Efficacy of Granulocyte Colony-stimulating Factor in the Management of Steroid-Nonresponsive Severe Alcoholic Hepatitis: A Double-Blind Randomized Controlled Trial
Abstract
Severe alcoholic hepatitis (SAH) is often a progressive disease with high mortality and limited steroid responsiveness. Management options of steroid nonresponsive SAH (day 7 Lille score > 0.45) are limited. We assessed the efficacy and safety of granulocyte colony-stimulating factor (G-CSF) in steroid nonresponders. A randomized, double-blind, single-center trial (NCT01820208) was conducted between March 2013 and June 2016 in patients with histologically proven SAH, nonresponsive to 40 mg/day of prednisolone were randomized to G-CSF (12 doses, 300 μg each in 28 days) or placebo. Responders were continued with prednisolone. Of the 430 patients with SAH, 132 received steroid therapy. Of these, 33 (25%) were nonresponders and were randomized to G-CSF or placebo (14 in each group after exclusions). The baseline characteristics of both groups were comparable. The 28-day mortality was comparable between the groups (21.4%, G-CSF; 28.6%, placebo; P = 0.69). At 90 days, in the G-CSF but not in the placebo group, the Model for End-Stage Liver Disease reduced from 24.6 ± 3.9 to 19.4 ± 3.7 (P = 0.002) and Maddrey’s discriminant function from 74.8 ± 22.8 to 57.4 ± 31 (P = 0.26). Infections were less common (28% versus 71%; P < 0.001) with lower 90-day mortality (35.7% versus 71.4%; P = 0.04) in the G-CSF than in the placebo group. On Cox regression analysis, receiving G-CSF (hazard ratio, 0.37; SD, 0.14-0.98; P = 0.04), and high baseline serum creatinine (hazard ratio, 4.12; SD, 1.7-10.3; P = 0.002) predicted day-90 outcomes in steroid nonresponsive SAH. Patients tolerated G-CSF without any major adverse events. Conclusion: Approximately one-quarter of patients with SAH do not respond to corticosteroid therapy. Administration of G-CSF is safe and helps to reduce the disease severity and 90-day mortality in these patients.
Abbreviations
-
- BMI
-
- body mass index
-
- CTP
-
- Child-Turcotte-Pugh
-
- DF
-
- (Maddrey’s) discriminant function
-
- GAH
-
- Glasgow alcoholic hepatitis
-
- G-CSF
-
- granulocyte colony-stimulating factor
-
- HR
-
- hazard ratio
-
- IBW
-
- ideal body weight
-
- INR
-
- international normalized ratio
-
- MELD
-
- Model for End-Stage Liver Disease
-
- MELD-NA
-
- MELD-sodium
-
- SAH
-
- severe alcoholic hepatitis
-
- TLC
-
- total leukocyte count
Alcoholic liver disease is one of the most common forms of liver disease, ranging from simple steatosis to alcoholic steatohepatitis to severe alcoholic hepatitis (SAH) with high mortality and limited treatment options. SAH is often associated with systemic inflammatory response syndrome (SIRS) and multi-organ failure with a short-term mortality of up to 40%. Severe hepatic inflammation occurs as a result of cellular necrosis, which may not only exacerbate liver damage but also may prevent tissue repair and regeneration. Corticosteroids have anti-inflammatory properties and are used for the treatment of SAH. The results are, however, quite variable. The Lille score of ≥ 0.45 on day 7 indicates nonresponse.1 A recent large, multicenter trial showed modest (P = 0.06) benefit at 28 days in only 60% of patients with SAH with no advantage over placebo at 90 days.2 Nonresponders, in contrast, have an increased risk of infection and mortality.
Treatment options for steroid-nonresponsive SAH remain inconclusive. The role and feasibility of liver transplant in such patients, with SAH and nonresponse to steroids, with very high short-term mortality remain uncertain with limited clinical data and many ethical issues.3, 4 New treatment options for difficult-to-manage patients with SAH not responding to conservative management are urgently needed.
Over the last decade, the importance of bone marrow–derived stem cell (BMSC) activation during liver disease has become apparent. Ineffective liver regeneration has recently been proposed to contribute to and correlate with severity of liver injury5 and poor outcomes using conventional care in patients with SAH.1 Some studies have documented the safety and usefulness of granulocyte colony-stimulating factor (G-CSF) in the management of SAH6, 7 by mobilization of BMSCs and improving liver regeneration and also possibly by improving the neutrophil function.8 However, there is no information on the role of G-CSF in steroid-nonresponsive SAH.
The options for steroid-nonresponsive SAH are limited. We undertook this study to assess the efficacy and safety of G-CSF in steroid-nonresponsive patients with SAH.
Patients and Methods
All consecutive patients with SAH (Maddrey’s discriminant function [DF] score > 32) over the age of 18 years admitted to the Institute of Liver & Biliary Sciences (ILBS) between March 2013 and June 2016 were screened and evaluated for corticosteroid therapy. Patients with SAH were included after a liver biopsy confirmation of the presence of alcoholic hepatitis. Patients were excluded if there was evidence of active infection (culture proven or clinical suspected bacterial infections), recent or past evidence of tuberculosis, history of chronic hepatitis B or hepatitis C infections, acute variceal bleed within 5 days (melena or hematemesis), uncontrolled diabetes, or deranged renal functions (creatinine > 1 mg/dL). Patients were considered for steroids after a week of resolution of infections either before or during the course of steroids. Before starting the steroid therapy, a detailed clinical assessment was done, including laboratory investigations, tumor necrosis factor 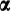 , arterial ammonia, arterial blood gas, blood and urine culture, chest X-ray, urine microscopy, hepatitis B surface antigen, antibody to hepatitis C virus, immunoglobulin M (IgM)–anti-hepatitis A virus, IgM–anti-hepatitis E virus, procalcitonin, and Mantoux test. Abdominal imaging and upper gastrointestinal (UGI) endoscopy were performed, and an informed consent was taken.
, arterial ammonia, arterial blood gas, blood and urine culture, chest X-ray, urine microscopy, hepatitis B surface antigen, antibody to hepatitis C virus, immunoglobulin M (IgM)–anti-hepatitis A virus, IgM–anti-hepatitis E virus, procalcitonin, and Mantoux test. Abdominal imaging and upper gastrointestinal (UGI) endoscopy were performed, and an informed consent was taken.
Every eligible patient was considered and taken up for transjugular liver biopsy along with measurement of hepatic venous pressure gradient before the start of steroids. Biopsy-proven patients with SAH were treated with prednisolone 40 mg once daily for 7 days as inpatients. Lille score was calculated on day 7. Responders (Lille score < 0.45) were continued on steroids for a total of 4 weeks, followed by 2 weeks of tapering before stopping the therapy. In patients who were nonresponsive to steroids (Lille score > 0.45) at day 7, steroids were stopped at day 7 and considered for randomization between G-CSF or placebo. For the purpose of the present study, steroid nonresponders (Lille score > 0.45 at day 7 of prednisolone treatment) were included. Exclusion criteria included patients requiring intensive care unit care due to any reason, grade 3 or 4 hepatic encephalopathy, active infection (patients with culture-positive infection), active bleeding, serum creatinine over 1.5 mg/dL, any previous known hypersensitivity to G-CSF, evidence of hemophagocytic lymphohistiocytic syndrome, presence of acute kidney injury, or lack of consent to participate in the study.
Study Protocol
This randomized controlled trial was conducted at ILBS New Delhi between March 2013 and June 2016. The institutional review board approved the study, and the trial was registered in the ClinicalTrials.gov with the study identifier NCT01820208. Patients with definite history of chronic alcohol abuse (>5 years) and presenting with jaundice were screened.9 SAH was diagnosed on the basis of Maddrey’s score > 32 and liver histology.10 We aimed to assess the efficacy of G-CSF (Emcure, Pune, India) in steroid-nonresponsive SAH.
In patients who were nonresponsive to steroids (Lille score > 0.45) at day 7, steroids were stopped at day 7, and these patients were considered for randomization to either of two groups in the next 48 hours to 72 hours. Group 1 received G-CSF, 5 μg/kg daily to a maximum of 300 μg per day for 5 doses followed by every third day until 4 weeks (a total of up to 12 doses). Group 2 received the placebo.
Randomization was done in 1:1 ratio. Sequence was generated using computer-generated random numbers, which was kept with the trial coordinator. The allocation was concealed in sealed opaque envelopes. The patient and the investigators were both blinded to the allocation. The trial coordinator provided the prefilled syringes (0.5 mL) (stored in refrigerator at 2℃-8℃) with either the drug (G-CSF) or placebo (normal saline), labeled as drug A or B, to the patients. After discharge, patients collected the prefilled syringes (labeled drug A/B) from the coordinator on an outpatient basis.
During the first week of the clinical trial, complete blood count was performed every day, and liver and kidney function tests and prothrombin time were repeated on days 4 and 7. Chest X-ray, blood culture, and urine culture were repeated as required. Patients also received conservative management in the form of diuretics, antibiotics, protein, and nutritional supplements per clinical requirements. A qualified nutritionist, specialized in critical care nutrition, was assigned to assess the nutritional needs of the patients. Patients received hospital-based diet (seven meals per day), providing 35 kcal/kg/day of ideal body weight (IBW) and 1.2 g of protein/kg/day of IBW (approximately 2,500 kcal and 85 g protein, adjusted per requirements by IBW). In those patients whose oral intake was less than 80% but more than 50% of target calories and protein, additional polymeric nutritional supplement (modular kitchen feed/commercial formula) was given as oral supplements to achieve 100% nutritional requirement goals. If the previous day’s intake was less than 50% of the requirements, a nasogastric tube (soft Freka) was placed and the remaining requirements were met by a modular kitchen feed or commercial formula as acceptable to the patient. Thereafter, a daily assessment of cumulative calorie and protein intake was recorded, and the volume, along with the modality of feeding, was changed according to the targets and compliance of the patient. At discharge, a detailed nutritional chart was provided to the patient and was reassessed further on subsequent outpatient visits.
The schedule of treatment was tailored in individual patients, especially with respect to rising total white cell counts increasing beyond 40,000/cmm, high levels of serum ferritin (>1,000 mcg/dL), rapidly reducing hemoglobin levels, and rising serum creatinine levels.
The patients were kept hospitalized for the initial 5 days of the intervention period, when they received daily injections of G-CSF or placebo. Subsequently, all patients were followed weekly for the initial 4 weeks and every fortnight until 90 days. Details beyond 90 days were also collected as part of routine follow-up data. All patients were closely monitored for the development of infections, vomiting of blood, or any other complications, and they were advised to report in person or telephonically as and when needed. Telephonic communication was made whenever patients missed more than two follow-up visits.
Definitions
Alcoholic hepatitis: Alcoholic hepatitis was diagnosed11 on a background of heavy regular alcohol intake as a variable combination of an aspartate aminotransferase (AST) level that is elevated (but <500 IU/mL), a ratio of the AST level to the alanine aminotransferase level equal to or more than 2, and a total serum bilirubin level of more than 5 mg/dL (86 μmol/L); an elevated international normalized ratio (INR) greater than 1.5; and neutrophilia. Baseline liver biopsy correlation was also done to confirm alcoholic hepatitis.
SAH: SAH was defined as a Maddrey’s DF score greater than 32.
Steroid response: Steroid responders and nonresponders were defined at day 7 of steroid therapy by a Lille score1 of less than or equal to 0.45 or greater than 0.45, respectively.
Endpoints: Primary endpoint was survival at day 28. Secondary endpoints were survival at day 90 and changes in the liver disease severity scores from the time of randomization until days 28 and 90 with respect to Child score, Model for End-Stage Liver Disease (MELD), and DF.
Active infection: SIRS was defined12 when two or more of the following conditions were met: (1) fever (oral temperature > 38°C) or hypothermia (<36°C); (2) tachypnea (>24 breaths/minute); (3) tachycardia (heart rate >90 beats/minute); (4) leucocytosis (>12,000/mm3), leucopoenia (<4,000/mm3), or more than 10% bands with a proven or suspected bacterial etiology. Prophylactic antibiotic use was not considered as active infection.
Acute bleeding: Acute bleeding was defined13 as hematemesis or melena within the previous 120 hours and renal insufficiency as serum creatinine greater than 1.5 mg/dL.
Liver biopsy confirmation of alcoholic hepatitis10 was done independently by the hepatopathologist, primarily based on semiquantitative assessment of the features of ballooning degeneration, Mallory Denk bodies, lobular inflammation, and cholestasis.
Statistical Analysis
Comparison of variables between G-CSF and placebo was performed using the Mann-Whitney nonparametric U test, chi-square test, and Student t test. Correlations between the parameters were evaluated using Spearman’s test. The delta change was compared by applying Student t test, paired t test/Mann-Whitney test, and the generalized estimating equations to account for the missing values wherever applicable. Maddrey’s DF and the MELD score were calculated at baseline and at follow-up, and the Lille model was calculated 7 days after initiation of treatment. The formulas for the scores were as follows: Maddrey’s DF = 4.6 × (patient prothrombin time − control prothrombin time [seconds] + serum bilirubin [mg/dL]); MELD score = 9.57 × loge creatinine (mg/dL) + 3.78 × loge bilirubin (mg/dL) + 11.20 × loge INR + 6.43; Lille score = Exp(−R)/(1+Exp[−R)]), where R = 3.19 − 0.101 × age (years) + 0.147 × albumin (g/L) + 0.0165 × evolution (bilirubin, μmol/L) − 0.206 × renal insufficiency − 0.0065 × bilirubin (μmol/L) − 0.0096 × prothrombin time (seconds). In the original study, we had planned to enroll 112 patients with SAH to have 56 patients with steroid nonresponse (Lille score > 0.45), and hence 28 patients with steroid nonresponse in each limb (G-CSF and placebo, 28 each). Due to stringency in our selection process and higher than expected response rate for steroids in our patients with SAH, and despite continuing the study for 3 years (planned initially for 1 year), we enrolled 28 patients for the steroid nonresponder group (i.e., 14 patients in each of the limbs).
Results
Of the 430 patients with SAH admitted during the study period, 132 (26.8%) were steroid eligible and received steroids (Fig. 1). Of these, 99 (75%) patients were considered steroid responders based on day 7 Lille score (<0.45) and were continued on prednisolone 40 mg per day for 3 more weeks with further tapering off over consecutive 2 weeks. The steroid responders (Lille score < 0.45) had comparable baseline liver disease severity and clinical profile at baseline as steroid nonresponders (Lille score > 0.45). The details of the steroid responders with Lille score less than 0.45 is presented in Supporting Table S1.
Prednisolone was stopped in 33 steroid nonresponders (Lille score > 0.45) after 7 days of therapy. Of these 33, one underwent liver transplantation, 2 became very sick within the next week with development of sepsis and multiorgan failure, and 2 others did not give consent for further randomization in the G-CSF/placebo protocol. The remaining 28 patients were randomized within the next 3 days to receive either G-CSF or placebo with a double-blinding and concealed allocation technique (14 in each group).
Baseline Parameters in Steroid Nonresponders
Nonresponders to steroids who were included in the G-CSF trial (n = 28) had a mean age of 40.2 ± 10.3 years, and all had mild to moderate ascites at presentation. Only 1 patient was female. All of the steroid nonresponders had advanced liver disease with Child-Turcotte-Pugh (CTP) 10.8 ± 1.2, MELD 26.2 ± 4.3, Glasgow alcoholic hepatitis (GAH) 8.5 ± 0.9, DF of 81.1 ± 26, and Lille score of 0.68 ± 0.2. There was no significant difference between the two groups with respect to any of the baseline clinico-biochemical parameters and the liver disease severity as measured by CTP, MELD, MELD-sodium (MELD-Na), DF, or Lille score (Table 1).
| Parameter | G-CSF (n = 14) | Placebo (n = 14) | P Value |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (years) | 39.6 (9.0) | 40.7 (11.7) | 0.83 |
| BMI (kg/m2) | 29.4 (8.4) | 24.5 (4.1) | 0.25 |
| Total bilirubin (mg/dL) | 23.4 (7.10) | 32.0 (10.5) | 0.34 |
| AST (IU/L) | 175.3 (73.5) | 175.9 (91.9) | 0.98 |
| ALT (IU/L) | 122.5 (94.5) | 99.6 (48.7) | 0.43 |
| AST/ALT ratio | 1.48 (0.5) | 1.32 (0.26) | 0.55 |
| Total protein (g/dL) | 6.6 (0.69) | 6.7 (0.54) | 0.81 |
| Serum albumin (g/dL) | 2.66 (0.55) | 2.77 (0.47) | 0.58 |
| INR | 2.67 (1.7) | 2.1 (0.4) | 0.44 |
| Hb (g/dL) | 9.4 (1.2) | 9.8 (1.6) | 0.42 |
| TLC (thousand cells/uL) | 19.4 (5.6) | 20.2 (7.9) | 0.78 |
| Platelet count (thousand cells/uL) | 176.8 (107.8) | 108.7 (64.0) | 0.06 |
| Blood urea (mg/dL) | 46.4 (33.2) | 70.4 (29.9) | 0.07 |
| Serum creatinine* (mg/dL) | 0.40 (0.15-0.58) | 0.60 (0.25-0.86) | 0.17 |
| Serum sodium (mEq/L) | 130.9 (5.6) | 131.6 (4.8) | 0.76 |
| Serum potassium (mEq/L) | 4.3 (0.7) | 4.5 (0.9) | 0.53 |
| HVPG (mm Hg) | 17.7 (3.5) | 21.5 (5.3) | 0.37 |
| CTP score | 10.9 (1.3) | 10.7 (1.2) | 0.64 |
| MELD score | 24.6 (3.9) | 27.6 (4.4) | 0.10 |
| MELD-Na score | 27.6 (2.8) | 29.7 (2.0) | 0.07 |
| GAH score | 8.4 (1.9) | 8.7 (0.5) | 0.51 |
| DF score | 74.8 (22.8) | 87.5 (28.7) | 0.29 |
| Lille score | 0.74 (0.18) | 0.66 (0.12) | 0.19 |
- * Data presented as median (interquartile range).
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; Hb, hemoglobin; and HVPG, hepatic venous pressure gradient.
Survival of Steroid-Nonresponsive SAH Patients Randomized Between G-CSF and Placebo
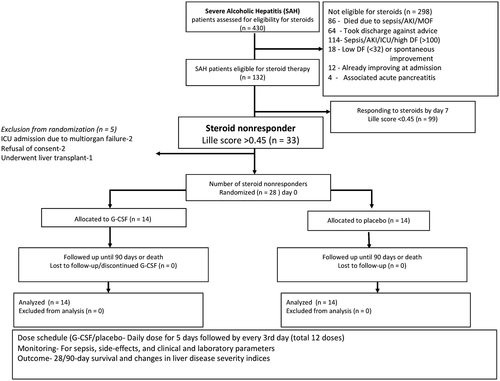
The 28-day survival between the G-CSF and placebo groups of steroid-nonresponsive SAH (11 of 14 [78.6%] in G-CSF versus 10 of 14 [71.4%] in placebo) was not different (P = 0.69). While at day 90, 9 of 14 (64.3%) patients receiving G-CSF survived compared with only 4 of 14 (28.6%) achieving this in the placebo group (P = 0.04). On post hoc analysis, the survival continued to be better in the G-CSF group even at 180 days (57.1% versus 28.6%; P = 0.04), with the overall mean survival in the G-CSF group of 256 days (SEM = 56.3 days) versus 82 days (SEM = 30.01 days) (P = 0.04) in the placebo group (Fig. 2).
Changes in the Clinical Parameters After G-CSF/Placebo in Steroid-Nonresponsive SAH
As expected, the total leukocyte count (TLC) started to increase from the very next day after initiation of G-CSF, reaching a maximum by the end of the second week. The TLC remained significantly elevated in the G-CSF group even at day 28 compared with baseline (at randomization 19.4 ± 5.6 thousand cells/μL versus 26.9 ± 8.4 thousand cells/μL at day 28; P < 0.02). The G-CSF group had stable liver disease severity indices (DF, MELD, and MELD-Na) until day 28 (Table 2) after the randomization. Furthermore, these showed an improving trend beyond the first month until the third month. With regard to disease-severity parameters (baseline versus day 90), CTP improved from 10.91 ± 1.30 to 10.17 ± 1.5 (P = 0.67), MELD from 24.6 ± 3.9 to 19.4 ± 3.7 (P = 0.02), MELD-Na from 27.6 ± 2.8 to 22.4 ± 3.6 (P = 0.03), and DF from 74.79 ± 22.8 to 57.4 ± 31 (P = 0.02) by the end of 3 months of G-CSF therapy, with total bilirubin decreasing from 23.4 ± 7.1 mg/dL to 14.5 ± 8.8 mg/dL.
| Parameter | G-CSF (n = 14) | Placebo (n = 14) | ||||
|---|---|---|---|---|---|---|
|
Baseline (n = 14) |
Day 28 (n = 11) |
P Value |
Baseline (n = 14) |
Day 28 (n = 10) |
P Value | |
| Total bilirubin (mg/dL) | 23.4 (7.10) | 25.9 (10.4) | 0.59 | 32.0 (10.5) | 25.5 (10.0) | 0.53 |
| AST (IU/L) | 175.3 (73.5) | 165.5 (69.8) | 0.34 | 175.9 (91.9) | 144.4 (84.4) | 0.11 |
| ALT (IU/L) | 122.5 (94.5) | 93.2 (46.0) | 0.25 | 99.6 (48.7) | 67.8 (36.9) | 0.27 |
| Total protein (g/dL) | 6.6 (0.69) | 6.87 (0.68) | 0.52 | 6.7 (0.54) | 6.0 (0.48) | 0.06 |
| Serum albumin (g/dL) | 2.66 (0.55) | 2.56 (0.43) | 0.61 | 2.77 (0.47) | 2.93 (1.3) | 0.39 |
| INR | 2.67 (1.7) | 1.96 (0.44) | 0.54 | 2.1 (0.4) | 2.23 (0.38) | 0.07 |
| TLC (thousand cells/uL) | 19.4 (5.6) | 26.9 (8.4) | 0.02 | 20.2 (7.9) | 21.8 (10.9) | 0.83 |
| Platelet count (thousand cells/uL) | 176.8 (107.8) | 155.7 (54.8) | 0.21 | 108.7 (64.0) | 74.14 (42.5) | 0.46 |
| Blood urea (mg/dL) | 46.4 (33.2) | 30.4 (10.5) | 0.80 | 70.4 (29.9) | 82.7 (50.4) | 0.54 |
| Serum creatinine (mg/dL)*, * | 0.40 (0.15-0.58) | 0.55 (0.34-0.77) | 0.09 | 0.60 (0.25-0.86) | 0.95 (0.54-1.07) | 0.06 |
| CTP score | 10.9 (1.3) | 10.67 (1.51) | 0.18 | 10.7 (1.2) | 11 (0.58) | 0.37 |
| MELD score | 24.6 (3.9) | 24.5 (5.4) | 0.74 | 27.6 (4.4) | 29.9 (3.5) | 0.03 |
| MELD-Na score | 27.6 (2.8) | 28.0 (4.5) | 0.62 | 29.7 (2.0) | 33.0 (2.0) | 0.03 |
| DF score | 74.8 (22.8) | 72.4 (33.0) | 0.82 | 87.5 (28.7) | 102.7 (25.2) | 0.05 |
Note:
- P values are calculated using generalized estimating equations to account for the missing data.
- * Data presented as median (interquartile range).
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase.
In contrast, in nonresponders who received placebo, the liver disease severity parameters worsened within the first month (baseline versus day 28): MELD from 27.6 ± 4.4 to 29.9 ± 3.5 (P = 0.3), MELD-Na from 29.7 ± 2.0 to 33.0 ± 2.0 (P = 0.03), and DF from 87.5 ± 28.7 to 102.7 ± 25.2 (P = 0.05). The placebo group showed worsening of the liver severity parameters in the first month, with only slightly more than 28% (4 of 14) surviving until 90 days; therefore, we did not compare data of day 90 in the placebo group.
Predictors of Mortality in Steroid-Nonresponsive Patients with SAH
Progressive liver failure complicated by multiorgan dysfunction was the most common cause of death. In the G-CSF group, 2 patients died after recidivism and progressive liver failure after initial recovery, 1 died of massive UGI bleed with spontaneous bacterial peritonitis (SBP), and the remaining 3 died with sepsis and multiorgan failure. In the placebo group, 1 patient succumbed to recidivism, and the other 10 to sepsis (the most common being pneumonia and SBP) and multiorgan failure. G-CSF administration reduced the 90-day mortality by 63% (HR, 0.37 [SD, −0.14-0.98]; P = 0.04). The other baseline parameter before randomization that helped to predict 90-day mortality was serum creatinine (HR, 4.12; SD, 1.7-10.3; P = 0.002). No other clinical or biochemical parameters, including age, serum total bilirubin, prothrombin time, Lille score, and severity of liver disease (e.g., CTP, MELD, GAH, DF), could predict mortality once a patient showed nonresponse to 7-day steroids (Lille score > 0.45) (Table 3).
| Variable | Univariate Analysis | |
|---|---|---|
| HR (SD) | P Value | |
| Lille score | 3.27 (0.17-61) | 0.43 |
| Age | 1.04 (0.98-1.10) | 0.19 |
| BMI | 0.99 (0.88-1013) | 0.99 |
| Baseline HVPG | 1.03 (0.93-1.15) | 0.55 |
| Total bilirubin | 0.98 (0.93-1.02) | 0.32 |
| Blood urea | 1.02 (1.002-1.04) | 0.03 |
| Serum creatinine*, * | 4.12 (1.7-10.3) | 0.002 |
| CTP score | 1.14 (0.73-1.78) | 0.58 |
| MELD score | 1.13 (0.97-1.32) | 0.13 |
| GAH score | 1.81 (0.68-4.81) | 0.24 |
| DF score | 0.99 (0.97-1.02) | 0.88 |
| G-CSF | 0.37 (0.14-0.98) | 0.04 |
- * Denotes change in creatinine of 1.0 mg/dL.
- Abbreviation: HVPG, hepatic venous pressure gradient.
Adverse Effects of Administration of G-CSF
Patients tolerated G-CSF therapy without any major adverse effects. TLC increased significantly in patients receiving G-CSF as expected. Daily dose of G-CSF was omitted in 2 patients in whom the TLC rose to over 40,000/cm3. One patient developed severe bone pains with every injection of G-CSF, which necessitated decreasing the frequency and the number of doses of G-CSF. In the rest of the patients, G-CSF therapy was given per protocol and was well tolerated.
Discussion
Corticosteroid therapy remains the standard of medical care in managing patients with SAH. There are limited options for steroid nonresponders, and mortality remains high in the absence of liver transplantation.5, 7, 14, 15 The results of this randomized controlled trial show that G-CSF therapy is efficacious in improving clinical indices and outcomes in steroid-nonresponsive patients with SAH. The therapy was also well tolerated.
In the current study, although the primary endpoint (28-day survival) was not different between the groups, we could see encouraging results, with significantly improved 90-day survival with G-CSF, in this very difficult-to-treat subgroup of steroid-nonresponsive patients with SAH who otherwise have no other accepted option. The reasons for not achieving improved survival at day 28 could possibly be because the G-CSF treatment protocol itself was for 28 days and the last injection was administered on day 28. It is conceivable that G-CSF therapy requires a longer time to show efficacy and survival benefits. This is evident from our patient cohort, as those who received G-CSF had significantly improved 90-day survival compared with placebo (64.3% versus 28.6%; P = 0.04). In fact, the survival continued to be better in the G-CSF group even at 180 days, with the overall mean survival in the G-CSF group of 256 days compared with 82 days (P = 0.04) in the placebo group. In an earlier study, our group had shown improved survival6 with G-CSF compared with placebo (66% versus 26%; P = 0.01) in patients with recently progressive liver failure, of whom 73.9% of the patients had acute deterioration of liver disease due to alcoholic hepatitis. In this previous study, the patients with SAH had, however, not received steroids before G-CSF therapy. Similar results on survival benefit with G-CSF in liver failure are also shown in a recently published meta-analysis.16
It has recently been shown that patients with SAH not responding to the medical management have altered local cytokine milieu, favoring aberrant liver regeneration in which the hepatic progenitor cells cannot form mature hepatocytes.5 In the earlier published trials,7, 17, 18 including our own,6 G-CSF has been shown to mobilize hematopoietic stem cells and improve hepatic progenitor cell proliferation, and hence survival in patients with alcoholic hepatitis. Improved survival in patients with SAH using G-CSF has additionally been postulated to be due to reduced number of infections and improvement in the immune paralysis.8 We also observed a significantly lower infection rate with the use of G-CSF (71% versus 28%; P < 0.001). G-CSF might have decreased further deterioration of liver functions due to infections and provided some extra time for liver regeneration. Further studies should focus on delineating the exact role and mechanism of G-CSF in alcoholic hepatitis. In a recent study, Newsome et al.19 showed no improvement at 90 days in stable Child A/B patients with cirrhosis who received G-CSF for 5 consecutive days, with a mean MELD of less than 13. Although half of the patients in their study had cirrhosis due to alcohol, none of their patients had alcoholic hepatitis (as they included only those with alcohol taken beyond 6 months). The infection rate in their study was not significant, as very few patients developed infections during the 90-day follow-up. The beneficial effects of G-CSF may be more pronounced in carefully selected patients with alcoholic hepatitis who have a potentially reversible acute deterioration of liver functions and increased risk of infections, rather than selecting patients who are stable with chronically decompensated alcoholic cirrhosis.
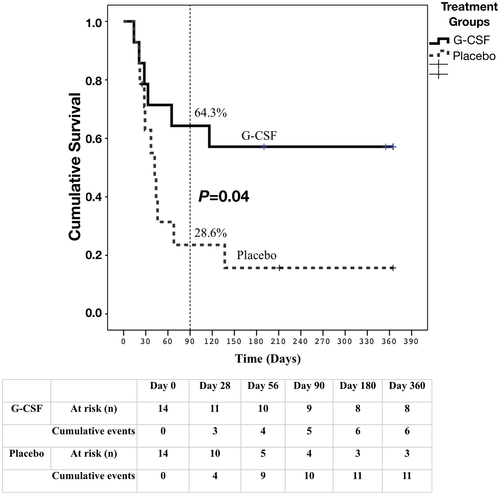
Although we did not look into the basic mechanisms of G-CSF in this trial (mobilization of stem cells/improved regeneration or improvement in immune paralysis), as these were reported in previous trials by our group,6, 20 we could demonstrate increased peripheral leukocytosis, improvement in liver disease severity (e.g., CTP, MELD, MELD-Na), and decreased rate of infections in the group receiving G-CSF. G-CSF is expected to improve survival in steroid-nonresponsive SAH by improving hepatic regeneration through modulation of the bone marrow and local resident hepatic progenitors, and by decreasing the rate of infections, as represented in the concept diagram (Fig. 3). In addition, we have previously shown that steroid nonresponse could be due to failure of cellular energy metabolism. High levels of acetyl carnitine in SAH are associated with high mortality.21 It is possible that G-CSF therapy could help in restoring cellular energy and hepatic regeneration.
One of the limitations of our study was the small sample size. However, this has to be taken in the context of the seriousness of the disease and the stringent selection criteria. We had screened 430 patients with SAH, 132 (26.8%) of whom were eligible and enrolled for steroid therapy. However, higher than our expectations, 99 (75%) patients responded, and only 33 (25%) were nonresponders, despite continuing the study for 3 years (planned initially for 18 months). Of these 33, we could enroll 28 patients for the study. Although this study proves the safety and possible benefits of G-CSF in steroid nonresponders, the small sample size limits our ability to detect variables that might predict survival at baseline (e.g., serum creatinine). There certainly is a need for further studies, including development of personalized therapeutic dosing schedules, for G-CSF administration.
In summary, G-CSF could be an appropriate rescue therapy in patients with SAH who do not respond to steroids and have no transplant options. Our results should be evaluated by other groups in a carefully selected subset of patients with SAH. Needless to say, dedicated research into the mechanisms of impaired hepatic regeneration in patients with SAH and approaches to overcome these are required.




