Aging-Related Expression of Twinfilin-1 Regulates Cholangiocyte Biological Response to Injury
Abstract
Disorders of the biliary tree develop and progress differently according to patient age. It is currently not known whether the aging process affects the response to injury of cholangiocytes. The aim of this study was to identify molecular pathways associated with cholangiocyte aging and to determine their effects in the biological response to injury of biliary cells. A panel of microRNAs (miRs) involved in aging processes was evaluated in cholangiocytes of young and old mice (2 months and 22 months of age, respectively) and subjected to a model of sclerosing cholangitis. Intracellular pathways that are common to elevated miRs were identified by in silico analysis. Cell proliferation and senescence were evaluated in Twinfilin-1 (Twf1) knocked-down cells. In vivo, senescence-accelerated prone mice (Samp8, a model for accelerated aging), Twf1-/-, or their respective controls were subjected to DDC (3,5-diethoxycarbonyl-1,4-dihydrocollidine). Cholangiocytes from DDC-treated mice showed up-regulation of a panel of aging-related miRs. Twf1 was identified by in silico analysis as a common target of the up-regulated miRs. Twf1 expression was increased both in aged and diseased cholangiocytes, and in human cholangiopathies. Knock-down of Twf1 in cholangiocytes reduced cell proliferation. Senescence and senescence-associated secretory phenotype marker expression increased in Twf1 knocked-down cholangiocytes following pro-proliferative and pro-senescent (10-day lipopolysaccharide) stimulation. In vivo, Samp8 mice showed increased biliary proliferation, fibrosis, and Twf1 protein expression level, whereas Twf1-/- had a tendency toward lower biliary proliferation and fibrosis following DDC administration compared with control animals. Conclusion: We identified Twf1 as an important mediator of both cholangiocyte adaptation to aging processes and response to injury. Our data suggest that disease and aging might share common intracellular pathways.
Abbreviations
-
- ANOVA
-
- analysis of variance
-
- DDC
-
- 3,5-diethoxycarbonyl-1,4-dihydrocollidine
-
- DMEM
-
- Dulbecco’s modified Eagle’s medium
-
- FBS
-
- fetal bovine serum
-
- Igf
-
- insulin-like growth factor
-
- Il
-
- interleukin
-
- LPS
-
- lipopolysaccharide
-
- miR
-
- microRNA
-
- NRC
-
- normal rat cultured cholangiocytes
-
- ntRNA
-
- nontargeting RNA
-
- PBC
-
- primary biliary cholangitis
-
- PCNA
-
- proliferating cell nuclear antigeph
-
- PSC
-
- primary sclerosing cholangitis
-
- Samp8
-
- senescence accelerated mouse prone 8
-
- Samr1
-
- SAM resistant 1
-
- SASP
-
- senescence-associated secretory phenotype
-
- SBB
-
- Sudan Black-B
-
- siRNA
-
- small interfering RNA
-
- SRB
-
- sulforhodamine B
-
- Twf1
-
- Twinfilin-1
The aging of a biological system is the universal process that is determined by the time-dependent accumulation of DNA damage, telomere shortening and epigenetic alterations. As a result of aging, the functions of organs inevitably decline in time, and death eventually occurs.1 Interest in understanding the aging process has risen recently in the scientific community. Aging is indeed the major risk factor for the development of many chronic diseases, from degenerative disorders to cancer.2 With the progressive increase in life expectancy, the development of therapeutic interventions to modulate aging-related alterations is eagerly sought and may prove essential in the management of general health care.3
To date, little is known about the influence of aging in the pathophysiology of hepatic and biliary disorders. Previous studies have demonstrated that liver structure and functions are altered in elderly patients, with possible important implications for disease development.4, 5 From a clinical perspective, many aspects of cholangiopathies tend to be influenced by advanced age. Clinical course of primary sclerosing cholangitis (PSC) is significantly different according to age at diagnosis, with increased occurrence of cholangiocarcinoma.6 Moreover, the development of biliary complications after orthotopic liver transplantation is influenced by donor age.7, 8 Important molecular clues supporting the role of aging in the modulation of cholangiocyte pathology are also emerging.9 Cholangiocytes of PSC and primary biliary cholangitis (PBC) patients show increased expression of several markers of cellular senescence, with a possible important role in the pathogenesis of the diseases.10-13 Senescence is defined as the replicative arrest of cells that follows a variety of oncogenic stimuli, such as DNA damage, telomere erosion, and strong or persistent mitogenic signals. Despite being first involved in cancer surveillance, senescence has been recognized recently as a part of the complex molecular alterations accompanying aging.14 Senescent cells not only appear to accumulate during aging, but they also secrete a number of proinflammatory cytokines and growth factors in a process known as senescence-associated secretory phenotype (SASP), which contributes to altering the tissue microenvironment and favors age-related diseases.14, 15 Unraveling the possible contribution of aging in cholangiocyte adaptation to injury may have important therapeutic implications. New drugs selectively targeting senescent cells are currently being investigated in a number of pathologies.16
The aim of the current study was to identify a molecular signature in reactive cholangiocytes associated with the aging process, and to verify a possible role of these pathways in the biological response to injury of biliary cells. We therefore aimed to address the following questions: (1) Do reactive cholangiocytes overexpress aging-related microRNAs (miRs)? (2) What are the targets of up-regulated miRs? (3) Do the identified targets influence biliary proliferation and senescence? and (4) Does aging affect the development and progression of biliary injury in vivo?
Materials and Methods
miRs Selection and Testing in Cholangiocytes
We performed a search in the Medline database through PubMed to find relevant studies regarding the involvement of miRs in the aging process, regardless of the organ or system. A total of 12 miRs were selected for further testing based on subjective evaluation of the manuscripts. Table 1 provides a list of the selected miRs, their putative roles, and pertinent references to the original studies.17-26
| miR | Role | Ref. |
|---|---|---|
| miR-1a | Regulates Igf-1 transcription; up-regulated in Zmpste24-/- progeroid mice | 19,20 |
| miR-29b | Up-regulated in response to DNA damage in a p53-dependent manner; up-regulated in Zmpste24-/- progeroid mice | 21,22 |
| miR-30e | Represses B-Myb expression (oncogene) | 23 |
| miR-669c | Increased in aged livers; targets glutathione S-transferases | 24 |
| miR-709 | Increased in aged livers | 24 |
| miR-214 | Increased in aged livers | 24 |
| miR-93 | Increased in aged livers; targets glutathione S-transferases; involved in oxidative stress (mitochondrial damage) | 24 |
| miR-34a | Tumor suppressor gene; inhibits Sirt1 expression (cell cycle and apoptosis) | 25 |
| miR-146a | Up-regulated; targets Irak1 and limits Il-6 and Il-8 expression; also down-regulated (increases ROS production) | 26 |
| miR146b | ||
| miR-20a | Represses Lrf, thus activating p19AFR (inhibition of cell proliferation and induction of senescence) | 27 |
| miR-24 | Decreased in replicative senescence; associated with increased expression of p16 | 28 |
- Abbreviation: ROS, reactive oxygen species.
In Silico Analysis
To predict the putative mRNA targets and pathways common to more than one miR, the String IDentifier (SID1.0) software was used.27 This program is based on an exhaustive search strategy and is specifically designed to screen shared data (target genes, miRs, and pathways) available from the TargetScan and DIANA-MicroT 3.0 databases. We analyzed the common targets of the six miRs found to be up-regulated (mmu-miR-146b, mmu-miR-30e, mmu-miR-93, mmu-miR-20a, mmu-miR-34a, and mmu-miR-1a). Common pathways of the same up-regulated miRs were identified using the DIANA-microT 3.0 target prediction program (http://diana.cslab.ece.ntua.gr/microT/).
Expression of TWF1 in Cholangiocytes
TWF1 expression was evaluated by real-time PCR in liver tissue from patients affected by PSC (n = 10) and PBC (n = 10). Normal liver tissue surrounding colorectal hepatic metastases (n = 17) served as internal controls. Cholangiocyte isolation was performed as previously described.28 TWF1 was also assessed by immunohistochemistry staining in liver sections of PSC patients (n = 5) compared with control livers (n = 3 alcoholic liver disease and n = 2 cryptogenic liver disease). Ethical approval was obtained from the research ethics committees at each participating medical center in accordance with the Declaration of Helsinki (S-08872b). After acquisition of informed consent, samples were collected at the time of liver biopsy, surgical liver resection, or liver transplantation. In vitro, expression levels of Twf1 were evaluated by real-time PCR in normal rat cultured cholangiocytes (NRCs) and in cholangiocytes isolated from both control mice (young and old) and mice (young and old) fed with 0.1% DDC (3,5-diethoxycarbonyl-1,4-dihydrocollidine).29
Role of TWF1 in Cholangiocytes Proliferation
NRCs were exposed for 48 hours to small-interfering RNA (siRNA) against Twf1 or to nontargeting RNA (ntRNA). The day after seeding, cells were stimulated with Dulbecco’s modified Eagle’s medium (DMEM)/F12 added with increasing concentration (0%-5%-20%) of fetal bovine serum (FBS). Changes in cells proliferation were assessed by measuring the mRNA expression level of Ki67 and the protein expression level of PCNA (proliferating cell nuclear antigeph). Variations in cell growth were also evaluated with the sulforhodamine B (SRB) assay (Sigma-Aldrich, St. Louis, MO) in NRCs—either or not subjected to siRNA against Twf1. Briefly, cells were seeded on 96-well plates in DMEM/F12 and transfected with siRNA against Twf1 or with ntRNA. The day after seeding, cells were stimulated with 5% FBS and grown for up to 72 hours. After fixing and washing, cells were air-dried and incubated with 0.4% SRB solution for 30 minutes. The incorporated dye was then solubilized by adding the SRB solubilization solution in an equal volume of original culture medium. Growth rates were evaluated spectrophotometrically using a microplate reader (Tecan, Mannedorf, Switzerland).
Role of TWF1 in Cholangiocytes Senescence
To address the potential role of Twf1 in cholangiocyte senescence, NRCs were seeded in DMEM/F12 and exposed for 48 hours to siRNA against Twf1 or to corresponding ntRNA. Variations of mRNA expression levels of senescence and SASP components were evaluated by real-time PCR. Cellular senescence was also assessed by quantification of Sudan Black-B (SBB) lipofuscin staining in Twf1 knock-down cholangiocytes and controls, as previously reported.30 The putative role of Twf1 in cholangiocyte senescence modulation was assessed in an in vitro model of cholangiocyte senescence induced by endogenous stimulus, as previously reported.10 Briefly, NRCs were seeded in DMEM/F12 and subsequently exposed to siRNA against Twf1 or to ntRNA as control, and the senescence model was induced by persistent stimulation (10 days) of NRCs with LPS (200 ng/mL). The levels of senescence markers and SASP components were evaluated by real-time PCR. SASP components levels were also assessed by enzyme-linked immunoadsorbent assay in the same setting of experiments. Culture media were collected and centrifuged to remove cell debris and used for interleukin (Il)-1a and insulin-like growth factor (Igf)-1 level measurements according to the manufacturer’s instructions (Rat IGF-1 ELISA Kit; XpressBio, Frederick, MD). The absorbance was read at 450 nm against 620 nm, as reference wavelength, using a microplate reader (Tecan, Mannedorf, Switzerland).
Animal Experiments
Samp8 mice,31 which spontaneously develop accelerated aging,32, 33 and Twf1-/- mice and their respective controls (Samr1 and Twf1+/+) (n = 5 per each experimental group) were subjected to 0.1% DDC feeding for 8 weeks as a model of sclerosing cholangitis, as previously reported.29 Changes in cholangiocyte proliferation were determined by measuring differences in intrahepatic bile duct mass (by quantitative immunohistochemistry for CK-19 in liver sections).34 Changes in collagen deposition were assessed by quantitative histochemistry for sirius red,35 and hepatic content of collagen was evaluated by hydroxyproline assay.36 Animal study protocols were performed in compliance with local institution guidelines and in accordance with the ARRIVE guidelines.
Statistical Analysis
Data were expressed as mean ± SD, and the 95% confidence interval (CI) was calculated. Differences between groups were analyzed by Student t test or analysis of variance (ANOVA), as appropriate. Differences between groups were considered significant when the P value was less than 0.05.
Results
Age-Related miRs Are Up-Regulated in Diseased Cholangiocytes
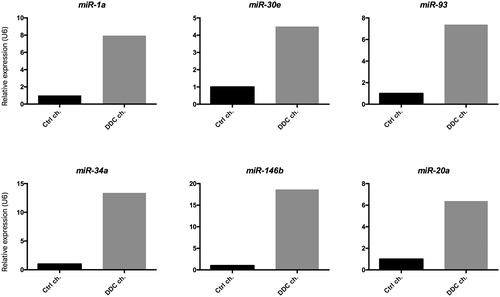
A number of miRs involved in the regulation of aging processes were selected from the literature. The role of each miR is summarized in Table 1. We screened the expression levels of each age-related miR in cholangiocytes isolated from mice subjected to 8-week DDC feeding as a model of sclerosing cholangitis. As shown in Fig. 1, we found an increase in the expression levels of miR-1a, miR-30e, miR-93, miR-34a, miR-146b, and miR-20a in cholangiocytes of DDC-treated mice as compared with control animals. No differences could be detected in the expression of remaining miRs.
TWF1 is the Molecular Target of Up-Regulated miRs
In silico evaluation was performed to identify putative pathways and molecular targets commonly regulated by the up-regulated miRs. We found 30 signaling pathways to be commonly regulated by all six up-regulated miRs (miR-1a, miR-30e, miR-93, miR-34a, miR-146b, and miR-20a) (Supporting Table S1). In addition, as presented in Supporting Table S2, based on the analysis of the TargetScanHuman6.2 database, we could identify six targets that are common to three of the six up-regulated miRs (any target was common to all six miRs). In particular, the acting-binding protein Twinfilin 1 (Twf1) is the common putative target of miR-1a, miR-20a, and miR30e.
TWF1 and Related miRs are Up-Regulated in old and Diseased Cholangiocytes
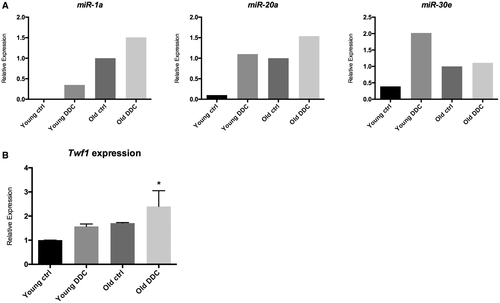
To assess a possible modulation of Twf1 by both aging and injury, expression levels of Twf1 and its related miRs (miR-1a, miR-20a, and miR-30e) were evaluated in cholangiocytes isolated from “young” and “old” mice subjected to DDC diet as a model of sclerosing cholangitis. As shown in Fig. 2A, expression levels of miR-1a, miR-20a, and miR-30e were modulated in both injured and aged cholangiocytes and increased as compared with control young cholangiocytes. The mRNA expression level of Twf1 showed a similar trend and was increased in old, diseased cholangiocytes compared with young control mice (Fig. 2B). Interestingly, Twf1 expression tended to be progressively higher from young, diseased cholangiocytes to old, diseased cholangiocytes, with intermediate levels in aged cholangiocytes of control mice. These results indicate that the analyzed miRs are not directly targeting Twf1 but are involved in its modulation, probably targeting other genes related to the pathways indicated in Supporting Table S1.
TWF1 is Expressed in Human Cholangiocytes
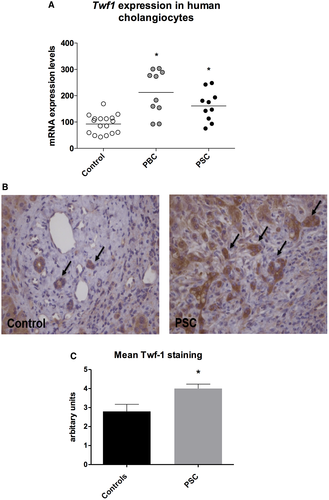
TWF1 expression was assessed in liver tissue of patients affected by primary cholangiopathies. Cholangiocytes isolated from PBC and PSC patients showed an increased expression of TWF1 compared with control cholangiocytes (Fig. 3A). Expression of TWF1 was also assessed by immunohistochemistry in liver sections of PSC patients and human samples collected from patients affected by other liver diseases for comparison. As depicted in Fig. 3B, immunohistochemistry showed a positive staining for TWF1 in cholangiocytes of the portal triads. Moreover, TWF1 expression tended to be higher in cholangiocytes of proliferating bile ducts of PSC patients as compared with the relative control. We also performed a semiquantitative evaluation of TWF1 expression on PSC and control livers, assigning a score ranging from 0 to 5 based on staining intensity for each patient (Fig. 3B,C). These data suggest that expression of TWF1 is not restricted to PSC cholangiocytes, but is linked to cholangiocyte proliferation, which also occurs in lower-level liver diseases other than cholangiopathies.
TWF1 Sustains Cholangiocyte Proliferation In Vitro
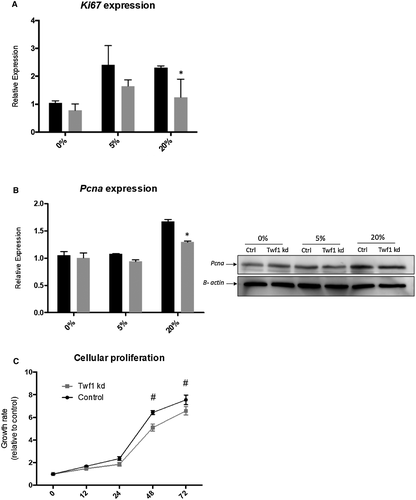
To verify whether Twf1 influences biliary proliferation, Twf1 knocked-down cholangiocytes and control cells were stimulated with 5% and 20% FBS. As shown in Fig. 4A,B, the knockdown of Twf1 expression by siRNA reduced the proliferation of stimulated cultured cholangiocyte assessed by Ki67 expression and PCNA protein evaluation. We also tested the role of Twf1 on biliary proliferation on a functional level with the SRB assay; the growth curve of Twf1 knockdown cholangiocyte was lower than control cells measured at both 48 hours and 72 hours after seeding of the cells (Fig. 4C).
Senescence and SASP are Increased in TWF1 Knocked-Down Cholangiocytes
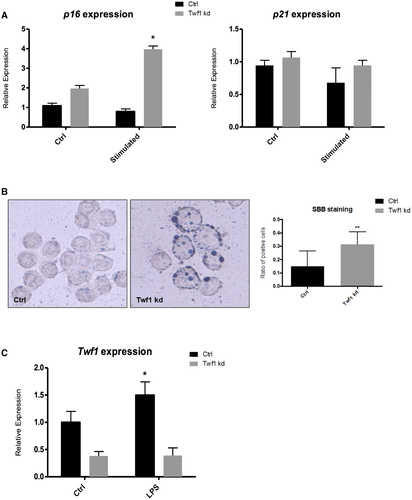
As a readout of cellular aging on a molecular level, the induction of senescence and SASP markers was evaluated in cholangiocytes knocked down for the expression of Twf1. Silencing of Twf1 determined the induction of senescence, as demonstrate by the up-regulation of p16 expression in cells incubated with a pro-proliferative stimulus (Fig. 5A). Similarly, as shown in Fig. 5B, quantification of lipofuscin deposition assessed by SBB staining was markedly increased in Twf1 knocked-down cholangiocytes compared with control cells, indicating an enhanced cellular senescence.30 In this context, however, we could not detect any change in the alternative senescence marker p21 on a mRNA level. As for the induction of SASP, we could demonstrate an increase in the expression levels of Il-1a, Il-1b, and Igf-1 in Twf1 knocked-down cholangiocytes, whereas there was no difference in Pai-1 (Supporting Fig. S1A). Il-1a protein levels increased significantly as a secreted factor in the culture medium of Twf1 knocked-down cells as compared with control (Supporting Fig. S1B), whereas Il-1b and Igf-1 levels were below detection limit (data not shown). Twf1 expression was also assessed in cholangiocytes exposed to persistent stimulation (10 days) with LPS, a well-known in vitro model that is able to induce cell senescence. Expression of Twf1 was increased in cholangiocytes with LPS-induced senescence compared with control (Fig. 5C). This evidence leads us to speculate that Twf1 expression is induced in response to cellular senescence possibly as a compensatory mechanism that stimulates cholangiocyte proliferation. The levels of senescence and SASP components, particularly p16, p21, Il-1b and Igf-1, were also significantly increased in LPS-induced cholangiocyte senescence (Supporting Fig. S2A,B). However, no difference was observed in the expression of Il-1a and Pai-I. Altogether, these data show that Twf1 induction could dampen the activation of senescence in response to harmful stimuli.
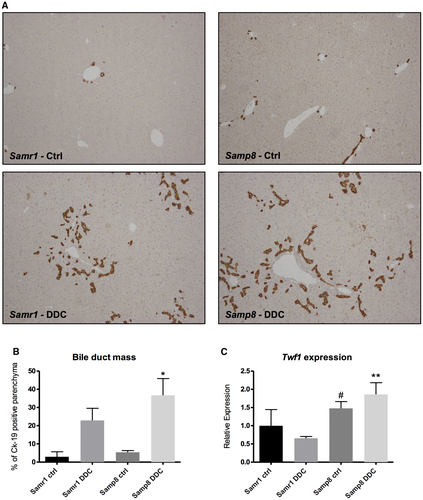
Accelerated Aging Enhances Biliary Response to Injury In Vivo
To test the influence of aging processes in biliary response to injury, cholangiocyte damage was induced by DDC administration for 4 weeks in Samp8 mice, which spontaneously present accelerated aging,32, 33 and in control Samr1 animals. As shown in Fig. 6A,B, the increase in biliary mass was higher in Samp8 mice compared with control animals, as assessed by Ck-19 immunohistochemistry and quantification. Collagen deposition assessed by sirius red quantification also tended to be higher in Samp8 mice subjected to DDC feeding compared with Samr1 mice, but this evaluation did not reach statistical significance (Supporting Fig. S3A). We also evaluated the hydroxyproline content in the livers of the same animals and found a similar trend (Supporting Fig. S3B). Twf1 protein expression levels have been evaluated in liver samples of Samp8 mice and their respective controls (Samr1), either subjected to DDC or not. As described in literature, Samp8 mice at about 2 months of age phenotypically resemble old mice of about 14 months of age. The protein expression level of Twf1 was significantly higher in Samp8 DDC-fed mice compared with controls, as well as in Samp8 mice subjected to control diet as opposed to their respective controls, indicating that the expression of Twf1 is modulated by both the aging and the damage of cholangiocytes in a mouse model of premature aging (Fig. 6C and Supporting Fig. S3C).
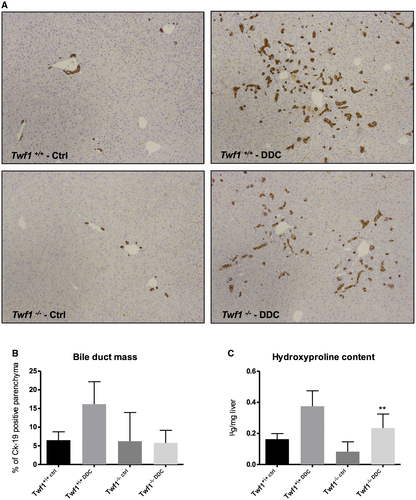
Lack of TWF1 Reduces Biliary Response to Injury In Vivo
To evaluate the influence of Twf1 in biliary response to injury, cholangiocyte damage was induced by DDC administration for 8 weeks in Twf1-/- mice and respective control Twf1+/+ mice. As shown in Fig. 7A,B, the increase in biliary mass tended to be lower in Twf1-/- mice compared with control animals, as assessed by Ck-19 immunohistochemistry and quantification. These data were further confirmed by the analysis of the collagen deposition assessed by sirius red quantification (Supporting Fig. S4) and by the evaluation of the hydroxyproline content, in which collagen deposition was significantly lower in Twf1-/- mice than the level observed in Twf1+/+ DDC feeding mice (Fig. 7C). Altogether, these data suggest that fibrosis occurrence is driven by both bile duct injury and more importantly by the aging process. Intriguingly, cholangiocyte response to injury in terms of fibrosis is exacerbated when the dangerous stimulus is triggered in the bile duct of aged mice.
Discussion
In the present study, we show that (1) the aging process and the injury of cholangiocytes are both associated with the upregulation of Twf1; (2) Twf1 is involved in the induction of biliary proliferation in response to injury and prevents cholangiocyte senescence; (3) biliary damage in response to injury is enhanced in a mouse model of accelerated aging; and (4) Twf1-/- mice show reduced reactive proliferation and collagen deposition in response to damage.
Altogether, in this study we provide evidence that the regulation of the aging process and the response to injury of cholangiocytes may share similar pathophysiological mechanisms and progress through common pathways.
The aging of a biological system is a multifaceted process that eventually leads to the loss of function of organs.1 Intense research in recent years has characterized a number of mechanisms to be involved in the regulation of aging. However, many aspects remain to be fully elucidated; in particular, the complexity of the process and paucity of specific molecular targets have hampered the current clinical applicability of recent discoveries.37, 38 In this context, it remains to be explored whether the alterations that come with the aging of a tissue could also affect the molecular changes that occur in pathological conditions. This aspect is particularly relevant given the fact that aging is the major risk factor for the development of most human diseases, from cancer to degenerative chronic diseases.39 Regarding hepatic and biliary disorders, important clues pointing towards a relevant role of aging in the modulation of biliary response to injury already exist. Old mice have recently been shown to be more susceptible to alcoholic liver injury through the down-regulation of sirtuin 1.40 From a clinical point of view, it is well known that donor age is among the most important risk factors predisposing to the development of biliary complications after liver transplantation7, 8; moreover, the clinical course of PSC has been demonstrated recently to differ according to age at diagnosis, with older patients at increased risk of developing cholangiocarcinoma.6
To screen for a large number of pathways possibly involved in aging, we selected from the literature a number of miRs known to regulate the aging process at different levels and in various organs (Table 1). miRs are short, noncoding RNA molecules that bind directly to target sequences located in a number of mRNAs, thereby fine-tuning the expression of multiple genes.41 The expression levels of selected miRs were then evaluated in cholangiocytes isolated from mice subjected to DDC feeding, a murine model of sclerosing cholangitis.29 This approach enabled us to preliminarily verify the possible involvement of aging-related miRs in the mechanisms underlying biliary response to damage, in a model of pathological alteration of the biliary tree. As shown in Fig. 1, of the 12 miRs tested, we found an increase in the expression levels of six aging-related miRs (i.e., miR-1a, miR-30e, miR-93, miR-34a, miR-146b, and miR-20a) in pooled samples of cholangiocytes isolated from DDC-treated animals, compared with controls. This result provides conceptual proof of a possible link, on a molecular level, between disease and aging. To substantiate this finding, the expression of up-regulated miRs was subsequently evaluated in cholangiocytes of young and old mice, either subjected to damage through DDC administration or not. In this setting, we could demonstrate that modulation of miR-1a, miR-20a, and miR30e expression occurred both in response to age (i.e., in old cholangiocytes of control fed mice) and in response to injury (i.e., in young mice subjected to DDC), with a possible additive effect when damage was inflicted to old cholangiocytes (Fig. 2A).
The assessment of putative targets of selected miRs was conducted by in silico analysis with the aim of identifying both intracellular pathways and molecular targets that are commonly affected by up-regulated aging-related miRs. As indicated in Supporting Table S1, up-regulated miRs converged on the regulation of a variety of intracellular pathways, many of which have been studied extensively for their role in biliary response to injury.42 Moreover, analysis of the target prediction database TargetScanHuman6.2 identified Twf1 as a common putative target of miR-1a, miR-20a, and miR30e. Twf1 is a highly conserved cytosolic protein that binds to and sequesters large amounts of actin monomer, thereby affecting the assembly of the actin cytoskeleton.43 Twf1 has also been demonstrated to directly bind and sever actin filaments, promoting degradation and turnover of actin structures.44, 45 The expression of Twf1 in cholangiocytes was significantly increased in old mice subjected to damage compared with young control mice; of note, Twf1 expression tended to be higher both in young diseased cholangiocytes and in control old cholangiocytes, when compared with controls (Fig. 2B). These data were further supported by the evidence found in the liver samples of Samp8 mice and their respective controls (Samr1). The protein expression level of Twf1 was significantly higher in Samp8 DDC-fed mice compared with controls, as well as in Samp8 mice subjected to control diet, indicating that the expression of Twf1 is modulated by both the aging and the damage of cholangiocytes in a mouse model of premature aging (Fig. 6C).
Induction of TWF1 expression could also be relevant in human cholangiopathies. Expression levels of TWF1 were indeed higher in the liver tissue of PBC and PSC patients when compared with control subjects (Fig. 3A). As shown in Fig. 3B, TWF1 immunohistochemistry resulted in being positive in reactive cholangiocytes of PSC patients and tended to be higher compared with the relative control, as shown by the staining intensity in Fig. 3C.
The response to damage by biliary cells is characterized by relevant changes in their phenotype and biology. Among others, proliferation is an essential aspect of biliary reaction to injury.46 The initial proliferation of cholangiocytes is functional to maintain the biliary mass, and therefore the secretory functions of the biliary epithelium. However, cell death eventually prevails with consequent development of ductopenia, while the constant inflammatory stimulation is believed to contribute to cholangiocarcinoma development.47 When cultured cholangiocytes were exposed to increasing pro-proliferative concentrations of serum, knockdown of Twf1 resulted in decreased Ki-67 expression and PCNA protein expression (Fig. 4A,B). Biliary proliferation was also reduced in Twf1 knocked-down cholangiocytes on a functional and dynamic level, as assessed by the SRB assay (Fig. 4C). The results of these experiments suggest that the up-regulation of Twf1, which occurs in response to both injury and the aging of cholangiocytes, could serve as a compensatory mechanism that sustains biliary mass in the case of cholangiocyte dysfunction. As shown in Fig. 5, the effect of Twf1 on biliary proliferation in vitro is at least in part mediated by the modulation of cellular senescence. The expression of the senescence marker p16 was indeed increased in proliferating cholangiocytes subjected to Twf1 knockdown (Fig. 5A), although we could not demonstrate a difference in the alternative senescence marker p21. Specific lipofuscin expression detected by SBB staining resulted in a significant increas in Twf1 knocked-down cholangiocytes (Fig. 5B), also indicating induction of cellular senescence.30 Senescent cells are known to acquire the so-called SASP. The mediators released by senescent cells, through complex effects on inflammation, growth and extracellular matrix remodeling, are believed to play pivotal roles in determining many of the age-related alterations of tissues.48 In our experiments, knockdown of Twf1 also caused an increase in the expression of a number of SASP markers, such as Il-1a, Il-1b, and Igf-1 (Supporting Fig. S1A). Il-1a protein levels increased significantly as a secreted factor, whereas no differences could be found in the secretion of Il-1b and Igf-1 (Supporting Fig. S1B). Similar results were also obtained in a well-known in vitro model that was able to induce cell senescence based on persistent stimulation (10 days) with LPS. Twf1 expression was increased in cholangiocytes with LPS-induced senescence compared with control (Fig. 5C). This evidence leads us to believe that Twf1 expression is induced in response to cellular senescence, possibly as a compensatory mechanism that stimulates cholangiocyte proliferation. The level of senescence and SASP components, particularly p16, p21, Il-1b and Igf-1, was also significantly increased in LPS-induced cholangiocyte senescence (Supporting Fig. S2A,B). Noteworthy, cholangiocyte senescence is induced in PSC cholangiocytes and has recently been demonstrated to play an important pathogenic role in the disease development.10, 12
Altogether, our data provide evidence that cellular adaptation to injury and to aging may imply the activation of similar pathways. In cholangiocytes, the age-dependent and damage-dependent up-regulation of Twf1 is functional to sustain biliary proliferation, possibly by reducing the activation of cellular senescence and SASP in response to injury. It remains to be elucidated whether tissue-specific dysregulation of such mechanism could predispose to the development of disease or to premature manifestations of aging.
In the last part of our study, we investigated in vivo whether aging increases biliary susceptibility to damage per se. Samp8 mice, an inbred mouse strain developed to study aging processes, and their respective controls, Samr1, were fed a DDC-containing diet to induce biliary alterations resembling PSC.31, 32 As shown in Fig. 6A,B, the biliary mass assessed by quantitative immunohistochemistry for Ck-19 was significantly increased in Samp8 mice subjected to damage as compared with age-resistant Samr1 controls. In addition, deposition of collagen tended to be increased in Samp8 mice, as observed by sirius red quantification (Supporting Fig. S3A) and by analysis of the hydroxyproline content in the liver (Supporting Fig. 3B), although it did not reach statistical significance. In contrast, in Twf1-/- mice, biliary reaction to damage tended to be dampened, as demonstrated by reduced proliferation assessed by Ck-19 immunohistochemistry and quantification (Fig. 7A,B). These data were further confirmed by analysis of the collagen deposition assessed by sirius red quantification (Supporting Fig. S4) and by evaluation of the hydroxyproline content, which was significantly lower in Twf1-/- mice than in controls (Fig. 7C).
These results suggest that aging of the biliary tree induces a stronger activation of compensatory responses to injury, per se. These include an increased expression of Twf1, which in turn is partially responsible for driving cholangiocyte proliferation and limiting biliary senescence. The final long-term effects of such modulations remain open to further research. Although enhanced proliferation may exert beneficial effects in acute settings, a sustained reactive phenotype of cholangiocytes may perpetuate the inflammation-associated damage of bile ducts, given the enormous secretory capacities of proliferating cholangiocytes.49 However, Twf1-induced decrease in cellular senescence may play a fundamental role in altering cancer surveillance in these cells, predisposing to cholangiocarcinoma development.50
In conclusion, we found Twf1 to be a key mediator of both biliary disease and aging. Both processes may proceed through similar pathways and share a common molecular signature. The understanding of such mechanisms may be a useful target for the treatment of cholangiopathies such as PSC.




