Intestinal Microbiota Mediates the Susceptibility to Polymicrobial Sepsis-Induced Liver Injury by Granisetron Generation in Mice
Abstract
Sepsis-induced liver injury is recognized as a key problem in intensive care units. The gut microbiota has been touted as an important mediator of liver disease development; however, the precise roles of gut microbiota in regulating sepsis-induced liver injury are unknown. Here, we aimed to investigate the role of the gut microbiota in sepsis-induced liver injury and the underlying mechanism. Cecal ligation and puncture (CLP) was used to induce polymicrobial sepsis and related liver injury. Fecal microbiota transplantation (FMT) was used to validate the roles of gut microbiota in these pathologies. Metabolomics analysis was performed to characterize the metabolic profile differences between sepsis-resistant (Res; survived to 7 days after CLP) and sepsis-sensitive (Sen; moribund before or approximately 24 hours after CLP) mice. Mice gavaged with feces from Sen mice displayed more-severe liver damage than did mice gavaged with feces from Res mice. The gut microbial metabolic profile between Sen and Res mice was different. In particular, the microbiota from Res mice generated more granisetron, a 5-hydroxytryptamine 3 (5-HT3) receptor antagonist, than the microbiota from Sen mice. Granisetron protected mice against CLP-induced death and liver injury. Moreover, proinflammatory cytokine expression by macrophages after lipopolysaccharide (LPS) challenge was markedly reduced in the presence of granisetron. Both treatment with granisetron and genetic knockdown of the 5-HT3A receptor in cells suppressed nuclear factor kappa B (NF-кB) transactivation and phosphorylated p38 (p-p38) accumulation in macrophages. Gut microbial granisetron levels showed a significantly negative correlation with plasma alanine aminotransferase (ALT)/aspartate aminotransferase (AST) levels in septic patients. Conclusion: Our study indicated that gut microbiota plays a key role in the sensitization of sepsis-induced liver injury and associates granisetron as a hepatoprotective compound during sepsis development.
Abbreviations
-
- 5-HT
-
- 5-hydroxytryptamine
-
- ABX
-
- antibiotics
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- BMDM
-
- bone-marrow–derived macrophage
-
- Ccl
-
- chemokine (C-C motif) ligand
-
- CLP
-
- cecal ligation and puncture
-
- Cxcl
-
- chemokine (C-X-C motif) ligand
-
- CYP1A1
-
- cytochrome P450, family 1, member A1
-
- FMT
-
- fecal microbiota transplantation
-
- H&E
-
- hematoxylin and eosin
-
- Il
-
- interleukin
-
- LPS
-
- lipopolysaccharide
-
- MAPK
-
- mitogen-activated protein kinase
-
- α-NF
-
- α-naphthoflavone
-
- NF-кB
-
- nuclear factor kappa B
-
- PBS
-
- phosphate-buffered saline
-
- PCA
-
- principal component analysis
-
- PCoA
-
- principal coordinates analysis
-
- PLF
-
- peritoneal lavage fluid
-
- p-AKT
-
- phosphorylated AKT
-
- p-ERK
-
- phosphorylated extracellular signal-regulated kinase
-
- p-JNK
-
- phosphorylated c-Jun N-terminal kinase
-
- p-p38
-
- phosphorylated p38
-
- Res
-
- sepsis resistant
-
- Sen
-
- sepsis sensitive
-
- siRNA
-
- small interfering RNA
-
- TLR4
-
- Toll-like receptor 4
-
- Tnf-α
-
- tumor necrosis factor alpha
-
- TUNEL
-
- terminal deoxynucleotidyl transferase dUTP nick end labeling
Sepsis that manifests from infection is one of the leading causes of morbidity and mortality in patients in intensive care units.1 It is estimated that more than 1.5 million patients suffer from sepsis annually,2 and the mortality of sepsis is approximately 20%-30%.3 Upon infection, host innate immunity is activated in response to pathogens, which is followed by the occurrence of a “cytokine storm” and immune dysregulation.4, 5 Such uncontrollable inflammation further leads to cell dysfunction and death, ultimately causing multiple organ dysfunction syndrome and death.6, 7 The liver is the key organ that maintains host homeostasis and plays an important role in host-defense activities8; hence, it is one of the most important “target” organs upon microbiota infection during sepsis. Unfortunately, sepsis-induced liver dysfunction is associated with poor clinical outcomes.8, 9 Few therapeutic approaches are currently available to reduce the burden of this life-threatening complication. Thus, investigating the pathogenesis of sepsis-induced organ injury, including liver dysfunction, is important to develop more-effective prevention and treatment strategies.
In the past decade, the gut microbiota has been proven to modulate injury responses in a number of organs remote from the gastrointestinal tract, especially in liver.10-14 Microbial metabolites are believed to be the main mediators for this progression. We previously found that gut bacteria–derived saturated fatty acids exerted protective effects against alcohol-induced liver injury.15 Moreover, certain dicarbonyl compounds could regulate liver damage in the context of drug-induced acute liver failure.16 In addition, the intestinal microbiota is the upstream modulator of systemic immunity,17, 18 which plays key roles in sepsis development. Based on these findings, it is possible that the gut microbiota is a key contributor to the pathogenesis of sepsis and related liver injury. The detailed roles of the gut microbiota during sepsis remain largely undefined. In the present study, we aimed to investigate the roles played by gut microbiota in regulating the susceptibility of mice to liver injury induced by polymicrobial sepsis with the hope of indicating a therapeutic strategy to prevent sepsis-induced liver damage.
Materials and Methods
Animal Model
Six- to 8-week-old specific pathogen-free male C57BL/6 mice were used. Toll-like receptor 4 knockout (Tlr4–/–) mice were purchased from the National Resource Center of Model Mice and Model Animal Research Center of Nanjing University (Nanjing, China). Polymicrobial sepsis was induced by cecal ligation and puncture (CLP) as described.19 In brief, mice were anesthetized with diethyl ether, after which a 2-cm midline laparotomy was performed under aseptic conditions to expose the cecum. A single through-and-through puncture was performed by a 21- (medium) or 18-guage (severe) needle between the ligation site and end of the cecum, and then a small amount of fecal material was extruded through the puncture. The cecum was repositioned into the peritoneal cavity, and the laparotomy was closed. Sham-operated animals underwent laparotomy and bowel manipulation without ligation and puncture. All mice were resuscitated with 1 mL of saline. For screening resistant and sensitive mice, half of the cecum was ligated and punctured with a 21-gauge needle. For the survival study, 75% of the cecum was ligated and punctured with an 18-gauge needle. Upon initiation of the sepsis models, mice in the granisetron treatment group received an intraperitoneal injection of granisetron at a dose of 1 mg/kg (the dose was modified based on a previous report20 and our pilot experiments) dissolved in phosphate-buffered saline (PBS) in addition to undergoing CLP. α-Naphthoflavone (α-NF) was dissolved in dimethyl sulfoxide and administrated to mice at a dose of 30 mg/kg for 7 days. All mice had free access to food and water and were housed in a temperature-controlled colony room on a 12/12-hour light-dark cycle. All experimental procedures were in accord with the National Institutes of Health guidelines and were approved by the local Animal Care and Use Committee of Southern Medical University (Guangzhou, China).
Cell Culture
RAW264.7 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Cultures were incubated at 37°C in a humidified atmosphere containing 5% CO2. Bone-marrow–derived macrophages (BMDMs) were isolated and grown in DMEM supplemented with 10% FBS, 1% penicillin-streptomycin, and 20 ng/mL of macrophage/colony-stimulating factor as reported.21 After 7 days, BMDMs were ready for further experimentation. Peritoneal macrophages were isolated by anti-F4/80 microBeads (Miltenyi Biotec, Shanghai, China), according to the manufacturer’s instructions.
Fecal Microbiota Transplantation
Fecal microbiota transplantation (FMT) was performed according to the modified method described.22, 23 Briefly, 6- to 8-week-old male C57BL/6 mice received antibiotics (vancomycin, 100 mg/kg; neomycin sulfate, 200 mg/kg; metronidazole, 200 mg/kg; and ampicillin, 200 mg/kg) intragastrically once a day for 5 days to deplete the gut microbiota. Feces from donor mice (resistant and sensitive groups) were collected and resuspended in PBS at 0.125 g/mL, then 0.15 mL of this suspension was administered to mice by oral gavage once a day for 3 days. After 3 days, mice were subjected to CLP and sacrificed 8 hours later, at which time tissues were collected.
Other materials and methods are described in the Supporting Materials and Methods section.
Statistical Analysis
The results are expressed as the mean ± standard error of the mean (SEM). Two-tailed Student’s t-test was used for statistical evaluation. Statistical differences between groups were analyzed using a significance level set at P < 0.05.
Results
Characterization of Sepsis-Resistant and -Sensitive Mice
We first performed medium severity CLP surgery to screen the response of mice to polymicrobial sepsis. We defined mice that were moribund before or at approximately 24 hours as sepsis sensitive (Sen), whereas animals that survived to 7 days were defined as sepsis resistant (Res; Fig. 1A). We further compared the extent of organ injury between these two mouse groups. Organs from Sen mice were harvested when they were moribund, whereas organs from Res mice were obtained at 7 days after CLP. Histological analysis (hematoxylin and eosin [H&E] and terminal deoxynucleotidyl transferase dUTP nick end labeling [TUNEL] staining) revealed that Sen mice displayed much more-severe organ injury and cell death (liver, lung, and kidney) than Res mice (Supporting Fig. S1A,B). Analysis of plasma biochemical parameters, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatinine, as well as the mRNA levels of inflammatory mediators measured in organs, also confirmed organ injury and increased inflammation in Sen mice (Supporting Fig. S1C,D). Other pathophysiological processes involved in organ damage during sepsis development, such as oxidative stress as monitored by superoxide accumulation (Supporting Fig. S2A), organ regeneration as detected by proliferating cell nuclear antigen (PCNA) expression (Supporting Fig. S2B), and intestinal injury and integrity as determined by colonic histological analysis and tight junction expression (Supporting Fig. S2C-E), were all improved in Res animals. Thus, Sen mice meet the definition of infection with organ dysfunction whereas Res mice had resolved organ injury or local inflammation when they were sacrificed.
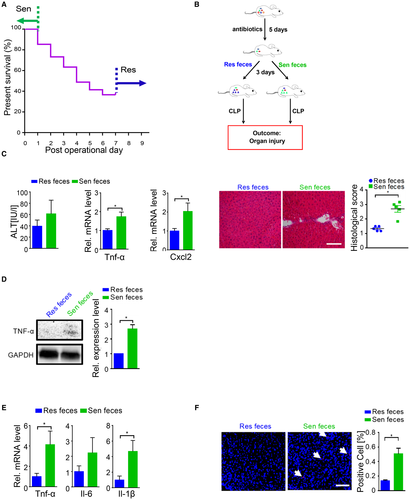
Susceptibility of Sepsis-Induced Liver Injury Was Transmissible by Gut Microbiota
Next, we explored the potential factors that mediated the difference in susceptibility. We speculate that gut microbiota may be one important modulator of the host response to polymicrobial sepsis. To verify our hypothesis, we performed an FMT experiment (Fig. 1B). Mice that received Sen feces developed more-severe liver injury than Res feces recipients after CLP surgery based on histological analysis and injury score quantification, and exhibited a trend toward higher ALT plasma levels (Fig. 1C). mRNA levels of almost all hepatic inflammatory factors showed obviously higher trends in Sen feces recipients than in Res feces recipients; in particular, tumor necrosis factor alpha (Tnf-α) and chemokine (C-X-C motif) ligand (Cxcl) 2 were significantly up-regulated (Fig. 1C and Supporting Fig. S1E). Increased hepatic TNF-α protein level confirmed the gene expression result (Fig. 1D). A similar inflammatory mediator expression profile was observed in peritoneal lavage fluid (PLF; Fig. 1E). More important, TUNEL staining revealed that mice transplanted with Sen feces exhibited more apoptotic cells compared to mice that received Res feces after CLP (Fig. 1F). We did not find obvious differences in lung and kidney injury after FMT between the recipient animal groups based on histological analysis (Supporting Fig. S3). These data suggested that susceptibility to sepsis-induced liver damage was transferrable by gut microbiota.
Gut Microbiota from Resistant and Sensitive Mice Showed Distinct Functions
We next analyzed the gut microbiota between Sen and Res mice to further understand how gut microbiota affected liver injury induced by polymicrobial sepsis. There was no difference in total bacterial load in the intestines of Sen and Res mice (Fig. 2A). The 16S diversity analysis showed that there was also no significant difference in alpha diversity between these two types of mice (Supporting Fig. S4A). Although the relative abundance of some strains, such as Actinobacteria at the phylum level and Helicobacter at the genus level, was significantly elevated in Sen feces compared to that in Res feces, weighted principal coordinates analysis (PCoA) analysis revealed that clusters of gut microbiota between Sen and Res mice were not completely separated (Fig. 2B and Supporting Fig. S4B,C), indicating that the intestinal microbiota exhibited a similar composition in Sen and Res mice.
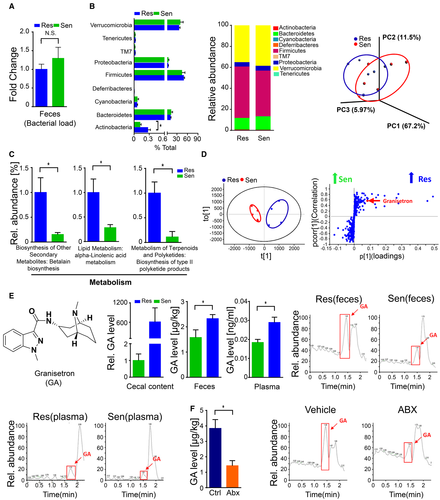
However, we conducted a Phylogenetic Investigation of Communities by Reconstruction of Unobserved States analysis, which evaluates and predicts microbial physiological function at the genomic level, and observed significant differences in levels of several genes responsible for metabolism. In particular, the genomic abundance of some pathways, such as biosynthesis of secondary metabolites, lipid metabolism, and metabolism of terpenoids and polyketides, was significantly enhanced in Res feces compared to that in Sen feces (Fig. 2C). These data suggested that gut microbial metabolic function may differ between Sen and Res mice. To further verify this finding, we performed nontargeted metabolomics analysis for gut microbiota (cecal content) in Sen and Res mice. Principal component analysis (PCA) and orthogonal partial least squares discrimination analysis showed that these mice exhibited different metabolite profiles (Fig. 2D). Granisetron, which serves as an antagonist for the 5-hydroxytryptamine (5-HT3) receptor,24 was markedly enriched in Res mice and was chosen for further targeted metabolomics analysis because it represented the largest difference in the nontargeted metabolomics analysis (Fig. 2E). Importantly, granisetron levels in plasma were also significantly higher in Res mice than in Sen mice, indicating that granisetron was released into the circulatory system (Fig. 2E). Collectively, our data revealed that gut microbiota from Res and Sen mice exhibited distinct functional differences, especially in metabolic processes.
Gut Microbiota Generated Granisetron
We then focused on the association between gut microbiota and granisetron. To confirm that the gut microbiota contributes to granisetron levels, we treated mice with antibiotics (ABX) to deplete the intestinal microbiota. Significantly reduced granisetron levels were observed in mice after ABX treatment compared to those in control mice (Fig. 2F). Moreover, we isolated some bacterial strains from human stool, cultured them in vitro, and then detected granisetron in the medium to monitor whether a single strain was responsible for the generation of this molecule. We found the presence of granisetron in the cultured medium from almost all bacterial strains; specifically, some bacteria, such as Clostridium difficile and Citrobacter freundi, produced greater amounts of granisetron, whereas other strains, such as Bacteroides thetaiotaomicron and Paenibacillus polymyxa, produced less based on the chromatograms (Supporting Fig. S5). Our data clearly demonstrated that the gut microbiota can generate granisetron.
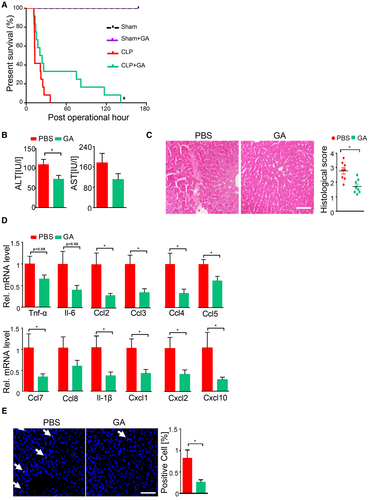
Granisetron Protected Mice against Polymicrobial Sepsis-Induced Liver Damage
To determine whether granisetron could modify the degree of liver injury during sepsis, we treated sham and CLP mice with granisetron when the surgery was finished. Granisetron-treated animals exhibited significantly longer survival times compared to those of control mice subjected to CLP (Fig. 3). Additionally, the ALT levels in granisetron-treated mice were markedly diminished compared to those in control mice at both 12 and 18 hours after CLP (Fig. 3B and Supporting Fig. S6A). Histological analysis revealed that livers from granisetron-treated mice had fewer pathological changes, including inflammation, necrosis, and thrombus formation, compared to livers from control mice after CLP (Fig. 3C and Supporting Fig. S6B). In agreement with these findings, expression of hepatic inflammatory factors was markedly decreased upon granisetron treatment (Fig. 3D and Supporting Fig. S6C). Additionally, granisetron treatment markedly reduced the number of apoptotic cells in the liver following CLP (Fig. 3E). Taken together, our data clearly demonstrated that granisetron can attenuate polymicrobial sepsis-induced liver damage.
Granisetron Did Not Affect Bacterial Clearance
We next explored the potential mechanism by which granisetron mediates its protective effects against polymicrobial sepsis-induced liver injury. We first determined whether granisetron could influence bacterial counts. Total bacterial load in both liver and cecum was not affected by granisetron administration (Supporting Fig. S7A). Moreover, microbiota isolated from cecum of mice was cocultured with granisetron under both anaerobic and aerobic conditions. We did not find any significant difference in total bacterial amount between granisetron-treated and nontreated groups as indicated by colony-forming unit (CFU) quantification (Supporting Fig. S7B). With ABX administration as a positive control, the OD600 absorbance results and 16S qPCR confirmed this finding (Supporting Fig. S7C,D). These data suggested that granisetron did not directly impact bacterial growth.
Bacterial clearance, which is mainly carried out by macrophages, plays an important role in development of sepsis and sepsis-induced organ injury.25 We considered the possibility that granisetron may affect macrophage bacterial clearance. Our data revealed that granisetron treatment did not affect the phagocytotic capacity of BMDMs (Supporting Fig. S7E). Additionally, there was no difference in bacterial killing by BMDMs between the granisetron-treated and untreated groups, as evidenced by CFU quantification (Supporting Fig. S7F). Collectively, these results suggested that the protective effect of granisetron during CLP is independent of macrophage-mediated bacterial clearance.
Granisetron Reduced Cytokine Overexpression After CLP
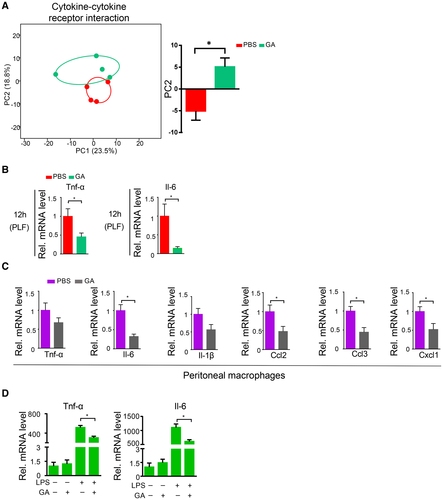
We next focused on the effects of granisetron on host. Because granisetron did not affect hepatic superoxide accumulation, hepatic regeneration, and colonic tight junction expression after CLP (Supporting Fig. S8), we ruled out these possibilities. Then, because inflammation, especially cytokine overproduction by immune cells, is a key driver of sepsis and sepsis-related liver injury progression,26, 27 we next investigated whether granisetron could attenuate cytokine overexpression during septic inflammation. We performed transcriptomic analysis of liver after CLP to provide the direct link between cytokines and liver cells during the protection of granisetron. We grouped all the genes involved in the cytokine/cytokine receptor interaction pathway, and found that the expression profile in granisetron-treated liver was markedly shifted compared to controls as determined by PCA analysis (Fig. 4A), suggesting that the effect of key cytokines on liver cells was associated with the beneficial effects of granisetron on septic liver injury development. Importantly, mRNA levels of Tnf-α and interleukin (Il)-6 were markedly reduced in the PLF in granisetron-treated mice compared to those in control mice at both 12 and 18 hours after CLP (Fig. 4B and Supporting Fig. S9A). Other key inflammatory factors, such as Il-1β and chemokine (C-C motif) ligand (Ccl) 2, were also reduced after granisetron treatment (Supporting Fig. S9B,C). Macrophages are believed to be the major source of cytokine overproduction during sepsis. We observed that peritoneal macrophages isolated from granisetron-treated mice showed lower cytokine expression than control mice after CLP (Fig. 4C). Additionally, based on our in vitro experiments, we found that granisetron administration was able to diminish Tnf-α, Il-6, Ccl2, Ccl3, and Cxcl1 gene expression in both RAW264.7 cells and isolated primary BMDM cells after lipopolysaccharide (LPS) challenge (Fig. 4D and Supporting Figs. S10A and S11A,B). These results indicated that granisetron reduces cytokine overexpression during sepsis progression.
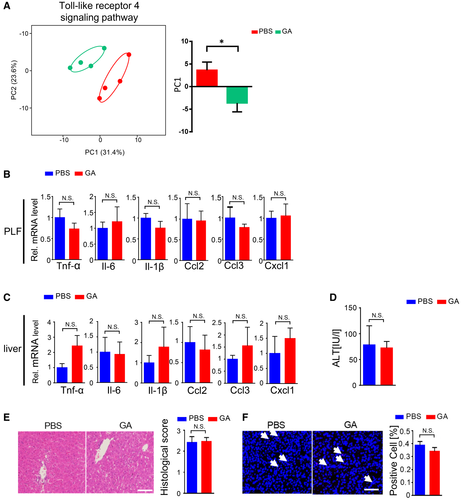
The Hepatoprotective and Anti-Inflammatory Effects of Granisetron Were Dependent on Toll-Like Receptor 4 Signaling
To further explore the detailed mechanism by which granisetron reduced cytokine overexpression and protected liver damage during polymicrobial sepsis progression, we performed transcriptomic analysis of macrophages from abdominal cavity after CLP. Figure 5A demonstrates that the expression profile of genes involved in the TLR4 signaling pathway, the main pathway that mediates the pathological effects of LPS, was markedly shifted upon granisetron administration. This suggested that the protective effects of granisetron may involve the regulation of TLR4 signaling. We used Tlr4–/– mice to further investigate the role of TLR4 in the protective effects of granisetron during sepsis. Cytokine levels in PLF was comparable between Tlr4–/– + PBS and Tlr4–/– + granisetron animals after CLP (Fig. 5B). In line with this finding, there was no difference in liver injury between Tlr4–/– + PBS and Tlr4–/– + granisetron groups as monitored by plasma ALT level, liver histological score, liver TUNEL staining, and hepatic inflammatory factor expression (Fig. 5C-F). These data demonstrate that the anti-inflammatory and hepatoprotective effects of granisetron during polymicrobial sepsis progression required the presence of TLR4.
TLR4 signaling leads to inflammatory mediator overexpression through activation of nuclear factor kappa B (NF-кB) and mitogen-activated protein kinases (MAPKs).28 We then explore whether granisetron would affect these pathways. NF-кB activation, as indicated by p65 translocation from the cytoplasm to the nucleus, was markedly reduced in the presence of granisetron after CLP challenge in isolated peritoneal macrophages and LPS-stimulated BMDMs and RAW264.7 cells (Fig. 6A-C and Supporting Fig. S10B,C). Because granisetron is an antagonist of the 5-HT3 receptor, we focused on protein kinase B (AKT) because of its potential as a downstream molecule of neurotransmitter signaling and its role as an upstream mediator of NF-кB signaling.29, 30 Granisetron reduced phosphorylated AKT (p-AKT) levels in peritoneal macrophages after CLP compared to controls (Fig. 6D). Moreover, Fig. 6E and Supporting Fig. S10D showed that AKT was activated upon LPS challenge and that granisetron markedly suppressed p-AKT levels in both RAW264.7 and BMDM cells. These data suggested that granisetron could down-regulate AKT activation after LPS administration. To further confirm that AKT inactivation was associated with a decreased inflammatory response, we pretreated LPS-stimulated macrophages with LY294002, an inhibitor of AKT signaling (Supporting Fig. S12). LY294002 markedly decreased NF-кB nuclear translocation and reduced mRNA levels of key cytokines and chemokines in both RAW264.7 and BMDM cells (Supporting Figs. S11C,D and S13). Collectively, our data indicated that granisetron is able to suppress AKT activation and restrict NF-кB transactivation during proinflammatory stimulation.
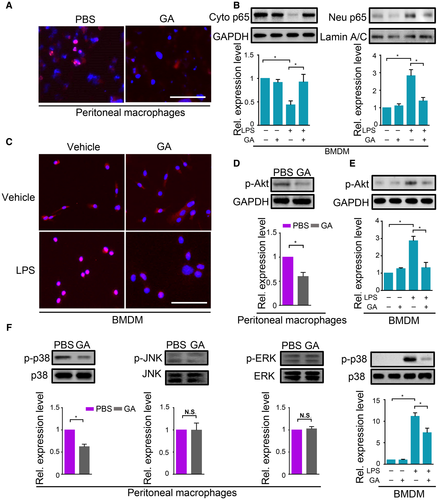
We next monitored the effect of granisetron on MAPK activation. Although phosphorylated extracellular signal-regulated kinase (p-ERK) and phosphorylated c-Jun N-terminal kinase (p-JNK) levels were not altered in the presence of granisetron in mouse peritoneal macrophages after CLP, phosphorylated p38 (p-p38) accumulation was markedly reduced upon granisetron administration compared to the PBS group (Fig. 6F). We further confirmed this finding in LPS-treated RAW264.7 cells and BMDMs, as presented in Fig. 6F and Supporting Fig. S14A. Phosphorylation of p38 was also markedly decreased in granisetron-treated cells as compared to controls after LPS stimulation. These results demonstrated that granisetron could diminish p38 activation during proinflammatory stimulation.
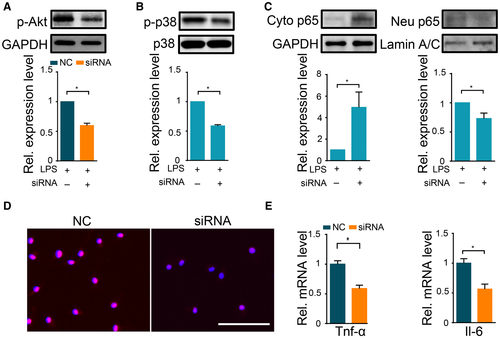
Genetic Knockdown of THE 5-HT3 Receptor Suppressed NF-κB Transactivation and p38 Activation
Granisetron is a specific antagonist for the 5-HT3 receptor. We next investigated the role of the 5-HT3 receptor in proinflammatory response after LPS challenge in macrophages. The 5-HT3 receptor has two subtypes: 5-HT3A and 5-HT3B. 5-HT3A is the predominant subtype.31 We first confirmed that macrophages expressed the 5-HT3A gene (Supporting Fig. S15A) and further used small interfering RNA (siRNA) to knock down 5-HT3A receptor gene expression (Supporting Fig. S15B). Genetic silencing of the 5-HT3A receptor led to significantly decreased p-AKT levels in both RAW264.7 and BMDM after LPS (Fig. 7A and Supporting Fig. S14B). In addition, NF-кB nuclear translocation and p-p38 accumulation were also markedly diminished upon 5-HT3A receptor genetic silencing (Fig. 7B-D and Supporting Fig. S14C-E). Moreover, 5-HT3A siRNA treatment could markedly reduce cytokine overexpression in both RAW264.7 and BMDM (Fig. 7E and Supporting Figs. S11E,F and S14F). Taken together, these results demonstrated that the 5-HT3A receptor suppresses LPS-induced proinflammatory responses, demonstrating that the effect of granisetron on inflammatory signaling may be dependent on inhibition of the 5-HT3A receptor.
Effect of Cytochrome P450, Family 1, Member A1 on Liver Injury during Polymicrobial Sepsis Development
It is reported that granisetron can be metabolized by cytochrome P450, family 1, member A1 (CYP1A1) in liver.32 Therefore, to evaluate the role of CYP1A1 in polymicrobial sepsis-induced liver injury development, we treated mice with α-NF, a well-established CYP1A1 inhibitor33, 34 and monitored the hepatic damage after CLP. α-NF administration did not significantly affect hepatic injury as characterized by comparable plasma ALT level and histological injury score, indicating that CYP1A1 may not directly contribute polymicrobial sepsis-induced liver injury (Supporting Fig. S16A-C). We further monitored plasma granisetron level and found that α-NF did not increase this microbial metabolite abundance in the circulatory system (Supporting Fig. S16D). This result may, at least in part, explain the liver injury phenotype observed in α-NF-treated mice. Indeed, granisetron undergoes hydroxylation and demethylation in liver in rodents and humans.35 It is reported that CYP1A1 is mainly responsible for 7-hydroxylation whereas other enzymes, such as cytochrome P450 family 3 subfamily A member 4, contributes for 9-demethylation.32, 36 Selective inhibition of CYP1A1 may induce alternative enzyme activity and thus maintain circulatory granisetron level. It is speculated that only by inhibiting all the metabolic enzymes would there be an impact on CLP-induced liver injury.
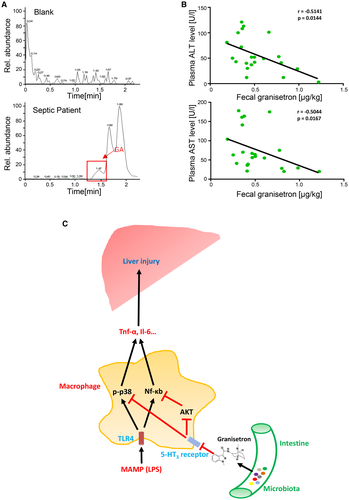
The Level of Gut Microbial Granisetron was Negatively Correlated with Liver Injury in Septic Patients
We finally sought evidence for the presence of granisetron in feces of septic patients (Fig. 8A). We observed that gut microbial granisetron levels showed a significantly negative correlation with plasma ALT and AST levels (Fig. 8B). Thus, granisetron levels were inversely associated with elevation of liver injury markers in humans with sepsis.
Discussion
This study was undertaken to establish whether organ protective factors could be transferred in gut microbiota from host to host in the setting of polymicrobial sepsis. The gut microbiota from mice resistant to sepsis-induced death produced a metabolite, granisetron, that protected naïve mice from sepsis-induced liver injury. Figure 8C depicts the major mechanisms identified by our work. These include inhibition of receptor 5-HT3A on macrophages by granisetron, leading to a suppression of NF-кB and p38 signaling. This, in turn, prevented cytokine production. This work supports the general concept of using FMT in the setting of sepsis, but, more specifically, that granisetron could be used to prevent excessive inflammation in sepsis.
Although our FMT experiment clearly demonstrated that gut microbiota was an upstream regulator of liver inflammatory and injury responses during sepsis, other factors involved in organ failure or resistance may also play a prominent role in polymicrobial sepsis progression. For example, reactive oxygen species (ROS), such as superoxide, may also participate in the cascade of injury processes during sepsis and influence the outcome of CLP.37 Our data indicated that gut microbiota may not influence ROS levels during sepsis development. In addition, the capacity of organ cell regeneration is another determinant of organ recovery, and efficiency of organ regeneration likely contributes to the severity and duration of the organ injury in response to polymicrobial sepsis.38 We further found that granisetron did not markedly affect PCNA expression in liver, suggesting that gut microbiome may not influence the organ cell-regeneration process. Therefore, many factors contribute to organ injury and the regeneration process during sepsis. Out work points to the microbial metabolite as just one of these factors. Other mediators and processes, such as redox balance or regeneration capacity, also contribute to susceptibility of polymicrobial sepsis-induced liver injury and death.
An interesting finding of the current work is that microbial composition was not markedly different between Sen and Res animals. In fact, Sen and Res mice were housed with each other (cohoused) before CLP; hence, the microbial composition in the two groups should have been similar, as observed in the present study. However, cohousing may not be sufficient to completely transfer all properties of the microbiota. For example, the bacterial composition and metabolomics profiles may not be parallel, meaning that different microbial compositions cannot be equated to different metabolomics profiles.39 Bacterial function may also be influenced by other independent factors, such as the physiological condition of the host intestine, food intake, and lifestyle. Another contributor to microbial responses is the release of stress factors from the gut into the intestinal lumen. Our results support the concept that disruption of colonic integrity was accompanied by increased liver damage during polymicrobial sepsis. Furthermore, the 5-HT3 receptor is highly expressed in the intestine (Supporting Fig. S15)40 and can regulate gut pathophysiology.41, 42 Hence, we speculate that the different susceptibility of intestinal injury observed in Sen and Res mice may partially involve serotonin and 5-HT3 receptor interaction in the gut and may modulate the 5-HT3 receptor antagonist, granisetron, production from the gut microbiota.
Granisetron has served as an antiemetic drug to treat nausea and vomiting for patients who are undergoing chemotherapy. In addition to its neuromodulation roles, granisetron has been reported to exert beneficial effects against the disease-associated inflammatory response. For example, granisetron could decrease colonic levels of Tnf-α, Il-6, and myeloperoxidase activity to protect rats against an experimental model of inflammatory bowel disease.20 In addition, this compound was able to alleviate inflammatory arthritis.43 Consistent with previous findings, our work further disclosed that granisetron also exhibited beneficial roles in sepsis-induced liver injury, which was linked to its anti-inflammatory effects. Macrophages were shown to be a target for granisetron in the present study. Macrophages can be broadly classified into two groups: M1 with a proinflammatory phenotype and M2 with an anti-inflammatory phenotype.44 M1 macrophages are characterized by production of proinflammatory factors, such as Tnf-α, Il-6, inducible nitric oxide synthase, and Il-1β, whereas M2 macrophages express higher levels of Il-4 and Il-10.45, 46 Macrophage activation has been proven to be plastic and reversible, which indicated that macrophage populations are dynamic and display functional changes in different microenvironments.47, 48 Our data showed that granisetron could directly suppress M1-related genes, such as Tnf-α, Il-6, and Il-1β, in murine macrophages; however, it had no effect on LPS-enhanced M2 profile, including Il-4 and Il-10 mRNA levels (data not shown), suggesting that granisetron regulates inflammatory responses in sepsis, at least in part, by reducing proinflammatory mediators production by M1 macrophages.
It is well known that excessive production and release of cytokines can cause liver injury during sepsis or endotoxemia. For example, Harbrecht et al. reported that systemic TNF-α could directly cause hepatocellular damage after LPS challenge.27 Indeed, macrophages, including Kupffer cells and BMDMs, are the major source for overproduced cytokines during sepsis. Thus, suppression of macrophage cytokine release is a reasonable strategy to alleviate liver injury during sepsis. Based on transcriptomic analysis and data using TLR4 knockout mice, our study identified TLR4 signaling, including NF-κB and p38 MAPK, as one of the targets for the effects of granisetron-mediated cytokine expression reduction during LPS or CLP challenge. By exploring AKT activation in macrophages, our findings suggest a link between neural signaling and inflammatory responses, in which the 5-HT3 receptor/AKT/NF-κB axis may play a key role in overexpression of inflammatory factors (Fig. 8C). For MAPKs, another key downstream pathway of TLR4 signaling, granisetron, selectively suppressed p38 activation, but had no impact on ERK and JNK activation, in macrophages. p38 activation would not only promote Tnf-α expression at the transcriptional level, but could also stabilize Tnf-α mRNA by regulating the zinc-finger protein, tristetraproline.49 Indeed, it has been reported that p38 inhibitor could significantly improve the animal’s survival and reduce inflammatory factor expression in macrophages during polymicrobial sepsis progression,50 demonstrating that p38 signaling suppression was able to directly reduce cytokine overexpression in macrophages and protect mice against polymicrobial sepsis after CLP. Hence, 5-HT3 receptor/p38/cytokine expression is another key pathway impacted by granisetron through 5-HT3 receptor inhibition (Fig. 8C). Future work will focus on the detailed modulatory mechanism of p38 by 5-HT3 receptor inhibition.
In summary, by associating a microbial metabolite, granisetron, as an anti-inflammatory compound, our work suggested the gut microbiota as an upstream regulator for polymicrobial sepsis-induced liver injury and uncovered an underlying mechanism by which the gut microbiota modulates susceptibility of sepsis-induced liver injury. These findings expand our knowledge of the “gut-liver axis” during sepsis development and indicate a therapeutic approach for septic liver injury.
Acknowledgment
We thank Zhanke He and Guoquan Wei from Southern Medical University for excellent technical assistance.




