Intrahepatic T-Cell Receptor β Immune Repertoire Is Essential for Liver Regeneration
Supported by the Scientific Research Foundation of Qingdao University (41117010262).
FASTQ files of VDJ sequences of TCRβ are deposited under Baidu Pan (https://pan.baidu.com/s/1c2Gs2sg; accession code: u06p). Further information about data and reagents used is available by request to the corresponding author.
Abstract
T lymphocytes synergize with the cellular immune system to promote hepatocyte regeneration. The T-cell receptor (TCR) immune repertoire is closely associated with the host immune response and regenerative proliferation. High-throughput sequencing of TCR provides deep insight into monitoring the immune microenvironment. Here, we aimed to determine the role of the TCRβ immune repertoire in liver regeneration (LR). We investigated hepatic regeneration in TCRβ chain-deficient (tcrb–/–) mice by two-thirds partial hepatectomy (PHx) method. Our results demonstrated that tcrb–/– mice revealed a reduced capacity for LR, which was characterized by impaired hepatocyte proliferation and enhanced hepatocyte apoptosis. Dysregulation of inflammatory signaling activation and inflammatory factors was observed in regenerated tcrb–/– livers. Simultaneously, significantly altered immunocyte levels and aberrant cytokine levels were observed during hepatic regeneration. In addition, we first determined the profile of the TCRβ immune repertoire during LR, indicating that PHx resulted in remarkably lower TCRβ diversity in intrahepatic T lymphocytes. Conclusion: Taken together, our data suggest that TCRβ deficiency gives a rise to aberrant intrahepatic immune microenvironment that impairs LR, and the TCRβ reconstitution is required for hepatic immunocyte recruitment and activation during LR.
Liver regeneration (LR) is one of the essential characteristics of this important organ. Although adult hepatocytes are quiescent and highly differentiated, they have great potential to regenerate when injured by various pathogens or chemical or mechanical damage.1, 2 The mechanism underlying this process is closely associated with multiple signalling pathways induced by a wide variety of inflammatory cytokines, growth factors, and transcription factors.2-5 Murine two-thirds partial hepatectomy (PHx), a widely used experimental model, induced regenerative proliferation of hepatocytes and nonparenchymal cells (stellate, biliary epithelial, and sinusoidal endothelial cells). Increasing evidence reveals that PHx robustly activates the immune system and triggers inflammatory mediator release, which leads to an acute phase.6 It is now generally accepted that various immunocytes (natural killer [NK] cells, dendritic cells, and macrophages) and paracrine factors (tumour necrosis factor-α [TNF-α], interleukin [IL]-6, IL-17, and interferon-γ [IFN-γ]) may be involved in this process.6-10 They cooperate with each other to synergistically promote hepatocyte proliferation by inducing signal transducer and activator of transcription 3 (STAT3) phosphorylation, which is directly mitogenic and pro-proliferative.11, 12
T cells can be divided into two subpopulations (αβ and γδ T cells) on the basis of different T-cell receptors (TCRs). A majority of T cells in adult blood expresses a TCR heterodimer consisting of α and β chains, of which the β chain has three different regions that correspond to the three complementary determined regions (CDRs). Specificity and diversity of αβ T cells depends on the CDR3 region of TCRβ, which originates from the recombination of somatic variable (V), diversity (D), and joining (J) gene segments and random nucleotide excision and addition at the VD and DJ junctions.13, 14 The hypervariable CDR3 region of TCRβ is critical for major histocompatibility complex (MHC)-peptide complex recognition and determines functional activation, clonal expansion, and selection for αβ T cells.15 Therefore, the characterization of the TCRβ-associated repertoire may reveal important information regarding immune responses during LR.
Accumulating studies point to the innate immune system as an important approach in regulating LR.6 In contrast, the role of the adaptive immune system in the process of LR has not been clarified. Emerging studies indicate that mice lacking nearly all T cells display 75% mortality post-PHx, whereas mice lacking only γδ T cells exhibit impaired hepatocyte proliferation during LR.16, 17 Hence, T cells are indispensable for normal LR. Nevertheless, the function of TCRβ in the process of LR has rarely been explored.
Next-generation high-throughput sequencing of the CDR3 region by a multiplex PCR system allows us to probe the complex TCRβ diversity and is a potent tool for evaluating the clonal composition of the αβ T-cell repertoire and for monitoring the immune microenvironment. In this study, we sequenced the immune repertoire of TCRβ in intrahepatic T lymphocytes post-PHx and discovered an essential role of the TCRβ immune repertoire in LR. In addition, an unrecognized mechanism between the TCRβ immune repertoire and inflammatory cell activation was uncovered, establishing a vital role of the T-cell–adaptive immune system in LR.
Materials and Methods
ANIMALS
TCRβ chain-deficient (tcrb–/–), tcrbflox/flox, and C57BL/6 mice were obtained from Second Military Medical University. For generation of Cre adenovirus-directed condition (AD-CMV-Cre) deletion in tcrbflox/flox mice (tcrbAD-Cre), AD-CMV-Cre (1 × 109 plaque-forming units in 100 µl phosphate-buffered saline) was injected into the tail vein of Tcrbflox/flox mice. Experiments were performed 7 days after injection. All mice were maintained on autoclaved chow diet in filter-topped cages in specific pathogen-free animal rooms. All animal experiments were approved by the Qingdao University of Medicine Institutional Animal Care and Use Committee.
PARTIAL HEPATECTOMY
Eight- to 12-week-old male mice were subjected to PHx as described.18 Briefly, all mice were anesthetized using isoflurane to avoid hepatotoxicity. The abdominal cavity was entered through an upper-midline incision just below the sternum. The large left lateral lobe and two median lobes were ligated and removed. The gallbladder was always removed during surgery to avoid damage. Mice were sacrificed at the indicated time post-PHx for subsequent analysis. Wild-type (WT) C57BL/6 mice were used as a control.
RNA ISOLATION, TCRβ REPERTOIRE AMPLIFICATION
Total RNA was isolated from liver tissue or intrahepatic lymphocytes using an RNAprep Pure Cell/Bacteria Kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s specifications. Concentrations of RNA were quantified using an Eppendorf BioPhotometer Plus (Eppendorf, Hamburg, Germany), and 100 ng of total RNA was converted into complementary DNA (cDNA) by reverse transcription using a Transciptor First Strand cDNA Synthesis Kit (Roche Applied Science, Penzberg, Germany), according to the manufacturer’s protocol, on a T100 Thermal Cycler (Bio-Rad Inc., Hercules, CA).
For TCRβ CDR3 library preparation, two-round nested amplicon arm PCR was performed with a Multiplex PCR Assay Kit Ver. 2 (TaKaRa, Dalian, China) using specific primers against each variable and constant gene (the multiplex primers in Supporting Table S1). The first round of PCR was performed using cDNA templates in a 50-μL reaction with the following program: one cycle of 94°C for 60 seconds, 30 cycles of denaturation at 94°C for 30 seconds and annealing at 60°C for 60 seconds, and a final extension for 10 minutes at 72°C. For the second round of PCR amplification, a 5-μL sample of the first-round PCR product was used, and the same PCR protocol was followed. PCR products were purified by agarose gel electrophoresis (Supporting Fig. S1), amplified using Illumina sequencing primers with different sample barcodes, and subjected to high-throughput sequencing using the Illumina HiSeq X Ten platform (Illumina, San Diego, CA) with a read length of 2 × 150 base pairs.
ANALYSIS OF HIGH-THROUGHPUT SEQUENCING DATA
The original data obtained from the Illumina HiSeq X Ten platform were converted to raw paired-end sequence reads by filtering the low-quality sequences. V, J, and CDR3 regions of TCRβ consensus sequences were identified using BLAST Plus in the international ImMunoGeneTics (IMGT) information system (IMGT: http://www.imgt.org/) by a standard algorithm.19 The diversity of TCRβ was measured by Gini coefficient, normalized Shannon diversity entropy, and Rank-abundance, which have been widely used for assessing the richness and diversity of TCR as described.20, 21
STATISTICAL ANALYSIS
All statistical analyses were performed using the GraphPad Prism 5 Package (GraphPad Software, La Jolla, CA). Data are presented as the mean ± SEM. The Mann-Whitney U test was performed to compare values obtained from two groups. Differences among three or more groups were determined using one-way analysis of variance with repeated measures or Friedman’s test. For immune repertoire data, statistical significance between prehepatectomy and posthepatectomy was assessed using a Student t test. Significance was accepted at P < 0.05.
OTHER METHODS
All other methods are described in the Supporting Methods.
Results
LR IS IMPAIRED IN THE ABSENCE OF TCRβ
Efficient deletion of TCRβ in Tcrb–/– and TcrbAD-Cre mice were confirmed at the protein level by immunofluorescence (IF) analysis (Supporting Fig. S2). To explore whether TCRβ plays an important role in LR, Tcrb–/– and TcrbAD-Cre mice were subjected to PHx. Livers of adult Tcrb–/– and TcrbAD-Cre mice were normal in appearance, size, and weight and had no significant difference in their architecture compared to WT mice (Supporting Fig. S3). Hepatocyte damage parameters, such as serum alanine transaminase (ALT) levels, were markedly decreased post-PHx in Tcrb–/– and TcrbAD-Cre mice compared to WT mice (Fig. 1A). Tcrb–/– and TcrbAD-Cre mouse livers displayed a significant reduction in vacuolar degeneration at 48 hours post-PHx (Fig. 1B,C). These results indicate that TCRβ is necessary for hepatic inflammatory response post-PHx.
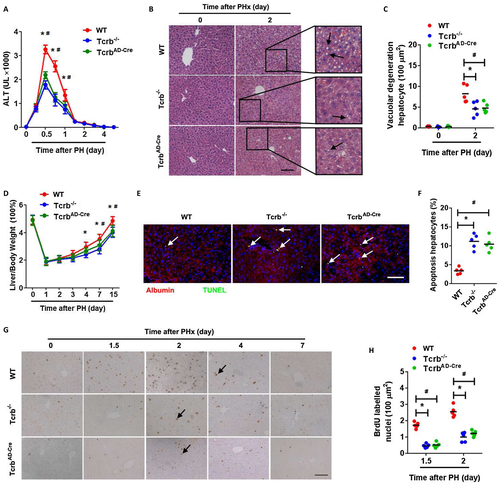
In addition, Tcrb–/– and TcrbAD-Cre mice exhibited a significant decrease in liver-to-body weight ratio post-PHx, which suggested impairment of LR (Fig. 1D). Hepatocyte proliferation was dramatically lower in Tcrb–/- and TcrbAD-Cre mice than in WT operated mice after PHx, as detected by multiple proliferative indices, including 5-bromodeoxyuridine (BrdU) incorporation (Fig. 1G,H), Ki67, and proliferating cell nuclear antigen (Supporting Fig. S4). We also measured cell apoptosis by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining and found that hepatocyte apoptosis was elevated in Tcrb–/– and TcrbAD-Cre mice 48 hours post-PHx (Fig. 1E,F). However, apoptotic αβ T or γδ T cells were barely detected in either WT or mutant mice post-PHx (Supporting Fig. S5). These data indicated that TCRβ is indispensable for both hepatocyte proliferation and survival in LR.
TCRβ DEFICIENCY INDUCES AN ABERRANT INFLAMMATORY MILIEU POST-PHx
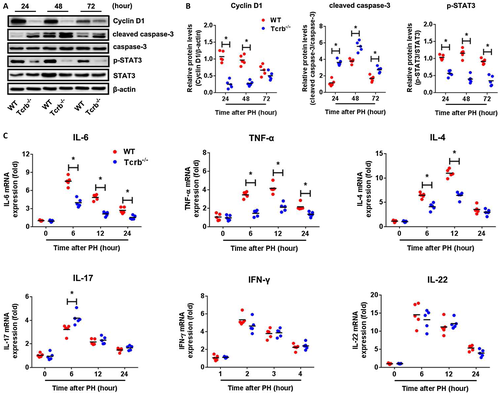
To determinate the mechanistic role of TCRβ in LR, we first examined cell-cycle progression. Cyclin D1, a critical regulator of cell-cycle progression from G1 to S phase, was expressed at substantially lower levels in Tcrb–/– and TcrbAD-Cre mice than in WT mice at 24 and 48 hours post-PHx (Fig. 2A,B and Supporting Fig. S6). Similarly, there was enhanced expression of cleaved caspase-3 in regenerating livers of Tcrb–/– mice at 24, 48, and 72 hours post-PHx. Given the critical role of TCRβ in hepatic inflammatory response during LR, we investigated inflammatory signaling in regenerating Tcrb–/– livers compared to WT livers. As expected, livers from Tcrb–/– mice exhibited diminished phosphorylation of STAT3 (p-STAT3; Fig. 2A,B). Moreover, levels of IL-6, TNF-α, and IL-4 were significantly attenuated in Tcrb–/– livers, all of which were robustly activated in WT livers from 6 to 12 hours post-PHx, whereas IFN-γ and IL-22 were not significantly affected by TCRβ deficiency. Interestingly, IL-17, which is associated with intrahepatic sterile inflammation, was enhanced in Tcrb–/– livers (Fig. 2C). Collectively, these data indicated that impaired proliferative signaling and abnormal expression of inflammatory mediators account for defective LR in the absence of TCRβ.
INTRAHEPATIC IMMUNE MICROENVIRONMENT MODULATES HEPATOCYTE SURVIVAL IN VITRO
In order to explore regulatory role of intrahepatic immune microenvironment on hepatocytes in vitro, cocultures of mouse normal liver cells (NCTC1469) with αβ T cells, isolated from intrahepatic immune cells (WT mice) prehepatectomy and posthepatectomy by flow cytometry, were performed for 2 days. Double IF analysis was performed on NCTC1469/αβ T cell cocultures. Interestingly, the proliferative capacity of hepatocyte did not change after coculture with αβ T cells alone either prehepatectomy or posthepatectomy (Supporting Fig. S7).
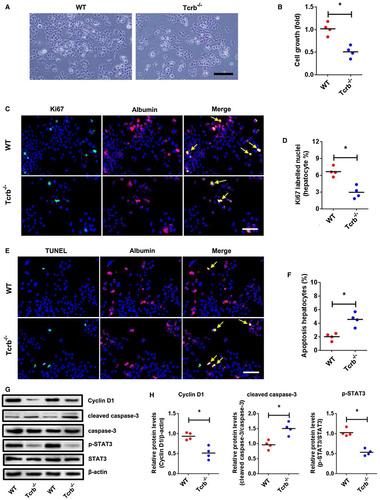
In contrast, double IF analysis showed an obvious decrease of growth and Ki67 expression in hepatocytes after coculture with intrahepatic immune cells isolated from Tcrb–/– liver post-PHx in comparison with hepatocytes cocultured with immune cells isolated from WT liver (Fig. 3A-D). TUNEL immunostaining has been performed to confirm the apoptosis in vitro. The percentage of apoptotic hepatocyte significantly increased after coculture with intrahepatic immune cells isolated from Tcrb–/– liver (Fig. 3E,F). In addition, the expression of cyclin D1, cleaved caspase-3, and p-STAT3 was confirmed by western blotting analysis of NCTC1469 cocultured with intrahepatic immune cells isolated from WT liver and Tcrb–/– liver post-PHx. As expected, significant decrease of cyclin D1, p-STAT3, and increase of cleaved caspase-3 were observed in hepatocytes cocultured with intrahepatic immune cells isolated from Tcrb-/- liver with respect to hepatocytes co-cultured with immune cells isolated from WT liver (Fig. 3G,H). Together, this demonstrated that hepatocyte proliferation and apoptosis are modulated by the intrahepatic immune microenvironment rather than αβ T cells alone.
TCRβ AFFECTS IMMUNE CELL ACTIVATION
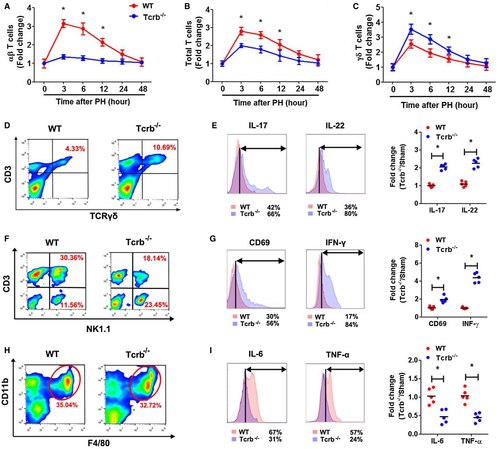
Because of the above results illuminating the aberrant inflammatory milieu in regenerating Tcrb–/– livers, we wondered whether TCRβ affects LR by influencing the recruitment and activation of neighboring immune subsets. As expected, Tcrb–/– livers displayed negligible αβ T-cell subset levels during LR (Fig. 4A and Supporting Fig. S8A,B). Conversely, a robustly elevated γδ T-cell number was observed post-PHx (Fig. 4B and Supporting Fig. S8C,D), although the total T-cell number was significantly decreased in Tcrb–/– livers (Fig. 4C). However, no significant difference of other immunocyte subsets (NK/NKT [natural killer T lymphocyte] and Kupffer cells [KCs]) was observed between WT and Tcrb–/– livers (Supporting Fig. S8E-J). We also evaluated the status of different immunocyte subsets post-PHx. γδ T-cell–secreted IL-17 and IL-22 levels were also markedly higher in hepatectomized Tcrb–/– mice than in WT mice (Fig. 4D,E). Moreover, the fractions of activated hepatic NK and NKT cells (CD69+, NK1,1+), which have been reported to suppress hepatic regeneration,10, 22, 23 were elevated in Tcrb–/– livers compared to WT livers, expressing higher IFN-γ levels (Fig. 4F,G). Interestingly, the fraction of invariant NKT cells (CD3+, CD1d-Tet+) was lessened post-PHx (Supporting Fig. S9). Similarly, TCRβ ablation led to a significant reduction in IL-6 and TNF-α levels secreted by KCs, though their proportions were not affected in regenerating liver by loss of TCRβ (Fig. 4H,I). These results suggest that TCRβ deficiency selectively activated NK, NKT, and KCs and decreases invariant NKT cell population, resulting in an expansion of γδ T cells in the regenerating liver and significantly impairs activation of hepatic inflammatory cells.
PROFILING OF Vβ AND Jβ GENE SEGMENTS DURING THE LR
To assess the variation of the TCRβ immune repertoire in intrahepatic αβ T cells during LR, we first measured this population pre-PHx and post-PHx in WT mice. The number of intrahepatic αβ T cells increased sharply 6 hours post-PHx (Fig. 5A,B). Subsequently, four paired PCR samples (pre-PHx and 6 hours post-PHx) were subjected to high-throughput sequencing (Supporting Table S2). We identified a total of 23 distinct Vβ gene segments and 13 distinct Jβ gene segments from all samples. The most frequent Vβ gene segments were TRBV13-2 (17.82% pre-PHx, 20.76% post-PHx) and TRBV1 (17.67% pre-PHx, 20.43% post-PHx). The most frequent Jβ gene segments were TRBJ2-7 (24.60% pre-PHx, 22.79% post-PHx) and TRBJ2-5 (14.52% pre-PHx, 12.84% post-PHx; Fig. 5C).
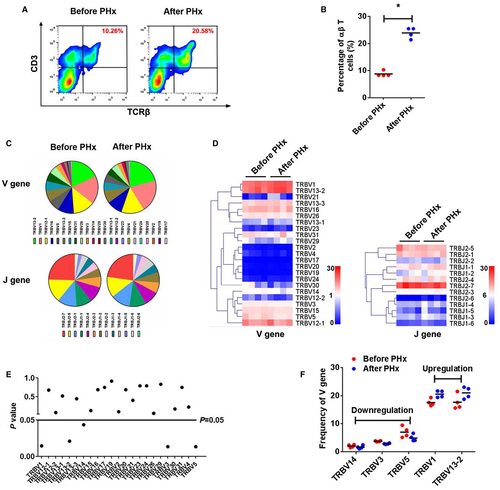
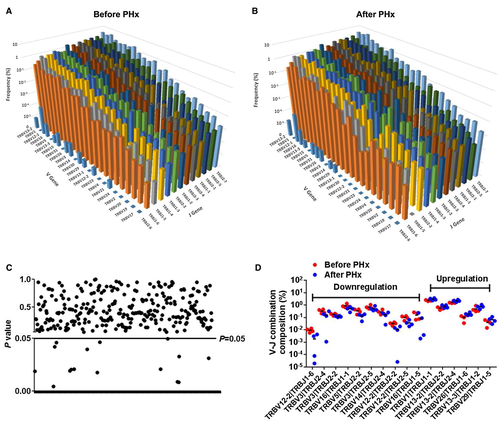
A heatmap was generated according to the usage frequency of Vβ and Jβ gene segments (Fig. 5D). Usage patterns of most Vβ and Jβ gene segments were similar pre-PHx and post-PHx (Fig. 5E and Supporting Fig. S10), whereas some Vβ gene segments revealed significant usage differences post-PHx, including TRBV1, TRBV13-2, TRBV12-2, TRBV3, and TRBV5 (Fig. 5F). Furthermore, we also detected the composition of V-J gene combinations and found a total of 290 distinct V-J gene combinations (Supporting Table S3). However, the composition of V-J gene combinations showed no difference pre-PHx and post-PHx (Supporting Fig. S10). Compared with prehepatectomy, the general usage patterns of V-J gene combinations were similar posthepatectomy (Fig. 6A,B), and there were only 16 V-J gene combinations (5.51%) that exhibited significant usage differences pre-PHx and post-PHx (Fig. 6C,D).
PHx INDUCED A LOWER DIVERSITY OF CDR3 AMINO ACIDS
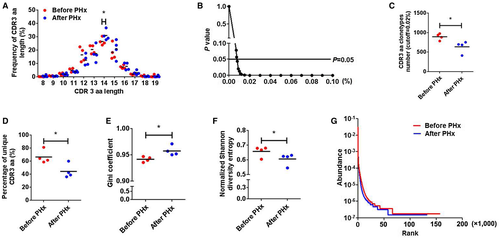
CDR3 amino acids (aa) clonotypes, which include a conserved cysteine in the Vβ region and a conserved phenylalanine in the Jβ region, determine the diversity of the TCRβ repertoire. In the current study, 418,944 distinct CDR3 aa clonotypes were identified pre-PHx and post-PHx (Supporting Table S4). We first analyzed CDR3 aa length in hepatic regeneration and found that CDR3 with a length of 14 aa posthepatectomy showed a higher usage frequency than that prehepatectomy (Fig. 7A). Although the total CDR3 aa clonotypes were nearly identical in both groups, PHx led to obvious reductions in high-frequency CDR3 aa clonotypes (cut-off threshold, >0.008%; P < 0.05; Fig. 7B,C) and the percentage of unique CDR3 aa (Fig. 7D). Furthermore, the Gini coefficient, normalized Shannon diversity entropy, and Rank abundance were used to compare CDR3 aa diversity. All results suggested a remarkably decreased CDR3 aa diversity post-PHx (Fig. 7E-G). These data indicated that PHx changes the usage of several key high-frequency CDR3 aa and lessens TCRβ diversity.
Discussion
In the current study, ablation of TCRβ led to a significant defect in LR, as manifested by reduction in pathological injury, impairment of hepatocyte replication, and increase in apoptotic hepatocytes. In addition, TCRβ deficiency drastically reduced the expression of inflammatory factors, which are essential for efficient LR. As a result, activation of STAT3, a key component in the inflammatory signaling cascade mediating the mitogenic response of hepatocytes to inflammatory factors post-PHx,24-26 is diminished. Accordingly, we observed diminished cyclin D1 induction in regenerating livers of tcrb–/– mice. Moreover, the enhanced hepatocyte apoptosis could also be ascribed to attenuated STAT3 activity, which promotes hepatocyte survival.3 These data showing that TCRβ is indispensable for hepatocyte proliferation in LR are consistent with a previous study revealing the crucial role of T cells in LR.16 The interesting observation, however, is that coculture with αβ T cells, which isolated from regenerative liver, did not exert impact on hepatocyte growth, whereas coculture intrahepatic immune cells significantly altered hepatocyte proliferation and apoptosis. This result demonstrated that hepatocyte survival is modulated by the intrahepatic immune microenvironment rather than αβ T cells alone.
Our work and a preceding study suggest that a robustly elevated T-cell population (both αβ T and γδ T cell) was observed in WT liver post-PHx. However, few proliferative αβ T or γδ T cells were found after isolation in vivo. It is indicated that an expanded T-cell population originated from extrahepatic recruitment. Strikingly, unusual expression of cytokines and distribution of immunocyte subsets were found in regenerating tcrb–/– livers. The phenomenon of aberrant immune status in regenerating livers of tcrb–/– mice parallels its effects in TCRδ–/– mice, in which regenerating TCRδ–/– livers have dampened inflammatory milieu and immunocyte activation. It is suggested that αβ T cells govern the inflammatory orchestration of hepatic regeneration by modulating the phenotype and recruitment of diverse intrahepatic immunocytes. Interestingly, our experimental results support the notion that the absence of αβ T cells increases the amount of γδ T cells and the presence of these cells mediates cytokines IL-17 and IL-22, which have been reported to promote hepatic regeneration.9, 17 This may be explained as a feedback mechanism responding to αβ T-cell deficiency, assuring a compromised LR process.
NK and NKT cells, two subsets of innate immune cells that account for 40% the intrahepatic lymphocytes in mice,27 play a critical role in bacterial and viral hepatitis and steatohepatitis.28, 29 Increasing evidence suggests that NK and NKT cells are increased and activated in the liver post-PHx. However, NK and NKT cell activation obviously retards hepatic regeneration, which is mediated through secretion of IFN-γ.10, 23 In this study, NK and NKT cells were more activated in regenerating Tcrb–/– livers producing higher IFN-γ. The finding that NK and NKT cell activation mediated by TCRβ-null impeded LR is consistent with previous studies. In addition, our results demonstrated that the fraction of invariant NKT cells (CD3+, CD1d-Tet+) was lessened post-PHx, which is in line with the conclusion that invariant NKT cells play a major role in LR.30 Accordingly, the effect could be also driven by a loss of invariant NKT cells as well. Apart from NK and NKT cells, KCs, another important component of the innate immune system in the liver, are also involved in LR. During the early phase post-PHx, KCs recruited from bone marrow are activated and produce TNF-α, which accelerates LR.31 In Tcrb–/– mice, KC activation seems quite weak, though its population was not clearly altered post-PHx. Taken together, these results suggest that TCRβ deficiency selectively decreases KC secretion of TNF-α and IL-6 and increases activated NK and NKT cells in regenerating livers, resulting in an impairment of hepatic regeneration.
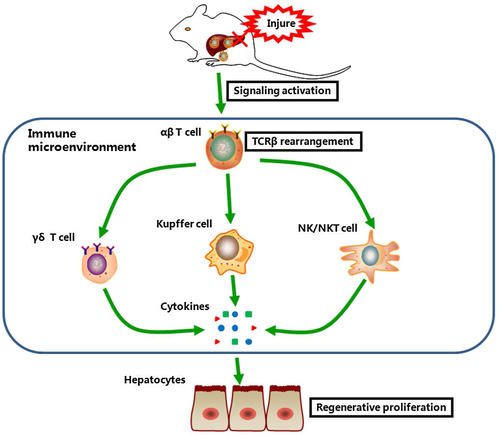
In past decades, more attention has been focused on a single parameter of the immune system, especially in the innate immune system. However, the interaction between immunocytes and the correlation between the innate immune system and adaptive immune system are still not clear. Interestingly, the abnormal activation of different subsets of immunocytes was found in both regenerative Tcrb–/– and TCRδ–/– livers.17 Given the highly aberrant inflammatory milieu and immune microenvironment, it has been postulated that the adaptive immune system acts more like a “commander” than like an executor during LR (Fig. 8). Meanwhile, we cannot exclude other mechanisms by which TCRβ regulates LR beyond immune status. Instead, more regulatory mechanisms and how TCRβ works with other immunocytes in LR need to be further examined.
The TCR immune repertoire, which has been associated with various liver diseases (including chronic virus hepatitis, hepatocellular carcinoma, and primary biliary cirrhosis), is a potential tool to monitor intrahepatic immune status and the microenvironment.20, 32-34 Accumulating studies indicate that PHx induces a series of inflammatory signaling cascades that interact to ensure complete LR. In this process, the immune response itself provides exact identification and has been associated with both hepatic parenchymal and nonparenchymal cell proliferation. The combination of the identities and distribution of PHx-responsive TCR represent a “footprint” of immune conditions. Thus, identifying and tracking TCR diversity has increased in priority as a strategy to understand the dynamics and distribution of immunocyte populations in LR instead of trying to correlate single parameters with the complex process.
Our results indicate an intact profile of Vβ, Jβ gene segments, and CDR3 clonotypes during LR. In the present study, significantly reduced TCRβ diversity and altered high-frequency CDR3 aa usage were observed post-PHx. However, no significant difference in distribution of Vβ, Jβ gene, and V-J combinations were found pre-PHx or post-PHx. Our work idicates the variation of TCRβ immune repertoire in the process of LR. Conventionally, αβ T cells mainly exert immune surveillance function by using its surface TCRs to recognize peptides bound to an MHC molecule on infected or otherwise altered cells. In contrast to γδ T cells, the immunoregulatory function of αβ T cells has rarely been explored. Nevertheless, this is not to say that the role of αβ T cells can be neglected. Our observation of lower clonality and diversity of αβ T cells post-PHx provides evidence of regeneration-associated immune response in the adaptive immune system. This finding may offer insight into the immunoregulatory function of the adaptive immune system that is independent of hepatic regenerative progression and synergistic with the innate immune system.
In conclusion, our work exhibits the diversification of the TCRβ immune repertoire during LR and demonstrates an impaired immune status, which at least partly accounts for the defect of LR in the absence of TCRβ. It provides insights into the regulation of hepatocyte regenerative proliferation and its relationship with the adaptive immune system.
Potential conflict of interest
Nothing to report.
REFERENCES
Author names in bold designate shared co-first authorship.
Abbreviations
-
- aa
-
- amino acids
-
- ALT
-
- alanine aminotransferase
-
- BrdU
-
- 5-bromodeoxyuridine
-
- cDNA
-
- complementary DNA
-
- CDR
-
- complementary determined regions
-
- D
-
- diversity
-
- IF
-
- immunofluorescence
-
- IFN-γ
-
- interferon-γ
-
- IL
-
- interleukin
-
- IMGT
-
- ImMunoGeneTics
-
- J
-
- joining
-
- KCs
-
- Kupffer cells
-
- LR
-
- liver regeneration
-
- MHC
-
- major histocompatibility complex
-
- NK
-
- natural killer
-
- NKT
-
- natural killer T lymphocyte
-
- PHx
-
- two-thirds partial hepatectomy
-
- p-STAT3
-
- phosphorylation of STAT3
-
- STAT3
-
- signal transducer and activator of transcription 3
-
- TCR
-
- T-cell receptor
-
- Tcrb–/–
-
- TCRβ chain-deficient
-
- TcrbAD-Cre
-
- Cre adenovirus-directed condition deletion in Tcrbflox/flox mice
-
- TNF-α
-
- tumour necrosis factor-α
-
- V
-
- variable
-
- TUNEL
-
- terminal deoxynucleotidyl transferase dUTP nick end labeling
-
- WT
-
- wild type




