Prognostic Role of Ammonia in Patients With Cirrhosis
Abstract
Ammonia is thought to be central to the pathogenesis of hepatic encephalopathy (HE), but its prognostic role in patients with cirrhosis and acute decompensation is unknown. The aims of this study were to determine the relationship between ammonia levels and severity of HE and its association with organ dysfunction and short-term mortality. We identified 498 patients from two institutions as part of prospective observational studies in patients with cirrhosis. Plasma ammonia levels were measured on admission and Chronic Liver Failure-Sequential Organ Failure Assessment criteria were used to determine the presence of organ failures. The 28-day patient survival was determined. Receiver operating characteristic analysis was used to identify the cutoff points for ammonia values, and multivariable analysis was performed using the Cox proportional hazard regression model. The 28-day mortality was 43.4%. Plasma ammonia correlated with severity of HE (P < 0.001), was significantly higher in nonsurvivors (93 [73-121] versus 67 [55-89] µmol/L, P < 0.001), and was an independent predictor of 28-day mortality (hazard ratio, 1.009, P < 0.001). An ammonia level of 79.5 µmol/L had sensitivity of 68.1% and specificity of 67.4% for predicting 28-day mortality. An ammonia level of ≥79.5 µmol/L was associated with a higher frequency of organ failures (liver [P = 0.004], coagulation [P < 0.001], kidney [P = 0.004], and respiratory [P < 0.001]). Lack of improvement in baseline ammonia at day 5 was associated with high mortality (70.6%). Conclusion: Ammonia level correlates with not only the severity of HE but also the failure of other organs and is an independent risk factor for mortality; lack of improvement in ammonia level is associated with high risk of death, making it an important biomarker and a therapeutic target.
Abbreviations
-
- ACLF
-
- acute on chronic liver failure
-
- AD
-
- acute decompensation
-
- AUROC
-
- area under the receiver operating characteristic curve
-
- CLIF-C
-
- Chronic Liver Failure Consortium
-
- HE
-
- hepatic encephalopathy
-
- INR
-
- international normalized ratio
-
- MELD
-
- Model for End-Stage Liver Disease
-
- OR
-
- odds ratio
-
- TLC
-
- total leucocyte count
-
- UCL
-
- University College London
-
- WH
-
- West Haven
Ammonia homeostasis is a multiorgan process involving not only the liver but also the kidneys, brain, gastrointestinal tract, and muscle. Hyperammonemia is thought to play a central role in the pathogenesis of hepatic encephalopathy (HE). In acute liver failure, elevated plasma ammonia levels are predictive of cerebral edema and herniation.1 In patients with cirrhosis with acute decompensation (AD), this relationship is less well established.2 Infection and inflammation, which are important contributory factors in the pathogenesis of HE in acute liver failure, are also thought to be the key mediators of HE in cirrhosis.3, 4 In patients with cirrhosis, clinically relevant cerebral edema is an infrequent occurrence, observed in only up to 5% of patients with acute on chronic liver failure (ACLF).5 However, in cirrhosis ammonia levels can predict risk and frequency of HE episodes6 and HE-related admissions, and in ACLF patients higher levels are observed in those with HE compared to those without. A failure in the reduction of ammonia levels in ACLF over time increases the probability for death.7 Even in ACLF survivors without HE, a serial decrease in ammonia levels over 7 days has been observed, possibly suggesting an additional role of ammonia in outcomes of ACLF other than HE.7 Admission ammonia levels have also been shown to be predictive of in-hospital survival in patients with alcoholic hepatitis8 and of transplant-free survival of AD patients with cirrhosis9 and are lower in ACLF patients who survive compared to those who die.10
In addition to the well-known neurotoxic effects, hyperammonemia has a deleterious effect on several organ systems, which may augment inflammation and/or organ injury.11 Hyperammonemia may directly induce hepatic injury,12 potentiate immune dysfunction13, 14 and hepatic stellate cell activation,15 and contribute to the pathogenesis of sarcopenia,16, 17 which is an independent predictor of mortality in cirrhosis.18
Thus, while a direct pathophysiological link between hyperammonemia and nonneurological organ injury is not well established, it is possible that ammonia may play an indirect role in mediating multiorgan dysfunction through stellate cell activation and inflammation, increased susceptibility to infections, and reduced capacity for recovery through sarcopenia in cirrhosis and AD other than HE, thereby contributing independently to mortality. The aims of this study were to (1) determine whether ammonia is increased in HE patients and whether this defines outcome, (2) determine whether ammonia is associated with organ dysfunction and therefore mortality, and (3) determine whether a change in ammonia levels defines outcomes.
Patients and Methods
Patients
The study included a total of 498 patients with cirrhosis from a combination of three different cohorts from two cities; London, UK, and New Delhi, India.
Cohort 1
Consecutive AD patients (n = 291) admitted to the Department of Gastroenterology, All India Institute of Medical Sciences, New Delhi, between January 2012 and December 2015, were prospectively recruited. All patients had baseline arterial ammonia measured at admission.
Cohort 2
This population was a nested cohort of 101 patients within a previously reported larger prospective cohort study of patients with cirrhosis with AD admitted to University College London (UCL) Hospital, London, between 2000 and 2006.7 Those requiring admission to the intensive care unit were included. All patients had arterial ammonia measured on admission.
Patients were included in both of these protocols if they had a clinical, radiological, or histological diagnosis of cirrhosis. The Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) score was used to determine the presence of organ failures and grade of ACLF.19 The West Haven (WH) classification20 was used to determine the severity of HE, and accordingly patients were divided into three groups; no or mild HE (WH grade 0-1), moderate HE (WH grade 2), and severe HE (WH grade 3-4). Exclusion criteria included admissions for reasons other than AD; severe comorbid diseases, especially established cardiovascular or renal disease; malignancy (extrahepatic or hepatocellular carcinoma); pregnancy; and history of any major surgery.
Cohort 3
This population of 106 patients was a part of a prospective study at UCL Hospitals between 2008 and 2010 to determine the characteristics and outcomes of patients with cirrhosis with grade 1 and minimal HE.21 This group comprised clinically stable patients with cirrhosis, and all had baseline venous ammonia measured at enrollment. This cohort comprised patients with cirrhosis without overt HE (less than WH grade 2). Exclusion criteria included active infection, presence of any organ failure, and hepatic or extrahepatic malignancy.
For the patients included in the study in New Delhi, India, the study was approved by the institute’s ethics committee, and patients were included prospectively. The UCL Hospitals ethics committee approved the studies in the UK. All patients included in the studies both in the UK and also in India provided informed consent and were recruited prospectively. If a patient did not have capacity to consent, assent from next of kin was obtained with retrospective consent from the patient, in accordance with the 1975 Declaration of Helsinki.
Management
Local protocols were used to manage hospitalized patients. All patients had protocol screening tests to identify possible sepsis, including blood, urine, and ascitic fluid cultures, and imaging as clinically indicated. Broad-spectrum antibiotics were used to treat infection as per hospital guidelines. Intravenous crystalloids and/or human albumin were used for fluid resuscitation and terlipressin if treating hepatorenal syndrome. In those with HE, bowel-cleansing agents (lactulose with or without phosphate enemas) were used to achieve two to three bowel motions per day. Organ support with continuous venovenous hemofiltration for renal failure, intubation, and ventilation for respiratory failure and vasopressors for circulatory failure were used as and when indicated.
Data Collection
Baseline clinical, demographic, and biochemical data were recorded prospectively at the time of enrollment. Prognostic scores (including Child-Pugh, Model for End-Stage Liver Disease [MELD], CLIF-SOFA, and CLIF Consortium [CLIF-C] ACLF) were subsequently calculated using parameters obtained at baseline. Follow-up was for 28 days from inclusion or until death or liver transplantation, if before. Ammonia samples were taken prior to institution of any organ support and transported on ice, and plasma ammonia was measured either by an enzymatic method (Randox Lab Ltd., UK) or spectrophotometrically (CobasMiraS; Hoffman-LaRoche, Switzerland). Where possible, repeat ammonia measurements were made at 5 days after baseline.
Statistical Analyses
Continuous variables were expressed as median (interquartile range). Categorical data were presented as proportions. Comparison of demographics and clinical features was done using chi-squared or Fisher’s exact test for categorical variables and the Mann-Whitney U test for continuous variables. Multivariable analysis was performed using the Cox proportional hazard regression model.
Receiver operating characteristic curves were used to identify the cutoff points for baseline ammonia values. The Kaplan-Meier method was used to generate survival curves. The data were analyzed using SPSS statistics software (version 20.0; SPSS, Chicago, IL), and Medcalc software (version 15.11.4; MedCalc Software, Ostend, Belgium).
Results
A total of 498 patients with cirrhosis were included. The majority of patients (72%) were male, with a median age of 48 (39-56) years. The most common etiology of cirrhosis was alcohol (53.4%), followed by hepatitis B (14.7%): 190 (38.2%) had no ACLF, 79 (15.9%) had ACLF grade 1, 135 (27.1%) had ACLF grade 2, and 94 (18.9%) had ACLF grade 3. Overall, 28-day mortality was 43.4%. Overt encephalopathy (grade 2 or above) was present in 39.6% (197) patients. Baseline demographic and clinical characteristics with respect to varying grades of HE are described in Table 1.
| Baseline Characteristic |
Grade 0/1 HE (n = 301) |
Grade 2 HE (n = 116) |
Grade 3/4 HE (n = 81) |
P |
|---|---|---|---|---|
| Predisposition | ||||
| Age (years) | 47 (39-56) | 52 (43-62) | 45 (35-55) | 0.001*, † |
| Male:female | 215 (71.4%):86 (28.6%) | 79 (68.1%):37 (31.9%) | 65 (80.2%):16 (19.8%) | 0.160 |
| Etiology (CLD) | 0.089 | |||
| Alcohol | 151 (50.2%) | 66 (56.9%) | 49 (60.5%) | |
| Autoimmune | 13 (4.3%) | 6 (5.2%) | 4 (4.9%) | |
| HBV | 47 (15.6%) | 13 (11.2%) | 13 (16.0%) | |
| HCV | 18 (6.0%) | 12 (10.3%) | 0 | |
| HBV + alcohol | 4 (1.3%) | 0 | 0 | |
| HCV + alcohol | 4 (1.3%) | 3 (2.6%) | 0 | |
| Cryptogenic | 34 (11.3%) | 7 (6.0%) | 11 (13.6%) | |
| Others | 30 (10.0%) | 9 (7.8%) | 4 (4.9%) | |
| Precipitating illness | ||||
| Alcohol | 77 (25.6%) | 10 (8.6%) | 21 (25.9%) | |
| Gastrointestinal bleeding | 15 (5.0%) | 16 (13.8%) | 11 (13.6%) | |
| Bacterial infection | 34 (11.3%) | 10 (8.6%) | 23 (28.4%) | |
| Acute hepatitis A/B/E | 49 (16.3%) | 13 (11.2%) | 11 (13.6%) | |
| Other/unidentified | 126 (41.9%) | 67 (57.8%) |
15 (18.5%) |
|
| Organ failures | ||||
| Liver | 155 (51.5%) | 33 (28.4%) | 41 (50.6%) | <0.001*, † |
| Kidney | 62 (20.6%) | 21 (18.1%) | 36 (44.4%) | <0.001†, ‡ |
| Coagulation | 64 (21.3%) | 22 (19.1%) | 33 (40.7%) | <0.001†, ‡ |
| Circulation | 17 (5.6%) | 7 (6.0%) | 5 (6.2%) | 0.978 |
| Respiratory | 70 (23.3%) | 33 (28.4%) | 46 (56.8%) | <0.001†, ‡ |
| Laboratory values | ||||
| Ammonia (μmol/L) | 73 (57-97) | 77 (60-99) | 97 (79-121) | <0.001†, ‡ |
| TLC (× 109) | 9.5 (6.4-14.6) | 9.5 (5.7-15.2) | 13.3 (8.8-19.1) | 0.001†, ‡ |
| Platelets (× 109) | 110 (70.0-184.0) | 106 (65-182) | 110 (69-162) | 0.621 |
| Bilirubin (mg/dL) | 13.0 (4.7-22.8) | 7.0 (3.3-16.0) | 10.5 (5.1-23.1) | 0.002*, † |
| INR | 1.8 (1.5-2.3) | 1.7 (1.4-2.1) | 2.3 (1.7-2.9) | <0.001†, ‡ |
| Albumin (g/dL) | 2.6 (2.2-3.0) | 2.8 (2.4-3.1) | 2.6 (2.3-3.0) | 0.568 |
| Creatinine (mg/dL) | 1.1 (0.7-1.7) | 1.0 (0.7-1.7) | 1.9 (1.1-3.6) | <0.001†, ‡ |
| Scores | ||||
| ACLF grades | <0.001 | |||
| ACLF 0 | 135 (44.9%) | 48 (41.4%) | 7 (8.6%) | |
| ACLF 1 | 47 (15.6%) | 29 (25.0%) | 3 (3.7%) | |
| ACLF 2 | 91 (30.2%) | 29 (25.0%) | 15 (18.5%) | |
| ACLF 3 | 28 (9.3%) | 10 (8.6%) | 56 (69.1%) | |
| MELD | 25 (20-31) | 22 (16-30) | 32 (23- 40) | <0.001*-‡ |
| Child-Pugh | 11 (9-12) | 9 (8-12) | 12 (11-13) | <0.001†, ‡ |
| CLIF-C ACLF (those with ACLF) | 49.0 (43.6-54.7) | 51.3 (44.7-56.8) | 54.2 (48.3-61.7) | <0.001†, ‡ |
| 28-day mortality (%) | 107 (35.5%) | 48 (41.4%) | 61 (75.3%) | <0.001†, ‡ |
- All data are expressed as n (%) or median (interquartile range), unless otherwise specified.
- * Significant between grade 0/1 HE and grade 2 HE.
- † Significant between grade 2 HE and grade 3/4 HE.
- ‡ Significant between grade 0/1 and grade 3/4 HE.
- Abbreviations: CLD, chronic liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus.
Factors Associated With HE
There were 301 patients (60.4%) who had no or mild HE (grade 0/1). Of the 197 patients with overt HE, 116 (58.9%) patients had grade 2 HE, whereas 81 (41.1%) had severe HE (grade 3 or 4). ACLF was more common in those with overt encephalopathy; 55.1% of patients with grade 0/1 HE had ACLF compared with 58.6% in those with grade 2 HE and 91.4% in those with grade 3/4. Bacterial infection was the most common precipitating event in those with grade 3/4 HE (28.4%).
Ammonia levels were significantly higher in patients with grade 3/4 HE (97 µmol/L [79-121]) compared to those with grade 2 (77 µmol/L [60-99]) and grade 0/1 HE (73 µmol/L [57-97]) (P < 0.001). On univariate analysis, total leukocyte count (TLC), creatinine, ammonia, respiratory, and kidney failures were predictive of overt HE (Supporting Table S1). Different multivariate models were made to analyze which factors remained associated with overt HE. Model one included TLC, creatinine, bilirubin, and ammonia. Ammonia (odds ratio [OR], 1.006; P = 0.022) and creatinine (OR, 1.189; P = 0.004) remained independently predictive of overt HE but in model two including TLC, ammonia, and liver, kidney, coagulation, and respiratory failures, only TLC (OR, 1.028; P = 0.032), coagulation (OR, 1.599; P = 0.044), and respiratory failure (OR, 2.059; P = 0.001) remained significantly associated. Factors associated with grade 3/4 HE were age, TLC, international normalized ratio (INR), creatinine, ammonia, kidney, coagulation, and respiratory failures (Table 2). In a multivariate model excluding organ failures, ammonia (OR, 1.010; P = 0.005), INR (OR, 1.353; P = 0.0044), and creatinine (OR, 1.463; P < 0.001) remained independently associated with grade 3/4 HE. In multivariate analysis including organ failures, kidney (OR, 2.384; P = 0.013) and respiratory (OR, 8.149; P < 0.001) failures remained associated with grade 3/4 HE.
| Univariate Model | Multivariate Model (All Significant Continuous Variables) | Multivariate Model (Includes All Organ Failures) | Multivariate Model (3): Without Respiratory Failure | P | ||||
|---|---|---|---|---|---|---|---|---|
| OR | P | OR | P | OR | P | OR | ||
| Age | 0.975 (0.958-0.993) | 0.005 | 0.976 (0.952-1.001) | 0.061 | 0.970 (0.946-0.995) | 0.018 | 0.976 (0.953-0.999) | 0.045 |
| Sex (female) | 0.588 (0.327-1.057) | 0.076 | 0.897 (0.415-1.937) | 0.782 | 0.892 (0.405-1.967) | 0.777 | 0.829 (0.391-1.757) | 0.625 |
| TLC | 1.049 (1.022-1.076) | <0.001 | 1.016 (0.982-1.051) | 0.360 | 1.011 (0.977-1.046) | 0.522 | 1.018 (0.986-1.052) | 0.273 |
| INR | 1.564 (1.245-1.964) | <0.001 | 1.353 (1.009-1.815) | 0.044 | NI | NI | ||
| Creatinine | 1.428 (1.252-1.628) | <0.001 | 1.463 (1.231-1.738) | <0.001* | NI | NI | ||
| Total bilirubin | 1.017 (0.997-1.037) | 0.090 | 0.978 (0.947-1.010) | 0.167 | ||||
| AST | 1.002 (1.000-1.003) | 0.100 | 1.000 (0.998-1.003) | 0.650 | 0.999 (0.997-1.001) | 0.467 | 1.000 (0.998-1.002) | 0.906 |
| ALT | 1.001 (0.999-1.003) | 0.230 | ||||||
| Albumin | 0.812 (0.530-1.244) | 0.340 | ||||||
| Ammonia | 1.011 (1.006-1.017) | <0.001 | 1.010 (1.003-1.017) | 0.005 | 1.002 (0.995-1.009) | 0.544 | 1.009 (1.002-1.015) | 0.008 |
| MAP | 0.992 (0.974-1.011) | 0.409 | ||||||
| Organ failures | ||||||||
| Liver | 1.249 (0.775-2.010) | 0.361 | ||||||
| Kidney | 3.219 (1.953-5.307) | <0.001 | 2.384 (1.200-4.737) | 0.013 | 3.239 (1.719-6.105) | <0.001 | ||
| Coagulation | 2.630 (1.591-4.348) | <0.001 | 1.882 (0.936-3.781) | 0.076 | 1.534 (0.803-2.931) | 0.195 | ||
| Circulation | 1.077 (0.399-2.912) | 0.883 | ||||||
| Respiratory | 4.007 (2.448-6.558) | <0.001 | 8.149 (3.933-16.887) | <0.001 | NI | |||
- Variables selected for multivariate model if P ≤ 0.10.
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; MAP, mean arterial pressure; NI, not included.
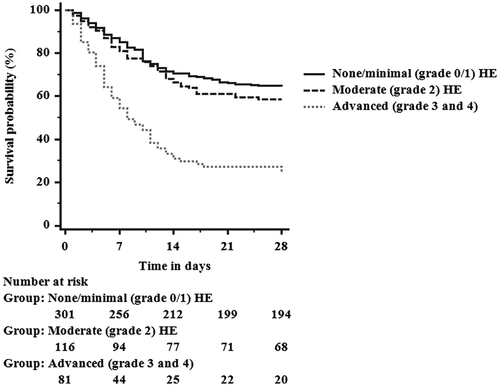
Those with grade 3/4 HE had higher MELD and CLIF-C ACLF scores compared to grade 0/1 HE (32 versus 25 and 54.2 versus 49.0, respectively; P < 0.001). Patients with more advanced stages of HE had increased mortality at 28 days (75.3%) compared to 35.5% mortality in those with grade 0/1 HE (P < 0.001); survival curves stratified by severity of HE are presented in Fig. 1.
Ammonia as a predictor of overt HE had an area under the receiver operating characteristic curve (AUROC) of 0.600 (0.550-0.650) (Supporting Fig. S1). The optimal cutoff (Youden’s index) of baseline ammonia as a predictor of overt HE was 79.5 µmol/L, with a sensitivity of 56% and a specificity of 57%. The AUROC of ammonia as a predictor of grade 3/4 was 0.690 (0.632-0.749). Overall, 239 patients (48.0%) had baseline ammonia ≥79.5 µmol/L (Table 3). In the group with ammonia ≥79.5 µmol/L, the incidence of overt HE was significantly higher at 46.1% compared to 33.6% in those with ammonia ≤79.5 µmol/L (P < 0.001).
| Ammonia <79.5 (n = 259) |
Ammonia ≥79.5 (n = 239) |
P | |
|---|---|---|---|
| HE | <0.001 | ||
| Grade 0/1 | 172 (66.4%) | 129 (54.0%) | |
| Grade 2 | 64 (24.7%) | 52 (21.8%) | |
| Grade 3/4 | 23 (8.9%) | 58 (24.3%) | |
| Organ failures | |||
| Liver | 103 (39.8%) | 126 (52.7%) | 0.004 |
| Kidney | 48 (18.5%) | 71 (29.7%) | 0.004 |
| Brain | 23 (8.9%) | 58 (24.3%) | <0.001 |
| Coagulation | 42 (16.3%) | 77 (32.2%) | <0.001 |
| Circulation | 10 (3.9%) | 19 (7.9%) | 0.057 |
| Respiratory | 58 (22.4%) | 91 (38.1%) | <0.001 |
| ACLF grade | |||
| Grade 0 | 131 (50.6%) | 59 (24.7%) | <0.001 |
| Grade 1 | 38 (14.7%) | 41 (17.2%) | |
| Grade 2 | 64 (24.7%) | 71 (29.7%) | |
| Grade 3 | 26 (10.0%) | 68 (28.5%) | |
| 28-day mortality | 69 (26.6%) | 147 (61.5%) | <0.001 |
Ammonia Level and its Association With Other Organ Failures
An ammonia level ≥79.5 µmol/L was associated with a higher frequency of organ failures (liver 52.7% versus 39.8% [P = 0.004], coagulation 32.2% versus 16.3% [P < 0.001], kidney 29.7% versus 18.5% [P = 0.004], and respiratory 38.1% versus 22.4% [P < 0.001]). Accordingly, in the higher ammonia group a larger proportion of patients had ACLF (75.3% versus 49.4% in the lower ammonia group, P < 0.001).
Ammonia as a Predictor of Survival
Overall mortality at 28 days was 43.4% (n = 216) (Table 4). Nonsurvivors were younger (45 versus 50, P < 0.001), more likely to be male (P = 0.009), and more likely to have alcohol-related cirrhosis and alcohol abuse as a precipitating illness. Ammonia levels were higher in nonsurvivors compared with survivors (93 versus 67 µmol/L, P < 0.001). Nonsurvivors had a higher frequency of all organ failures (liver, kidney, brain, coagulation, respiratory; P < 0.001) except circulatory failure (P = 0.052). Of those who died, 84.7% had ACLF at baseline compared to only 44.3% of survivors (P < 0.001). Baseline MELD, Child-Pugh, and CLIF-C ACLF scores were all higher in nonsurvivors compared to survivors (30.5 versus 22.4, 12 versus 10, and 53.1 versus 47.5, respectively; P < 0.001).
|
Survived (n = 282) |
Died (n = 216) |
P | |
|---|---|---|---|
| Predisposition | |||
| Age (years) | 50 (41-59) | 45 (36-55) | <0.001 |
| Male:female | 190 (67.4%):92 (32.6%) | 169 (78.2%):47 (21.8%) | 0.009 |
| Etiology (CLD) | <0.001 | ||
| Alcohol | 140 (49.6%) | 126 (58.3%) | |
| Autoimmune | 12 (4.3%) | 11 (5.1%) | |
| HBV | 43 (15.2%) | 30 (13.9%) | |
| HCV | 30 (10.6%) | 0 | |
| Cryptogenic | 27 (9.6%) | 25 (11.6%) | |
| HBV + alcohol | 4 (1.4%) | 0 | |
| HCV + alcohol | 7 (2.5%) | 0 | |
| Others | 19 (6.7%) | 24 (11.1%) | |
| Precipitating illness | |||
| Alcohol | 37(13.1%) | 71 (32.8%) | |
| Gastrointestinal bleeding | 17 (6.0%) | 20 (9.3%) | |
| Bacterial infection | 30 (10.6%) | 37 (17.1%) | |
| Acute hepatitis A/B/E | 43 (15.2%) | 30 (13.9%) | |
| Other/unidentified | 155 (55.0%) | 58 (26.9%) | |
| Organ failures | |||
| Liver | 98 (34.8%) | 131 (60.6%) | <0.001 |
| Kidney | 37 (13.1%) | 82 (38.0%) | <0.001 |
| Cerebral | 20 (7.1%) | 61 (28.2%) | <0.001 |
| Coagulation | 37 (13.2%) | 82 (38.0%) | <0.001 |
| Circulation | 11 (3.9%) | 18 (8.3%) | 0.052 |
| Respiratory | 61 (21.6%) | 88 (40.7%) | <0.001 |
| Laboratory values | |||
| Ammonia (μmol/L) | 67 (55-89) | 93 (73-121) | <0.001 |
| TLC (× 109) | 8.2 (5.4-11.7) | 13.5 (9.0-18.5) | <0.001 |
| Platelets (× 109) | 114 (81-184) | 106 (65-176) | 0.148 |
| Bilirubin (mg/dL) | 7.1 (3.4-17.8) | 16.9 (7.1-24.8) | <0.001 |
| INR | 1.6 (1.4-2.1) | 2.2 (1.7-2.9) | <0.001 |
| Albumin (g/dL) | 2.7 (2.4-3.1) | 2.5 (2.1-3.0) | <0.001 |
| Creatinine (mg/dL) | 1.0 (0.7-1.4) | 1.6 (0.9-2.7) | <0.001 |
| Scores | |||
| ACLF 0 | 157 (55.7%) | 33 (15.3%) | <0.001 |
| ACLF 1 | 46 (16.3%) | 33 (15.3%) | |
| ACLF 2 | 60 (21.3%) | 75 (34.7%) | |
| ACLF 3 | 19 (6.7%) | 75 (34.7%) | |
| MELD | 22.4 (16.5-27.5) | 30.5 (25.0-37.5) | <0.001 |
| Child-Pugh | 10 (9-12) | 12 (10-13) | <0.001 |
| CLIF-C ACLF (those with ACLF) | 47.5 (42.5-54.0) | 53.1 (47.0-59.7) | <0.001 |
- Abbreviations: CLD, chronic liver disease; HBV, hepatitis B virus; HCV, hepatitis C virus.
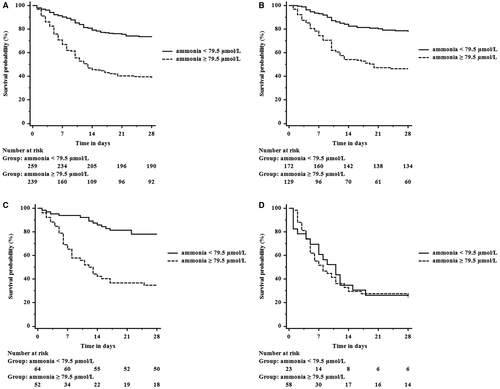
In univariate analysis, increased ammonia was significantly associated with reduced 28-day survival (hazard ratio [HR], 1.011; P < 0.001) (Table 5). On multivariate analysis ammonia remained independently predictive of death (HR 1.009, P < 0.001). Ammonia as a predictor of 28-day mortality had an AUROC of 0.726 (0.682-0.770) (Supporting Fig. S2). Those with an ammonia level of ≥79.5 µmol/L had a greater probability of death compared to those with an ammonia level of ≤79.5 µmol/L (61.5% versus 26.6%, P < 0.001) (Fig. 2A) (sensitivity of 68.1% and specificity of 67.4%). To determine whether ammonia levels affected mortality independently of the degree of HE, we calculated Kaplan-Meier curves within the three severity categories of HE using an ammonia level of 79.5 µmol/L as a cutoff (Fig. 2B-D). In patients with grade 0/1 or grade 2 HE, those with an ammonia level ≥79.5 µmol/L had significantly higher mortality compared to those with an ammonia concentration <79.5 µmol/L (P < 0.001).
| Unadjusted HR (95% CI) | P | Adjusted HR (95% CI) | P | |
|---|---|---|---|---|
| HE | ||||
| None, minimal | 1 | NI | ||
| Moderate | 1.208 (0.859-1.697) | 0.278 | ||
| Advanced | 3.213 (2.341-4.410) | <0.001 | ||
| Age | 0.983 (0.974-0.993) | <0.001 | 1.005 (0.994-1.016) | 0.390 |
| Sex | ||||
| Male | 1 | |||
| Female | 0.650 (0.470-0.898) | 0.009 | 0.632 (0.449-0.888) | 0.008 |
| MAP | 0.983 (0.973-0.994) | 0.002 | NI | |
| TLC | 1.065 (1.051-1.078) | <0.001 | 1.039 (1.024-1.055) | <0.001 |
| Creatinine | 1.187 (1.128-1.248) | <0.001 | NI | |
| Total bilirubin | 1.026 (1.016-1.037) | <0.001 | NI | |
| Ammonia | 1.011 (1.009-1.013) | <0.001 | 1.009 (1.006-1.012) | <0.001 |
| INR | 1.632 (1.462-1.822) | <0.001 | ||
| Organ failures | ||||
| Liver | 2.152 (1.637-2.829) | <0.001 | 1.686 (1.259-2.257) | <0.001 |
| Kidney | 2.791 (2.116-3.680) | <0.001 | 1.728 (1.284-2.326) | <0.001 |
| Cerebral | 3.041 (2.256-4.099) | <0.001 | 1.604 (1.151-2.235) | 0.005 |
| Coagulation | 2.672 (2.027-3.524) | <0.001 | 1.973 (1.452-2.680) | <0.001 |
| Circulation | 1.790 (1.105-2.900) | 0.018 | 1.459 (0.882-2.413) | 0.141 |
| Respiratory | 2.116 (1.612-2.777) | <0.001 | 1.433 (1.059-1.938) | 0.020 |
- Abbreviations: CI, confidence interval; MAP, mean arterial pressure; NI, not included.
Change in Ammonia Levels, Severity of HE, and Mortality
In 86 patients with AD, repeat ammonia measurements were available at day 5. Of these, 74% were male (n = 64), alcohol was the leading cause of chronic liver disease 37 (43%), and 71% (n = 61) had ACLF (Table 6). None of these patients were receiving renal replacement therapy. A decrease in ammonia at day 5 from baseline (n = 28) was associated with no change or improvement in HE for the majority of patients (85.7%). In those patients where there was no change or an increase in ammonia levels between admission and day 5 (n = 58), 82.7% had worsening of or no improvement in HE grade. Progression of HE from grade 0/1 on day 1 to grades 2-4 on day 5 was associated with an average increase in ammonia of 17 μmol/L (P = 0.025). However, no significant change in ammonia levels was observed if the grades of HE remained the same or improved (Supporting Table S2).
| Baseline Characteristic |
Ammonia Decreased (n = 28) |
Ammonia No Change (n = 17) |
Ammonia Increased (n = 41) |
P |
|---|---|---|---|---|
| Predisposition | ||||
| Age (years) | 35 (26-46) | 43 (39-55) | 41 (33-52) | 0.064 |
| Male:female |
16 (57.1%) 12 (42.9%) |
16 (94.1%) 1 (5.9%) |
32 (78.0%) 9 (22.0%) |
0.017*,† |
| Etiology (CLD) | 0.430 | |||
| Alcohol | 10 (35.7%) | 10 (58.8%) | 17 (41.5%) | |
| Autoimmune | 3 (10.7%) | 0 | 4 (9.8%) | |
| HBV | 7 (25.0%) | 3 (17.6%) | 6 (14.6%) | |
| Cryptogenic | 4 (14.3%) | 4 (23.5%) | 6 (14.6%) | |
| Others | 4 (14.3%) | 0 | 8 (19.5%) | |
| Precipitating illness | 0.662 | |||
| Alcohol | 9 (32.1%) | 8 (47.1%) | 13 (31.7%) | |
| Gastrointestinal bleeding | 2 (7.1%) | 2 (11.8%) | 1 (2.4%) | |
| Bacterial infection | 5 (17.9%) | 1 (5.9%) | 5 (12.2%) | |
| Acute hepatitis A/B/E | 6 (21.5%) | 3 (17.6%) | 10 (24.4%) | |
| Other/unidentified | 6 (21.3%) | 3 (17.6%) | 12 (29.3%) | |
| Organ failures | ||||
| Liver | 12 (42.9%) | 11 (64.7%) | 29 (70.7%) | 0.062 |
| Kidney | 6 (21.4%) | 7 (41.2%) | 15 (36.6%) | 0.293 |
| Brain | 6 (21.4%) | 3 (17.6%) | 11 (26.8%) | 0.724 |
| Coagulation | 9 (32.1%) | 5 (29.4%) | 18 (43.9%) | 0.464 |
| Circulation | 3 (10.7%) | 0 | 3 (7.3%) | 0.390 |
| Respiratory | 8 (28.6%) | 3 (17.6%) | 10 (24.4%) | 0.710 |
| Laboratory values | ||||
| Ammonia (μmol/L) | 104 (88-152) | 78 (68-105) | 91 (73-111) | 0.036* |
| TLC (× 109) | 9.2 (6.9-14.6) | 12.8 (8.9-18-4) | 10 (7.9-17.5) | 0.148 |
| Platelets (× 109) | 138 (73- 208) | 144 (76-225) | 114 (79-193) | 0.782 |
| Bilirubin (mg/dL) | 8.8 (4.7-19.9) | 18.4 (9.1-22.5) | 17.9 (8.7-24.9) | 0.116 |
| INR | 2.1 (1.7-3.3) | 2.1 (1.6-2.5) | 2.2 (1.8-3.3) | 0.621 |
| Albumin (g/dL) | 2.6 (2.0-3.0) | 2.5 (1.9-2.9) | 2.5 (2.2-3.0) | 0.832 |
| Creatinine (mg/dL) | 0.9 (0.8-1.9) | 1.2 (0.8-3.5) | 1.4 (0.9-2.5) | 0.118 |
| Scores | ||||
| ACLF grades | 0.311 | |||
| ACLF 0 | 9 (32.1%) | 4 (23.5%) | 12 (29.3%) | |
| ACLF 1 | 4 (14.3%) | 2 (11.8%) | 1 (2.4%) | |
| ACLF 2 | 10 (35.7%) | 8 (47.1%) | 13 (31.7%) | |
| ACLF 3 | 5 (17.9%) | 3 (17.6%) | 15 (36.6%) | |
| MELD | 27 (23-33) | 29 (24-37) | 31 (24-37) | 0.144 |
| Child-Pugh | 11 (10-13) | 11 (10-13) | 11 (10-13) | 0.907 |
| CLIF-C ACLF (those with ACLF) | 42.7 (40.0-46.2) | 47.3 (40.0-60.0) | 51.3 (45.6-59.7) | 0.005*, ‡,‡ |
| Delta ammonia (day 5- day 1) | 37 (17-55) | –1 (–3 to 2) | –23 (–50 to –14) | <0.001*,†,‡ |
| HE change (improved:no change:worsened) | 4 (14.3%):22 (78.6%):2 (7.1%) | 2 (11.8%):11 (64.7%):4 (23.6%) | 8 (19.5%):23 (56.1%):10 (24.4%) | 0.312 |
| 28-day mortality (%) | 10 (35.7%) | 11 (64.7%) | 30 (73.2%) | 0.007 |
- All data are expressed as n (%) or median (IQR), unless otherwise specified.
- * Significant between ammonia decrease and no change.
- † Significant between ammonia no change and increase.
- ‡ Significant between ammonia decrease and increase.
- Abbreviations: CLD, chronic liver disease; HBV, hepatitis B virus.
No change or worsening of ammonia at day 5 (n = 58) was associated with a 28-day mortality of 70.6% (n = 41) compared to 35.7% (n = 10) in those with an improvement in baseline ammonia (n = 28) (P = 0.007). In those who survived, the change in ammonia (days 1-5) was 10 μmol/L (–11 to 40) compared to an ammonia change (days 1-5) of –13 μmol/L (–26 to 2) in nonsurvivors (P = 0.004).
Discussion
This study of patients with cirrhosis, many with ACLF, adds significantly to the evidence that ammonia levels correlate with not only the severity of HE but also the failure of other organs in cirrhosis and is an independent risk factor for 28-day mortality. The data provide evidence that the ammonia level not only has a clinically relevant utility in determining the severity of HE but can provide important prognostic information, signifying its potential role as a biomarker in identifying patients at high risk of mortality. A reduction in ammonia level was associated with improved survival, confirming it as a potential therapeutic target.
Studies assessing the role of ammonia in HE have had variable results, in part due to differing patient cohorts and study designs.2, 3, 7, 22 This study demonstrates a significant correlation between increasing ammonia levels and severity of HE, which supports a role for ammonia in the development of HE. The data presented here confirm that the severity of HE is associated with increased mortality.4, 23 However, in patients with the same grade of HE, an ammonia level ≥79.5 µmol/L is associated with increased mortality, indicating an additional role of ammonia in dictating clinical outcomes other than HE severity. At present, it is not clear whether reducing ammonia levels would result in a reduction in mortality. Sidhu et al.24 compared L-ornithine L-aspartate with placebo in patients with HE and AD. Although there was a significant reduction in ammonia in the drug arm, no differences in survival were observed, possibly because of the relatively low sample size. In a meta-analysis of L-ornithine L-aspartate for prevention and treatment of HE that included 1,489 patients, L-ornithine L-aspartate administration was associated with a relative reduction in mortality compared to placebo (relative risk, 0.42; 0.24-0.72), suggesting that ammonia lowering may improve survival of patients with cirrhosis with AD.25 The data showing that an increase in ammonia levels between day 5 and admission was associated with an increased risk of death and with worsening of HE provide support for the argument that ammonia should be a therapeutic target. This hypothesis will need to be tested in prospective studies.
Our results are in agreement with two recent US single-center retrospective studies showing that ammonia levels on admission are predictive of in-hospital mortality in decompensated cirrhosis.8, 9 Our study adds to these previous studies through inclusion of a larger number of patients from two separate institutions in two countries and the large cohort of patients with ACLF of various grades, allowing analysis of the relationship between organ failure and ammonia. Although the data were obtained retrospectively from the studies, all patients were prospectively recruited. An ammonia level of ≥79.5 µmol/L correlated with liver, kidney, respiratory, and coagulation failure and trended toward an association with circulatory failure, although this did not reach statistical significance.
The pathogenic effects of ammonia in modulating the dysfunction of organs other than the brain may explain the association of its concentration with mortality. The kidneys can adapt in early hyperammonemia, but with worsening renal function exacerbated by clinical scenarios such as acidosis, hypokalemia,11 and upper gastrointestinal bleeding,26 renal ammoniagenesis exceeds its clearance capacity; thus, the kidneys are responsible for a net release in circulating ammonia.16 Ammonia levels are indicative of portosystemic shunting27; thus, higher ammonia levels may be due to worse portal hypertension, which is known to be associated with worse outcomes.28 Hyperammonemia may not only reflect the degree of underlying sarcopenia but be a driver for it18 as ammonia is critically involved in regulating myostatin.17 Sarcopenia is associated with worse outcomes in cirrhosis.29 Ammonia also impairs neutrophil function, thus predisposing to infection.14 It is, therefore, a potentially important therapeutic target. It was interesting to note that the patients in whom ammonia levels were reduced at day 5 had significantly better survival compared to the patients in whom this was unchanged or increased. These data are in keeping with previous studies in patients with ACLF and acute liver failure.7, 8, 30 From this study, it is not possible to ascertain whether the relationship between the change in ammonia and survival is a cause or an effect.
The limitations of this study include the merging of three distinct patient cohorts into one group over a large study time period. We compared the baseline demographic and clinical profiles of patients at both institutions (Supporting Table S3). The cohort from New Delhi compared with that from London was younger (43 versus 55, P < 0.001), comprised more male subjects (76.6% versus 65.7%, P = 0.008), had a greater incidence of hepatitis B–induced cirrhosis (19.6% versus 8%, P < 0.001), had higher MELD and Child-Pugh scores (29 versus 20 and 12 versus 9, respectively; P < 0.001), and comprised a greater incidence of ACLF patients (69.1% versus 51.7%, P < 0.001). Therefore, it was not surprising that there were significant differences in baseline ammonia values (91 versus 65 µmol/L, respectively; P < 0.001). Despite these potential limitations, the data were collected prospectively and contain a large number of patients with ACLF, which allows evaluation of the relationship between ammonia levels and organ failures. Although we combined results of venous and arterial ammonia, previous studies have shown no significant differences between venous and arterial ammonia values with respect to HE.2 Venous blood sampling was only performed in the minimal hepatic encephalopathy cohort due to ethical considerations. Renal replacement therapy such as continuous venovenous hemofiltration was used where required for patients with renal failure and can lead to a reduction in plasma ammonia. This is a potential confounding factor when assessing survival. However, renal failure (thus use of renal support) was higher in nonsurvivors (38%) compared to survivors (13%). Therefore, the use of renal replacement therapy would only potentially confound by decreasing the strength of any relationship between hyperammonemia and mortality. The fact that statistical significance exists between hyperammonemia and survival in this study despite a potential negative confounding bias only serves to strengthen the conclusions of the study.
In conclusion, this study supports a role for ammonia as an important toxin that independently defines the risk of organ failures and mortality of patients with cirrhosis with AD and ACLF both with and without HE. Improvement in HE and survival are strongly linked with a lowering of ammonia levels, suggesting that ammonia is a potential therapeutic target to improve not only HE but also other organ failures and survival. Further studies are required to assess whether targeted ammonia lowering can improve the mortality of this patient group.




