with perioperative outcome. However, we feel quite confident about our results, as we were able to validate the predictive potential of our microRNA (miRNA) signature in an independent validation cohort in whom no significant differences in albumin levels were observed. Of note, the differences in the exploration cohort depend on 4 patients with very low albumin levels. After excluding these 4 patients (allowing for comparable albumin levels in both groups), we observed similar results as in our entire exploration cohort, indicating that albumin levels do not appear to account for the predictive potential of miRNAs (Fig. 1).
As the aim of our study was to define the potential of a preoperative plasma miRNA signature to predict surgical outcome, we strongly disagree to include intraoperative characteristics in our analyses. It is simply not valid to stratify for intraoperative characteristics when we aim to use markers to predict liver dysfunction prior to resection. Furthermore, the identified combination of miRNAs appears to identify patients at high risk for postoperative complications with very high sensitivity. Obviously, high-risk patients have the highest intraoperative blood loss, the longest operative time, and the need for Pringle maneuver and blood transfusion. Consequently, if we were to stratify for these intraoperative variables, we would stratify for patients whom we would ultimately like to identify with our preoperative marker, and this certainly would not make sense. Therefore, we acknowledge the authors comments, but disagree with the statement that “the reliability and accuracy of the conclusions of this study need further evaluation,” at least not by including intraoperative variables.
Indeed, we are in the process of further validating the reliability, accuracy, and cost-effectiveness of our marker in a multicenter trial. These data are intended to provide evidence for the clinical utility of this biomarker and to assess the cost-effectiveness of an in vitro diagnostic quantitative real-time PCR assay with rapid turnaround times.